Abstract
Adolescence is marked by the development of personal identity and is associated with structural and functional changes in brain regions associated with Self processing. Yet, little is known about the neural correlates of self-reference processing and self-reference effect in adolescents. This fMRI study consists in a self-reference paradigm followed by a recognition test proposed to 30 healthy adolescents aged 13–18 years old. Results showed that the rostral anterior cingulate cortex is specifically involved in self-reference processing and that this specialization develops gradually from 13 to 18 years old. The self-reference effect is associated with increased brain activation changes during encoding, suggesting that the beneficial effect of Self on memory may occur at encoding of self-referential information, rather than at retrieval.
Keywords: Self-reference processing, self-reference effect, adolescents, fMRI.
Self-reference processing refers to the ability to distinguish self-relevant from non-self-relevant information and is proposed to be at the core of the Self (Northoff et al., 2006). The concept of Self can be defined as a set of personal complex and multidimensional mental representations about ourselves and the associated flow of self-consciousness (Duval et al., 2009). In the memory domain, the Self is represented by one’s episodic memories and personal semantic knowledge which are at the core of one’s identity (Conway & Pleydell-Pearce, 2000; Conway, 2005). Cerebral substrates of the self-reference processing have been largely investigated in adults using “self-reference” tasks in which participants evaluate whether personal attributes are self-descriptive. Much evidence indicates that self-reference processing in adults recruits cortical midline structures (CMS), including medial prefrontal cortex (mPFC), adjacent rostral anterior cingulate cortex (ACC), precuneus and posterior cingulate cortex (PCC) (Johnson et al., 2002; Northoff & Bermpohl, 2004; Northoff et al., 2011, 2006; van der Meer et al., 2010). However, it has been demonstrated that self-reference processing and appraisal of Others share a common network of CMS regions (Araujo et al., 2013; Benoit et al., 2010; Qin & Northoff, 2011). The mPFC and the PCC were found to be recruited during both self-reference processing and appraisal of Others, while the rostral ACC has been specifically involved in self-reference processing (Araujo et al., 2013; Murray et al., 2012; Qin & Northoff, 2011; Qin et al., 2012).
According to the Self-Memory System, the Self and autobiographical memory share a constant reciprocal interaction (Conway & Pleydell-Pearce, 2000; Conway, 2005, Conway et al., 2004). Autobiographical memories are important in shaping the Self and in providing a sense of continuity over time (Conway & Pleydell-Pearce, 2000; Conway, 2005). In turn, the Self modulates construction and access to autobiographical memories, both at encoding and at retrieval, and matches memory consistency with one’s current goals and beliefs (self-coherence, Conway et al., 2004). The relation between Self and memory is reflected in the self-reference effect, whereby memory benefits from self-referential information (Roger et al., 1977; for review see Klein, 2012) and has been confirmed by numerous studies (Heatherton et al., 2006; Kelley et al., 2002; Serbun et al., 2011; Symons & Johnson, 1997; Turk et al., 2008). Neuroimaging studies examining the self-reference effect in adults consistently showed that mPFC activity predicts subsequent memory performances of self-referential items (Fossati et al., 2003; Gutchess et al., 2010; Macrae et al., 2004; Philippi et al., 2012; Zhu et al., 2012). Neuroimaging studies on the self-reference effect during retrieval showed mPFC activation associated with the successful retrieval of self-encoded information (Benoit et al., 2010; Fossati et al., 2004; Morel et al., 2014). Fossati et al. (2004) suggested that the self-reference effect involves both commun and different brain regions at encoding and retrieval. Morel et al. (2014) examined the relative contribution of encoding and retrieval processes to the self-reference effect in young adults and showed that this effect is associated with brain changes at encoding only, including greater activity in the ventral mPFC and hippocampus. They proposed that the reactivation of self-related memories during the encoding of self-referential information may promote successful retrieval.
Adolescence is core period of life for the development of a cohesive sense of Self and for shaping one’s personal identity (for review, Pfeifer & Peake, 2012; Sebastian et al., 2008). Autobiographical memories pertaining to adolescence are crucial for the construction of identity which makes this period rich in “self-defining” experiences (Conway, 2005). From a neurodevelopmental point of view, self-development during adolescence might be associated with structural and functional changes in regions important for the Self, specifically the mPFC (Blakemore, 2012, 2008; Casey et al., 2005, 2000; Mills, Lalonde et al., 2014; Pfeifer & Blakemore, 2012; Pfeifer & Peake, 2012; Sebastian et al., 2008). Longitudinal structural neuroimaging studies show that the mPFC is one of the latest developing regions (Mills, Goddings et al., 2014; Mills, Lalonde et al., 2014; Tamnes et al., 2013). Developmental fMRI studies on metalizing (including self-reference processing studies described below) showed that mPFC activity increases between childhood and adolescence, followed by a decrease between adolescence and adulthood (Blakemore, 2012, 2008).
Only a few neuroimaging studies have examined brain substrates of self-reference processing in typically developing adolescents. Results confirmed the central role of the mPFC, including the rostral ACC, during self-reference processing in adolescents (Jankowski et al., 2014; Pfeifer, Kahn, et al., 2013; Pfeifer, Merchant, et al., 2013; Pfeifer et al., 2009; Schneider et al., 2012). Furthermore, the longitudinal study by Pfeifer, Kahn, et al. (2013) in the same adolescents at 10 and 13 years old showed that hyperactivation of ventral mPFC during self-reference processing increased between 10 and 13 years, compared to appraisal of a fictional other (i.e., Harry Potter). Although the proposal that neurodevelopmental changes in the mPFC through adolescence might be related to self-development (Sebastian et al., 2008), no study has yet evaluated if increase in mPFC activity during self-reference processing continues in adolescents older than 13.
Behavioural studies on the self-reference effect in childhood suggested that this effect emerges around 4 years old (Cunningham et al., 2013; Halpin et al., 1984) and then increases from middle to late childhood in line with the self-concept development (Halpin et al., 1984; Ray et al., 2009). Only one neuroimaging study examined the self-reference effect during childhood (Ray et al., 2009) and found that memory for self-referentially encoded trait words increased from ages 7 to 13. Moreover, they observed that the self-reference effect correlated positively with activations in rostral ACC. However, brain activity was recorded during the encoding phase only. Hence, no study has yet examined brain regions underlying the self-reference effect at retrieval in adolescents. Moreover, whereas adolescence is a core period for self-development, no study has examined the self-reference effect development in adolescents older than 13.
Here, we examined the neural correlates of self-reference processing and self-reference effect at encoding and retrieval using fMRI in a group of healthy adolescents. The first aim of our study was to investigate common and specific brain regions involved in appraisal of Self (self-reference processing) and Others. Based on results of previous studies on self-reference processing, we hypothesized that appraisal of Self and Others would recruit a common network of CMS regions, but that ACC would be more specifically recruited during self-reference processing than appraisal of Others. Moreover, according to results by Pfeifer, Kahn, et al. (2013) in adolescents younger than 13 years old, we hypothesized that hyperactivation of ventral mPFC, including the rostral ACC, during self-reference processing compared to appraisal of Others would continue to increase through adolescence. The second aim of our study was to examine the self-reference effect and its neural correlates during encoding and recognition to determine at which phase (i.e., encoding or retrieval) the Self may have a beneficial effect on memory. Based on previous behavioural findings in children (Cunningham et al., 2013) and in adults (Serbun et al., 2011), we hypothesized that self-referencing would enhance recognition and source memory performances in adolescents. Because, on one hand, the self-reference effect is proposed to increase with the self-concept development during childhood (Halpin et al., 1984; Ray et al., 2009) and, on the other hand, adolescence is a core period for self-development (Pfeifer & Blakemore, 2012; Pfeifer & Peake, 2012; Sebastian et al., 2008), we hypothesized that the self-reference effect would continue to increase through adolescence. According to the study of Morel et al. (2014) in adults, we hypothesized that Self would have a beneficial effect on memory during the encoding of Self-referential information, but not at retrieval. Finally, we expected to find functional brain changes in regions involved in the self-reference effect through adolescence.
Method
Participants
Thirty typically developing Caucasian adolescents (15 females; WISC-IV (Wechsler, 2005): Perceptual Reasoning Index = 108.3 ± 3.3; Verbal Comprehension Index = 112.7 ± 3.3) aged 13 to 18 years old (mean age = 15.92 ± 1.66 years) were recruited from several junior high schools in Caen (Normandy, France) between April 2011 and January 2013. All were right handed and were French native speakers. None of them reported any prior or current neurological or psychiatric disorders, learning disabilities, head trauma, psychoactive medication, and MRI contraindications. Families were given a comprehensive description of the research. We obtained written informed consent from adolescents and their parents, in line with the guidelines of the relevant ethics committees. The study was approved by the Regional Ethics Committee (CPP Nord Ouest III).
fMRI task and procedure
In the scanner, participants performed an event-related self-reference paradigm. They were instructed to indicate whether or not trait adjectives i) characterized themselves (“Self” condition), ii) characterized two well-known French celebrities, Johnny Hallyday (French singer and actor) in the first run and Nicolas Sarkozy (French politician, former president of France) in the second run (“Other” condition) or iii) whether adjectives were positive or not (“Semantic” condition). The use of two celebrities, one political figure and one artist, permitted us to study the judgement of a famous person in general and not oriented to one specific figure, i.e. precluding any preferential judgement in favour of one or the other. We verified, in a prior interview, if participants had any personal affinity with either celebrity, which was not the case.
The present paradigm was adapted from a study on self-reference processing and self-reference effect in adults (Morel et al., 2014). Principal changes concerned the celebrity used for the Other condition (Nicolas Sarkozy instead of Jacques Chirac, an older president which adolescents may not know) and trait adjectives which were adapted to adolescent vocabulary. Trait adjectives (total = 206) were selected from a pool of 463 personality trait adjectives issued from a French language dictionary. This selection of adjectives was based on their familiarity and valence ratings obtained from a preliminary experiment on 114 adolescents aged 12 to 16 years (mean age: 13.5 ± 0.69 years) which have not taken part in the fMRI study. No adjectives shorter than 4 letters and longer than 12 letters (mean length of adjectives: 7.83 ± 1.83 letters) were selected.
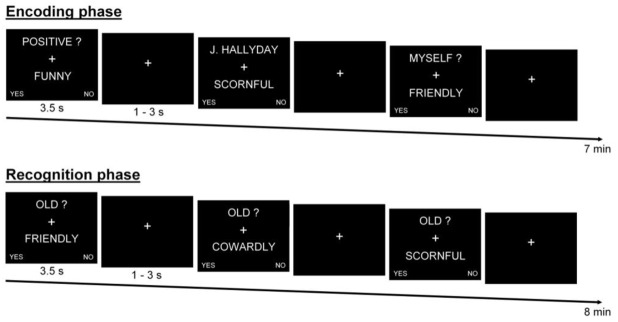
After a training session performed outside the scanner to familiarize the participants with the task, the encoding phase took place and consisted of two runs (7 minutes each) with presentation of 72 stimuli (24 by condition) and the associated cue word informing of the condition to appear (Self, Other or Semantic). Each adjective was presented for 3.5 seconds, followed by a fixation cross jittered between 1 and 3 seconds (see Figure 1).
Figure 1.
Trial structure. During the encoding session (top), personality traits adjectives were presented and participants had to indicate whether or not the adjective characterized themselves (Self condition), characterized a French celebrity (J. Hallyday in the first run and N. Sarkozy in the second run; Other condition) or were positive or not (Semantic condition). During the recognition session (bottom), participants had to indicate if the presented adjective had already been seen during the encoding phase or not (by answering if the adjective was “Old?”, i.e. had been seen, or not).
After the encoding phase, an unannounced recognition task took place, including two runs (8 minutes each) with presentation of 54 adjectives (18 by condition) of the encoding phase and 30 new adjectives (Distractors). Participants had to indicate if the presented adjective had already been seen during the encoding phase or not (i.e., recognition task). Adjectives were presented for 3.5 seconds, followed by a fixation cross jittered between 1 to 3 seconds (see Figure 1). Of note, “old” adjectives of the first recognition run were seen during the first encoding run, while adjectives of the second encoding run were presented during the second recognition run.
Trait adjectives were presented visually using E-Prime (Psychology Software Tools, Pittsburgh, PA) implemented in IFIS System Manager (In vivo, Orlando, FL). Lists of adjectives displayed for each condition and the yes/no answers on the keyboard were counterbalanced across participants and for each participant, valence of trait adjectives was counterbalanced across conditions (i.e., Self, Other, Semantic and Distractor).
Post-scanning session: source retrieval test
After the scanning session, participants performed an unannounced source retrieval test outside the scanner. Each adjective correctly recognized during the recognition phase was presented again to the participant who had to indicate under which condition the adjective had been judged during the encoding phase. Participant answered by pressing one of five buttons corresponding to “Myself”, “N. Sarkozy”, “J. Hallyday”, “Positive?” and “I don’t know”. Because of a technical problem, one participant did not perform the post-scanning session. A limit of our study may be in the use of two celebrities which may increase task difficulty during the source retrieval test at debriefing (i.e., post-scanning, outside scanner). Yet, this is a secondary issue of our study, as our main interest resides in the cerebral substrates of encoding and recognition in the scanner.
MRI acquisition
Imaging data were collected using an 8 channel head coil on a Philips Achieva 3.0 T MRI scanner (Eindhoven, the Netherlands) through two scanning sessions. During the first acquisition session (anatomical session), a high resolution T1-weighted anatomic image was acquired using a three-dimensional fast field echo sequence (3D-T1-FFE sagittal; repetition time (TR): 20 ms; echo time (TE): 4.6 ms; flip angle: 10°; 180 slices; slice thickness: 1 mm; no gap; matrix: 256 × 256; field of view (FoV): 256 × 256 mm2; in-plane resolution: 1 × 1 mm2), followed by a high-resolution T2-weighted spin echo anatomical acquisition (2D-T2-SE sagittal; TR: 5500 ms; TE: 80 ms; flip angle: 90°; 81 slices; slice thickness: 2 mm; matrix: 256 × 256; FoV: 256 × 256 mm2; in-plane resolution: 1 × 1 mm2) and a non-Echo-Planar Imaging (non-EPI) T2-star volume (2D-T2*-FFE axial; TR: 3509 ms; TE: 30 ms; flip angle: 90°; 70 slices; slice thickness: 2 mm; matrix: 128 × 128; FoV: 224 × 224 mm2; in-plane resolution: 2 × 2 mm2). In the second acquisition session (functional session), a non-EPI T2* image was acquired similar to the anatomical session. Then, functional data were acquired with an interleaved two-dimensional T2* SENSitivity Encoding (SENSE) EPI sequence designed to reduce geometrical distortions and magnetic susceptibility artefacts (2D-T2*-FFE-EPI axial, SENSE factor: 2; TR: 2382 ms; TE: 30 ms; flip angle: 80°; 42 slices; slice thickness: 2.8 mm; no gap; matrix: 80 × 80; FoV: 224 × 224 mm2; in-plane resolution: 2.8 × 2.8 mm2; 172 and 199 volumes per run for encoding and recognition phases respectively). In total, four functional runs were acquired per participant: two for the encoding task and two for the recognition task.
Behavioural analysis
Behavioural data were analyzed using Statistica (Statsoft, Tulsa, OK). Response times were collected during encoding and recognition phases. To study self-reference processing, we performed a repeated measures ANOVA with conditions (Self x Other x Semantic) as within-subject factor on response times during encoding. Then, to explore the relation between age and self-related processes, we performed correlation analyses, between age and response times. To evaluate the self-reference effect, repeated measures ANOVAs with conditions (Self x Other x Semantic) and subjects’ answers (recognized x non-recognized) as within-subject factors were conducted on response times during the encoding and recognition phases. Memory performances scores (Hits/(Hits+Misses) × 100) were calculated for each encoding condition (Self, Semantic and Other) for both recognition and source retrieval tests. Firstly, memory performances for each condition were compared to random level (i.e., 50% for recognition and 20% for source retrieval) using t-tests. Then, repeated measures ANOVAs with conditions (Self x Other x Semantic) as within-subject factor were conducted on recognition and source retrieval performances. When ANOVAs revealed significant effects, post-hoc pairwise comparisons were performed using Fisher’s LSD test. Finally, to explore behavioural changes on the self-reference effect through adolescence, we performed correlation analyses, between age and response times during encoding and recognition phases, as well as between age and memory performance during recognition and source retrieval tests.
fMRI analysis: Pre-processing and statistical analysis
Data pre-processing and statistical analysis were performed with SPM5 (Wellcome Trust Centre for Neuroimaging, London, UK). During pre-processing steps, after checking EPI data for the lack of artefact and discarding the six first EPI volume of each run to allow signal stabilization, images of each run were corrected for slice timing and realigned to the first volume of the corresponding run. Then, coregistration was carried out as followed: first, the mean EPI image (calculated during realignment) was coregistred onto the non-EPI T2* volume of the functional session. Second, the non-EPI T2* volume of the functional session was coregistred onto the anatomical one. Third, the T2 image was coregistred on to the non-EPI T2* volume of the anatomical session. Fourthly, the T1 volume was coregistered onto the T2 image. Geometric EPI distortions were corrected by warping the mean EPI image to roughly match the non-EPI T2* volume using the methodology developed and validated by Villain et al. (2010). The T1 image was then segmented and normalized to an age-appropriate stereotactic template (NIHPD 13–18.5: http://www.bic.mni.mcgill.ca/ServicesAtlases/NIHPD-obj1, Fonov et al., 2011) and resulting normalization parameters were applied to the coregistred T1, EPI and non-EPI T2* volumes with a voxel size of 2 × 2 × 2 mm. Finally, the normalized EPI images were smoothed at 8 mm full width at half maximum (FWHM). Additionally, an individual grey matter (GM) mask was created for each participant by conjunction of two binary images: the GM segments from the T1 volumes including only voxels with values greater than 0.15 and the non EPI-T2* volume including only voxels with values greater than 0.05. This individual GM mask was used as an explicit mask during first level analysis.
For first level analyses, two general linear models, one for the encoding phase and one for the recognition phase, were applied to each participant and brain responses were modeled at each voxel. Based on the subject’s answers during the recognition session, recognized and non-recognized items of each condition (Self, Other and Semantic) were identified (see Appendix 1 for the number of trials and non-responses for each condition). Both encoding and recognition models included six experimental conditions of interest (Self recognized, Self non-recognized, Other recognized, Other non-recognized, Semantic recognized and Semantic non-recognized) and one condition of non-interest corresponding to non-responses. In addition, Self, Other and Semantic items seen during encoding, but not seen again at recognition, were added as three conditions of interest in the encoding model, whereas distractors correctly rejected and false alarms were included as two conditions of non-interest in the recognition model. All conditions were modelled from item onset to subjects’ response times. Moreover, valence for each item and movement parameters were modelled as regressors.
To study self-reference processing at encoding, the main effects of Self, Other and Semantic conditions (independently from the recognition of the items and including non-seen again items) were assessed for each participant for the encoding model. Then, these three contrasts were entered in a flexible factorial design with two factors at the second level with conditions (Self, Other and Semantic) as within-subject factor and subjects as random factor. To evaluate the self-reference effect at encoding and recognition, six contrasts corresponding to Self recognized, Self non-recognized, Other recognized, Other non-recognized, Semantic recognized and Semantic non-recognized were created for each participant for both encoding and recognition models and were entered in two flexible factorial designs (one for encoding and one for recognition) with three factors at the second level with conditions (Self, Other, Semantic) and subjects’ answers (recognized, non-recognized) as within-subject factors and subjects as random factor.
For all second level analyses, brain activity was examined for the Self and Other conditions in two steps: First, we contrasted the Self and Other conditions with the Semantic condition (Self > Semantic and Other > Semantic) to assess Self and Other activation networks. To explore whether Self and Other shared a common activation network, we conducted conjunction analyses between Self > Semantic and Other > Semantic contrasts. Second, we directly compared Self and Other conditions (Self > Other and Other > Self) to determine which regions are more engaged during Self than Other condition and inversely. Note that for the self-reference effect, we considered only recognized items at encoding (to evaluate successful encoding) and at recognition (to examine successful recognition), for each contrast (Self Recognized > Semantic Recognized, Other Recognized > Semantic Recognized, Self Recognized > Other Recognized and Other Recognized > Self Recognized). Group effects were masked using a GM mask based on the mean normalized GM maps from the T1 images of all participants. Resulting statistical maps were thresholded at an uncorrected voxel-level p value of 0.001 corrected for multiple comparisons at p<.05 using an extent threshold of 93 voxels for self-reference processing, 91 voxels for self-reference effect at encoding and 80 voxels self-reference effect at recognition. These extent thresholds have been determined using Monte Carlo simulations via the AFNI (Analysis of Functional NeuroImages software; Cox, 1996) tool 3dClustSim applied to each flexible factorial design. Coordinates are reported in the MNI stereotaxic space.
To examine brain activity changes during self-reference processing and self-reference effect through adolescence, we performed correlations between age and neural activity in clusters obtained from the direct comparison of Self and Other. To this aim, we extracted for each participant the parameter estimates of the canonical hemodynamic response function within these clusters for the linear combination of the contrast of interest (Self > Other for self-reference processing and Self Recognized > Other Recognized for the self-reference effect). Then, we conducted correlations between age and parameter estimates.
Results
Behavioural results
Self-reference processing
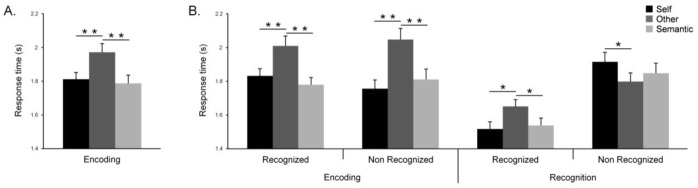
During the encoding phase, analyses revealed a significant main effect of condition on response times (F(2,58) = 18.21, p < .001; Figure 2) with longer response times for the judgment of trait adjectives in the Other than the Self and Semantic conditions (p < .001). Response times during Self and Semantic conditions were not significantly different (p = .45). Correlation analyses between age and response times showed no significant result.
Figure 2.
Response times for each condition during self-reference processing (A) and the self-reference effect (B) at the encoding and recognition phases (error bars represent standard error of the mean, * p < .05, ** p < .001).
Self-reference effect
During the encoding phase, analyses on response times revealed a significant main effect of condition (F(2,58) = 20.00, p < .001), no significant main effect of subjects’ answers (F(1,29) = .02, p = .89) and a significant interaction between condition and subjects’ answers (F(2,58) = 3.67, p = .03). Response times were shorter for encoding of subsequent correct recognition of Self and Semantic than Other adjectives (Self vs. Other: p < .001, Semantic vs. Other: p < .001). Response times for encoding of subsequent correct recognition Self and Semantic adjectives were not significantly different (p = .12). During the recognition phase, analyses on response times (Figure 2) revealed no significant main effect of condition (F(2,58) = .62, p = .54), a significant main effect of subjects’ answers (F(1,29) = 56.66, p < .001) and a significant interaction between condition and subjects’ answers (F(2,58) = 8.60, p < .001). Response times were shorter for Self recognized and Semantic recognized than Other recognized adjectives (Self vs. Other: p = .003, Semantic vs. Other: p = .01). Response times for Self recognized and Semantic recognized adjectives were not significantly different (p = .63). In addition, correlation analyses between age and response times showed no significant results during the encoding and recognition phases.
Memory performances for J. Hallyday and N. Sarkozy were not significantly different during the recognition phase (t(29) = 1.56, p = .13) and during the source retrieval test (t(28) = − .45, p = .65). In addition, memory performances for J. Hallyday and N. Sarkozy were higher than the random level during both the recognition phase (random level = 50%, J. Hallyday: t(29) = 4.28, p < .001; N. Sarkozy: t(29) = 2.85, p = .008) and the source retrieval test (random level = 20%, J. Hallyday: t(28) = 6.64, p < .001; N. Sarkozy: t(28) = 5.26, p < .001). Thus, responses for N. Sarkozy and J. Hallyday were collapsed in the Other condition.
During the recognition phase, memory performances of each condition were higher than the random level (i.e. 50%, Self: t(29) = 13.15, p < .001; Other: t(29) = 4.65, p < .001; Semantic: t(29) = 11.09, p < .001). We observed a significant main effect of condition (F(2, 58) = 18.16, p < .001; see Figure 3). Specifically, participants were better at recognizing adjectives pertaining to the Self than to the Other (p < .001) or to the Semantic condition (p = .033). Hence, our results show a significant self-reference effect on memory. Finally, recognition performances were greater for the Semantic than the Other condition (p < .001). In addition, correlation analyses between age and recognition performance showed no significant result.
Figure 3.
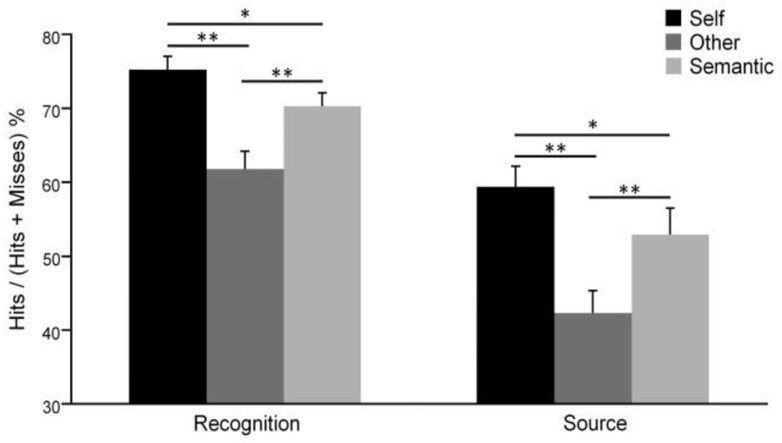
Memory performances for each condition during recognition and source retrieval tests (error bars represent standard error of the mean, * p < .05, ** p < .001).
During the source retrieval test, memory performances in each condition were higher than random level (i.e. 20%, Self: t(28) = 13.59, p < .001; Other: t(28) = 7.10, p < .001 Semantic: t(28) = 9.07, p < .001). Analyses revealed a significant main effect of condition on memory performances (F(2,56) = 18.30, p < .001) with better memory performances for the Self than the Other (p < .001) and Semantic (p = .027) conditions. This shows a source retrieval improvement for adjectives pertaining to the Self (Figure 3). As previously, memory performances were lowest for the Other condition (Other versus Semantic: p < .001). Furthermore, correlation analyses between age and source retrieval memory showed no significant result.
fMRI results
Self-reference processing
The first goal of this study was to determine which brain regions are specifically involved in self-reference processing. Hence, we examined common and specific activation networks recruited during self-reference processing and appraisal of Others.
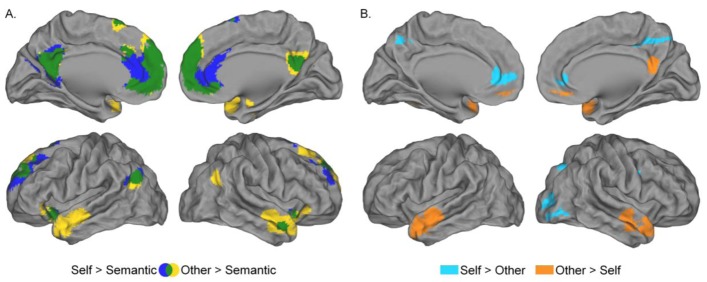
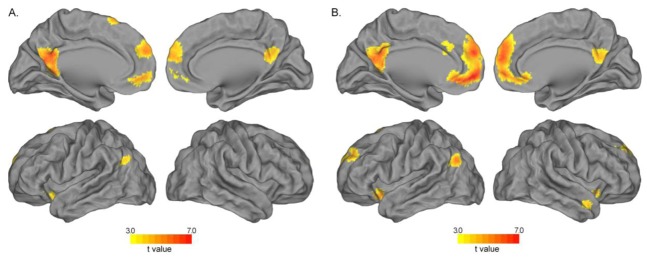
Contrasting the Self to the Semantic condition and the Other to the Semantic condition revealed a large common activation network (Table 1, Figure 4, Appendix 2A), including the PCC extending into the precuneus, the bilateral dorsal mPFC, left superior frontal and angular gyri, as well as the left insula. Additionally, the Other condition recruited the bilateral middle temporal pole, right angular gyrus and right insula.
Table 1. Self-reference processing.
Regions showing activation during appraisal of Self and Others, illustrating the self-reference processing, specifying for each peak the side, Brodmann area(s) (BA), MNI coordinates (x y z), cluster size (k), and t values at an uncorrected voxel-wise threshold of p <0.001. Results are corrected for multiple comparisons at the cluster-level at p <0.05 (corresponding to an extent threshold of 93 voxels, as calculated with the 3dClustSim tool).
| Region | Side | BA | MNI (voxels)
|
k | t values | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Self > Semantic | |||||||
| PCC / Precuneus | L | 23/30 | −4 | −64 | 18 | 1343 | 6.59* |
| mPFC | L | 10 | −4 | 60 | 20 | 2359 | 5.89* |
| Dorsal mPFC | L | 8 | −6 | 22 | 62 | 99 | 5.07 |
| Angular gyrus | L | 39 | −48 | −68 | 36 | 200 | 4.47 |
| Inferior frontal gyrus | L | 47 | −28 | 20 | −16 | 129 | 4.16 |
| Other > Semantic | |||||||
| PCC / Precuneus | R | 23 | 6 | −52 | 26 | 2683 | 10.38* |
| Middle temporal gyrus | L | 21 | −64 | −6 | −18 | 1127 | 10.07* |
| Medial orbital PFC | R | 11 | 2 | 48 | −16 | 629 | 8.86* |
| Middle temporal pole | R | 38 | 46 | 12 | −32 | 1502 | 8.36* |
| Dorsal mPFC | L | 9 | −8 | 62 | 32 | 1091 | 6.93* |
| Angular gyrus | L | 39 | −50 | −72 | 38 | 546 | 6.83* |
| Parahippocampal gyrus | R | 35 | 26 | −28 | −18 | 223 | 6.25* |
| Inferior frontal gyrus | R | 47 | 40 | 34 | −12 | 475 | 6.19* |
| Dorsal mPFC | R | 9 | 6 | 60 | 30 | 678 | 6.09* |
| Angular gyrus | R | 39 | 50 | −62 | 30 | 438 | 6.08* |
| Inferior frontal gyrus | L | 47 | −44 | 20 | −16 | 881 | 5.72* |
| Superior frontal gyrus | R | 9 | 14 | 38 | 56 | 147 | 5.44* |
| Insula | R | 13 | 40 | −24 | 24 | 108 | 4.46 |
| Lingual gyrus | R | 18 | 8 | −80 | −4 | 120 | 4.23 |
| Self > Semantic * Other > Semantic (conjunction) | |||||||
| PCC / Precuneus | L | 23 | −4 | −54 | 26 | 1204 | 6.27* |
| Dorsal mPFC | L | 10 | −4 | 60 | 22 | 338 | 5.70* |
| Medial orbital PFC | L | 11 | −6 | 46 | −12 | 394 | 5.22* |
| Superior frontal gyrus | L | 6 | −6 | 22 | 62 | 95 | 5.07 |
| Dorsal mPFC | R | 10 | 8 | 64 | 12 | 312 | 5.06 |
| Angular gyrus | L | 39 | −48 | −68 | 36 | 196 | 4.47 |
| Insula | L | 13 | −28 | 20 | −16 | 120 | 4.16 |
| Self > Other | |||||||
| Inferior occipital gyrus | R | 19 | 36 | −88 | −12 | 1074 | 6.94* |
| Inferior occipital gyrus | L | 19 | −30 | −84 | −16 | 203 | 4.99 |
| Superior parietal lobule | L | 7 | −10 | −80 | 52 | 100 | 4.42 |
| Rostral ACC | L | 32 | −2 | 44 | 2 | 303 | 4.35 |
| Other > Self | |||||||
| Middle temporal pole | R | 38 | 42 | 20 | −32 | 1575 | 7.74* |
| Middle temporal gyrus | L | 21 | −64 | −8 | −14 | 1026 | 7.15* |
| Precuneus / PCC | R | 23 | 6 | −52 | 26 | 619 | 7.00* |
| Medial orbital PFC | R | 11 | 4 | 46 | −18 | 184 | 6.83* |
| Angular gyrus | R | 39 | 48 | −68 | 28 | 557 | 6.04* |
| Parahippocampal gyrus | L | 37 | −28 | −34 | −20 | 429 | 6.00* |
| Inferior frontal gyrus | R | 47 | 44 | 36 | −18 | 147 | 4.84 |
| Dorsal mPFC | L | 9 | −6 | 48 | 44 | 116 | 4.31 |
Abbreviations: PCC = posterior cingulate cortex; mPFC = medial prefrontal cortex; ACC = anterior cingulate cortex.
Peaks surviving a FWE-corrected voxel-wise threshold of p <0.05.
Figure 4.
Regions showing greater activation during Self-reference processing (blue) and appraisal of Others (yellow) than during Semantic condition. Self-reference processing and appraisal of others involved a common brain network (green). Uncorrected p = .001, k ≥ 75 voxels.
Contrasting Self and Other conditions revealed differences in the activation pattern (Table 1, Figure 5A). The Self condition showed greater activity within inferior occipital gyrus, rostral ACC and the left superior parietal lobule, compared to the Other condition. On the contrary, the Other condition revealed greater activation within middle temporal pole, PCC extending into precuneus, medial orbital PFC, right inferior frontal gyrus as well as right angular gyrus, left parahippocampal gyrus and left dorsal mPFC compared to the Self condition.
Figure 5.
(A)Regions showing greater activation during Self-reference processing than appraisal of Others (cyan) and during appraisal of Others than Self-reference processing (orange). Same threshold as figure 4. (B) Activation difference between Self-reference processing and appraisal of Others in the rostral ACC ([−2 44 2], Z = 4.03, k = 303 voxels) increased from 13 to 18 years old. (C.) Activity in the rostral ACC decreased from 13 to 18 years old for appraisal of Others (black squares), but not for Self-reference processing (grey triangles). * p< .05.
Correlations between age and neural activity during self-reference processing (clusters from the contrast Self > Other) revealed a significant effect only in the rostral ACC cluster ([−2 44 2], t value = 4.35, k = 303) and showed a linear increase in activity within the rostral ACC cluster for the Self compared to the Other condition (i.e., Self > Other) which was positively correlated with age (r = .37, p = .04; see Figure 5B). We performed further analyses to examine how rostral ACC was involved in appraisal of Self and Other through adolescence. To address this issue, we extracted for each participant the parameter estimates of the canonical hemodynamic response function within the rostral ACC cluster for each condition of interest (i.e., Self and Other), and performed correlations between age and parameter estimates. Results showed that rostral ACC activation was significantly and negatively correlated with age only for the Other condition (Other: r = − .38, p = .03; Self: r = − .14, not significant; see Figure 5C). Steiger’s test (Steiger, 1980; see also Howell, 2009), which test for the difference between two dependent correlation coefficients, has been used and showed that these two correlations were significantly different from each other (t(27) = 1.74, p = .047). This suggests that rostral ACC is recruited in the same way during self-reference processing and appraisal of Others in younger adolescents, but then becomes specific to self-reference processing in older adolescents.
Self-reference effect
Encoding phase
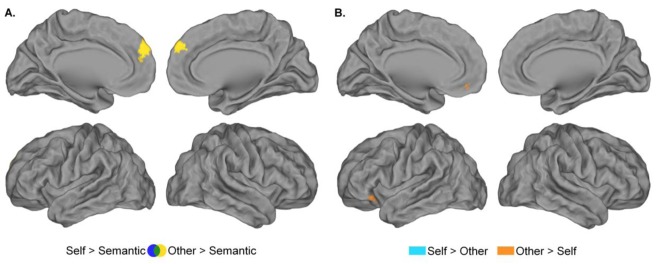
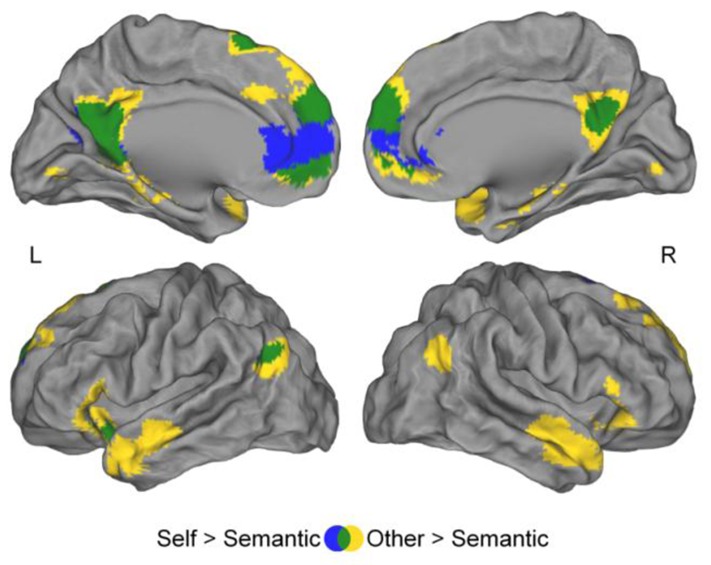
Contrasting the Self to the Semantic and the Other to the Semantic conditions for adjectives that will be correctly recognized during the subsequent recognition phase (successful encoding) revealed a common network of activation (Table 2, Figure 6A, Appendix 2B), including the mPFC, the PCC extending into the precuneus, left angular gyrus, bilateral inferior frontal gyri and right middle temporal gyrus. Successful encoding of Self-referential adjectives activated the rostral ACC, whereas successful encoding of Other-referential adjectives activated the left middle temporal pole. Hence, while successful encoding of Self- and Other-referential adjectives involved a common network compared to the Semantic condition, directly contrasting Self and Other revealed activation differences in specific brain regions (Table 2, Figure 6B). Successful encoding of Self-referential adjectives recruited the rostral ACC, right inferior and superior occipital gyri, precuneus and right inferior parietal lobule. Conversely, the successful encoding of Other-referential adjectives involved the bilateral middle temporal pole, medial orbital PFC, right PCC and left fusiform gyrus compared to the Self condition.
Table 2. Self-reference effect during encoding phase.
Regions showing activation during the successful encoding of Self- and Other-referential adjectives, illustrating the self-reference effect, specifying for each peak the side, Brodmann area(s) (BA), MNI coordinates (x y z), cluster size (k), and t values at an uncorrected voxel-wise threshold of p < .001. Results are corrected for multiple comparisons at the cluster-level at p < .05 (corresponding to an extent threshold of 93 voxels, as calculated with the 3dClustSim tool).
| Region | Side | BA | MNI (voxels)
|
k | t values | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Self Recognized > Semantic Recognized | |||||||
| mPFC / rostral ACC | L | 10 | −6 | 62 | 0 | 5585 | 9.74* |
| Precuneus / PCC | L | 23 | −4 | −66 | 22 | 1906 | 7.07* |
| Inferior frontal gyrus | L | 47 | −28 | 18 | −16 | 338 | 6.10* |
| Angular gyrus | L | 39 | −48 | −74 | 36 | 414 | 6.07* |
| Inferior frontal gyrus | R | 47 | 32 | 18 | −12 | 140 | 5.48* |
| Superior frontal gyrus | R | 6 | 10 | 16 | 68 | 134 | 5.30* |
| Superior frontal gyrus | L | 6 | −14 | 18 | 66 | 120 | 5.05* |
| Middle temporal gyrus | R | 21 | 50 | 10 | −28 | 109 | 4.76 |
| Other Recognized > Semantic Recognized | |||||||
| Medial orbital PFC | R | 11 | 2 | 44 | −18 | 3718 | 10.90* |
| Middle temporal pole | R | 38 | 42 | 20 | −36 | 1514 | 9.32* |
| PCC / Precuneus | R | 23 | 6 | −52 | 24 | 1600 | 9.27* |
| Middle temporal gyrus | L | 21 | −62 | −8 | −14 | 1147 | 8.25* |
| Angular gyrus | L | 39 | −50 | −74 | 34 | 447 | 6.34* |
| Inferior frontal gyrus | L | 47 | −36 | 24 | −14 | 529 | 6.28* |
| Inferior frontal gyrus | R | 47 | 42 | 32 | −14 | 452 | 5.54* |
| Superior frontal gyrus | L | 6 | −8 | 18 | 64 | 162 | 5.33 |
| Angular gyrus | R | 39 | 44 | −58 | 26 | 349 | 4.54 |
| Rostral ACC | L | 24 | −2 | 28 | 24 | 173 | 3.97 |
| Self Recognized > Semantic Recognized * Other Recognized > Semantic Recognized (conjunction) | |||||||
| Medial orbital PFC | L | 11 | −6 | 58 | −8 | 2877 | 7.05* |
| PCC / Precuneus | L | 23 | −2 | −62 | 22 | 1262 | 6.13* |
| Angular gyrus | L | 39 | −48 | −74 | 36 | 307 | 6.07* |
| Inferior frontal gyrus | L | 47 | −36 | 24 | −14 | 294 | 6.06* |
| Inferior frontal gyrus | R | 47 | 30 | 18 | −14 | 134 | 5.14 |
| Middle temporal gyrus | R | 21 | 50 | 10 | −28 | 109 | 4.76 |
| Rostral ACC | L | 24 | −2 | 28 | 24 | 141 | 3.97 |
| Self Recognized > Other Recognized | |||||||
| Rostral ACC | L | 32 | −8 | 46 | −2 | 594 | 6.00* |
| Precentral | R | 44 | 52 | 10 | 32 | 106 | 4.64 |
| Inferior occipital gyrus | R | 19 | 36 | −88 | −10 | 445 | 4.48 |
| Superior occipital gyrus | R | 7 | 26 | −72 | 44 | 597 | 4.29 |
| Inferior parietal lobule | R | 40 | 46 | −42 | 42 | 108 | 4.18 |
| Precuneus | L | 7 | −2 | −64 | 50 | 174 | 4.08 |
| Other Recognized > Self Recognized | |||||||
| Middle temporal pole | R | 38 | 42 | 20 | −36 | 960 | 8.54* |
| Medial orbital PFC | R | 11 | 2 | 42 | −20 | 188 | 7.85* |
| Middle temporal pole | L | 38 | −42 | 18 | −32 | 1119 | 6.22* |
| PCC | R | 23 | 6 | −52 | 26 | 168 | 5.24* |
| Fusiform gyrus | L | 37 | −38 | −44 | −22 | 96 | 4.33 |
Abbreviations: PCC = posterior cingulate cortex; mPFC = medial prefrontal cortex; ACC = anterior cingulate cortex; PFC = prefrontal cortex.
Peaks surviving a FWE-corrected voxel-wise threshold of p < .05.
Figure 6.
Self-reference effect during the encoding phase. (A)Regions showing greater activation during successful encoding of Self-referential adjectives (blue) and Other-referential adjectives (yellow) compared to successful encoding in the Semantic condition. Successful encoding of Self-referential and Other-referential adjectives involved a common brain network (green). (B)Regions yielding greater activation during successful encoding of Self-referential than Other-referential adjectives (cyan), and during successful encoding of Other-referential than Self-referential adjectives (orange). Same threshold as figure 4.
Correlation analyses between age and neural activity during the self-reference effect at encoding (i.e., clusters for the contrast Self Recognized > Other Recognized) showed no significant result.
Recognition phase
During the recognition phase, we did not observe brain activation changes relative to the successful recognition of Self-referential adjectives, neither when the Self condition was contrasted with the Semantic nor with the Other condition (Tables 3, Figure 6A and B). However, we observe brain activation associated with successful recognition of Other-referential adjectives in the two contrasts (Other recognized > Semantic Recognized and Other recognized > Self Recognized). Successful recognition of Other-referential adjectives activated the dorsal mPFC when compared to successful recognition in the Semantic condition (Table 3, Figure 7A) and the left inferior frontal gyrus and medial orbital PFC when compared to successful recognition of Self-referential adjectives (Table 3, Figure 7B).
Table 3. Self-reference effect during recognition phase.
Regions showing activation during the successful recognition of Self- and Other-referential adjectives, illustrating the self-reference effect, specifying for each peak the side, Brodmann area(s) (BA), MNI coordinates (x y z), cluster size (k), and t values at an uncorrected voxel-wise threshold of p < .001. Results are corrected for multiple comparisons at the cluster-level at p < .05 (corresponding to an extent threshold of 93 voxels, as calculated with the 3dClustSim tool).
| Region | Side | BA | MNI (voxels)
|
k | t values | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Self Recognized > Semantic Recognized | |||||||
| No significant activation | - | - | - | - | - | - | - |
| Other Recognized > Semantic Recognized | |||||||
| Dorsal mPFC | R | 9 | 4 | 58 | 34 | 96 | 4.75 |
| Dorsal mPFC | L | 9 | −6 | 58 | 36 | 230 | 4.33 |
| Self Recognized > Semantic Recognized *Other Recognized > Semantic Recognized (Conjunction) | |||||||
| No significant activation | - | - | - | - | - | - | - |
| Self Recognized > Other Recognized | |||||||
| No significant activation | - | - | - | - | - | - | - |
| Other Recognzsed > Self Recognized | |||||||
| Inferior frontal gyrus | L | 47 | −44 | 30 | −16 | 172 | 4.98* |
| Medial orbital PFC | L | 11 | 0 | 42 | −18 | 87 | 4.64 |
Abbreviations: mPFC = medial prefrontal cortex.
Peaks surviving a FWE-corrected voxel-wise threshold of p < .05.
Figure 7.
Self-reference effect during recognition phase. (A.) Regions showing greater activation during successful recognition of Self-referential adjectives (blue) and Other-referential adjectives (yellow) compared to successful recognition in the Semantic condition. Successful recognition of Self-referential and other-referential adjectives did not involve a common brain network (green). (B.) Regions yielding greater activation during successful recognition of Self-referential than Other-referential adjectives (cyan), and during successful recognition of Other-referential than Self-referential adjectives (orange). Same threshold as figure 4.
Discussion
The purpose of this study was to investigate self-reference processing and its interaction with memory (self-reference effect) during both encoding and retrieval of trait adjectives in healthy adolescents. First, we showed that the rostral ACC was more recruited during appraisal of Self (self-reference processing) than Others and that this hyperactivation increased through adolescence. Second, self-referencing enhancement for both recognition and source retrieval performances. Finally, the self-reference effect seems to be a consequence of better encoding of the information, rather than processes taking place during recognition.
Self-reference processing in adolescence
The first aim of our study was to investigate common and specific neural structures involved in self-reference processing and appraisal of Others in healthy adolescents. We observed that appraisal of Self (self-reference processing) and Others involved a common network of CMS regions including mPFC, PCC and precuneus that is consistent with findings in adults (Araujo et al., 2013; Benoit et al., 2010; Qin & Northoff, 2011). These regions seem implicated more generally in the evaluation and judgment of personality traits than specifically in self-reference processing; and appraisal of Self and Others involve, at least partially, the same processes. The mPFC, the PCC and the precuneus have been previously implicated in Self processing (Brewer et al., 2013; Northoff & Bermpohl, 2004; Northoff et al., 2006; Saxe et al., 2006) and social cognition (Amodio & Frith, 2006; Brewer et al., 2013; Saxe et al., 2006). Thus, making a judgment on another person may require the same cognitive processes as making a judgment of oneself. This is consistent with the “simulation” theories of social cognition which propose that we can use knowledge about one’s person to infer the mental states of others (Keysers & Gazzola, 2007; Mitchell et al., 2006, 2005).
Though recruiting common brain regions of CMS, appraisals of Self (self-reference processing) and Others activated distinctive regions. During self-reference processing, adolescents recruited specifically the rostral ACC, a region proposed to be at the core of the concept of Self (Martinelli et al., 2013; Murray et al., 2012; Pfeifer, Kahn et al., 2013; Qin & Northoff, 2011; Schneider et al., 2012). The rostral ACC, which is part of the PFC, undergoes structural and functional changes during adolescence (Blakemore, 2012, 2008; Casey et al., 2005, 2000) that might be related to self-development (Pfeifer & Peake, 2012; Sebastian et al., 2008). Our results showed that rostral ACC hyperactivation during appraisal of Self (self-reference processing) compared to Others increased linearly through adolescence. This result based on cross-sectional comparisons in adolescents aged 13–18 years old complements those of the longitudinal study by Pfeifer, Kahn, et al. (2013) which showed that activation in the ventromedial PFC increased between 10 and 13 years old. We showed that the increasing recruitment of rostral ACC in self-reference processing continues in adolescents older than 13 years old. In addition, this increasing difference in rostral ACC activity between appraisal of Self (self-reference processing) and Others was underlined by the progressive disengagement of the rostral ACC response for appraisal of Others through adolescence. Together, these results may suggest that rostral ACC becomes more specific to self-reference processing through adolescence. Pfeifer and Peake (2012) proposed that there may be more overlap between Self and Others during childhood and predict that longitudinal changes in brain regions recruited during self-reference processing may be related to the development of the Self, consistent with our results. Thus, we suppose that the specialization of the rostral ACC response for self-reference processing through adolescence could be related to the development of the Self.
We showed that appraisal of Others was associated with greater activation in the PCC, the temporal pole and the right dorsal mPFC, regions of the social cognition network in adults and adolescents (Blakemore, 2008; Burnett et al., 2011; Mitchell et al., 2005, 2004). Adopting the perspective of the Other (third-person perspective) has been associated with activation in the PCC (Brewer et al., 2013; Jackson et al., 2006; Ruby & Decety, 2004) and the dorsal mPFC(Mitchell et al., 2006, 2005, 2004; Ruby & Decety, 2003). The temporal pole has been involved in access to social knowledge and retrieval of semantic memory (Frith and Frith, 2003; Olson et al., 2007). This suggests that appraisal of Others implies taking a third-person perspective and memory retrieval of social knowledge. Our result is consistent with those of Pfeifer, Kahn, et al. (2013) in younger adolescents (aged between 10 and 13 years old) showing PCC and dorsal mPFC activations during appraisal of a fictional Other (i.e., Harry Potter). Interestingly, we observed in adolescents that dorsal mPFC activation was more associated with appraisal of Others than with appraisal of Self, unlike Pfeifer et al. (2007) in children (aged between 9 and 10 years old) showing that dorsal mPFC was recruited during both appraisals. Together, these observations provide support for their hypothesis that the dorsal mPFC may increasingly specialize from late childhood to adulthood with the development of social cognition.
Self-reference effect in adolescence
Our second aim was to examine the self-reference effect and its neural correlates during encoding and recognition to determine at which phase (i.e. encoding or recognition) the Self may have a beneficial effect on memory. First, we observed that self-referencing enhances both recognition and source retrieval performances, confirming the presence of the self-reference effect in adolescents. In other words, adolescents showed better memory performances during recognition and source recollection tests for items self-referentially encoded compared to those encoded under the Semantic and Other conditions. This result is consistent with the child (Cunningham et al., 2013; Ray et al., 2009) and adult literatures (Serbun et al., 2011).
Unexpectedly, memory performance for personality trait adjectives encoded under the Semantic condition was higher than for the Other condition. Studies on the self-reference effect find that generally memory performance is highest for Self, intermediate for Other and lowest for Semantic conditions (Benoit et al., 2010; Pauly et al., 2012). Yet, the low degree of intimacy between the participants and celebrities used in the Other condition and the emotional aspect of our Semantic task could explain the present results.
Second, our results suggest that the self-reference effect is associated with more brain activation changes during encoding than recognition phases, suggesting that the Self may have a beneficial effect on memory during the encoding of the self-referential information. This result is consistent with results by Morel et al. (2014) in young adults showing that successful encoding of self-referential adjectives recruited specifically the ACC and the precuneus. As we previously showed, rostral ACC was associated with self-reference processing and is a core region of the concept of Self (Araujo et al., 2013; Martinelli et al., 2013; Northoff et al., 2011). However, precuneus activation was observed only for successful encoding of self-referential adjectives (not during the self-reference processing), suggesting that the self-reference effect may be subtended by the additional recruitment of the precuneus during encoding. The precuneus has been involved in episodic memory retrieval (Sajonz et al., 2010; for review, Cavanna & Trimble, 2006), in particular in episodic autobiographical memories (Viard et al., 2010, 2007; for review, Cabeza & St Jacques, 2007). Thus, recollection of episodic autobiographical memories during self-reference processing may enhance encoding of self-referential information and lead to the self-reference effect. Successful recognition of Self-referential adjectives was not associated with significant greater activation in any brain regions compared to Semantic and to Other conditions, while successful recognition of Other-referential adjectives activated preferentially the dorsal mPFC. The dorsal mPFC has been associated with decision making processes (van der Meer et al., 2010), making judgments about the external world (Denny et al., 2012) and processes used for selecting higher level social and affective meanings (Olsson & Ochsner, 2008). The dorsal mPFC activation observed during successful recognition of Other-referential items could be associated with decision making processes in the social domain. Recognition of self-referential information seems to be a more automatic process than recognition of Other-referential information that may require an additional decision making process. Consistent to this suggestion, we observed that response times were shorter for the recognition of self-referential information than for the recognition of other-referential information. In addition, regarding encoding and recognition, we observe that the self-reference effect is essentially subtended by enhancement of encoding, whereas successful memorization of other-referential information requires both encoding and recognition.
Contrary to our hypothesis that the self-reference effect would continue to increase through adolescence, we found no correlation between this effect and age. In addition, we observed no functional change in brain regions recruited during the self-reference effect through adolescence. This could suggest that this effect undergoes no change during adolescence. Indeed, some authors propose that adolescents demonstrate a self-reference effect similar to adults (Halpin et al., 1984; Ray et al., 2009).
Methodological considerations and future directions
The present study is consistent with the proposal that brain changes during adolescence are associated with the refining of cognitive processes (Casey et al., 2005, 2000), in particular the shaping of one’s sense of Self and identity (Sebastian et al., 2008). Here, we showed that the specialization of the rostral ACC response for self-reference processing through adolescence may be related to the development of the Self. Further research on self-reference processing using other measures of self-development (e.g., self-esteem or self-descriptiveness changes with age) may be helpful to validate and better understand the relation between specialization of the rostral ACC response for self-reference processing and the development of the Self through adolescence. In addition, nothing allows us to conclude at which age the specialization ends. Future longitudinal investigations are needed to validate the present results and to clarify the trajectory of brain changes underlying the development of the Self from childhood to adulthood.
Some neuroimaging studies of Self processing in adults show that self-descriptiveness of stimuli modulates mPFC and ACC responses during self-reference tasks (Moran et al., 2009, 2006; Zhu et al., 2012). During adolescence, these regions still undergo functional and structural changes (Mills, Lalonde et al., 2014; Blakemore, 2012; Casey et al., 2005, 2000; Pfeifer & Peake, 2012; Sebastian et al., 2008). Further research should focus on disentangling the specific contribution of self-descriptiveness and development of PFC and ACC during self-reference tasks in adolescents.
Conclusion
Here, we show, first, the central role played by the rostral ACC in self-reference processing which was more recruited during appraisal of Self (self-reference processing) than Others and which increases in activity through adolescence. This specialization may be related to the development of a stable sense of Self and one’s personal identity during this life period. Second, self-referencing enhances both recognition and source retrieval performances, confirming the self-reference effect in adolescents. Finally, and to the best of our knowledge, this study is the first to explore brain substrates of the self-reference effect during both encoding and retrieval in adolescents. We observe that this effect is associated with more brain activation changes during encoding, suggesting that the beneficial effect of Self on memory may occur at encoding of the self-referential information, rather than at retrieval. Furthermore, the self-reference effect is associated with additional recruitment of the precuneus during encoding, suggesting that recollection of episodic autobiographical memories during Self-judgment may enhance encoding of self-referential information and lead to the self-reference effect.
Acknowledgments
This study was supported by the University Hospital of Caen, the Caen school district and the Mutuelle Générale de l’Education Nationale (mutual insurance company). We would like to thank S. Egret, F. Guénolé, F. Mézenge for their help in testing participants and B. Landeau, N. Morel, G. Rauchs and N. Villain for their help in the initial phases of this study. We are also thankful to the adolescents and institutions that took part in our research.
Appendix 1. Number of trials and non-responses for each condition
| Self
|
Other
|
Semantic
|
Distractors
|
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | |
| Encoding phase (presentation of 48 items by condition) | ||||||||
| Non-responses | 0.63 | 1.40 | 0.57 | 1.45 | 0.83 | 2.13 | - | - |
| Recognition phase (presentation of 36 items by condition and 60 distractors) | ||||||||
| Hits | 25.57 | 4.34 | 20.97 | 5.31 | 24.00 | 4.92 | 46.70 | 9.70 |
| Misses | 8.37 | 3.51 | 12.97 | 5.08 | 10.00 | 3.44 | 11.97 | 6.60 |
| Non-responses | 0.93 | 1.89 | 1.27 | 3.22 | 1.40 | 3.59 | 2.60 | 6.00 |
Hits = Recognized; Misses = Non-recognized or false alarms
Appendix 2: Conjunction analysis
Self and Other conditions involved a common brain network. (A)Regions commonly active during appraisal of Self and Others. (B)Regions commonly active during successful encoding of Self-referential and Other-referential adjectives (encoding phase). Uncorrected p = .001, k≥75 voxels.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews. Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Araujo HF, Kaplan J, Damasio A. Cortical midline structures and autobiographical-self processes: an activation-likelihood estimation meta-analysis. Frontiers in Human Neuroscience. 2013;7:548. doi: 10.3389/fnhum.2013.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Volle E, Burgess PW. When I think about me and simulate you: medial rostral prefrontal cortex and self-referential processes. NeuroImage. 2010;50:1340–9. doi: 10.1016/j.neuroimage.2009.12.091. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews. Neuroscience. 2008;9:267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. Imaging brain development: the adolescent brain. NeuroImage. 2012;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Garrison KA, Whitfield-Gabrieli S. What about the “Self” is Processed in the Posterior Cingulate Cortex? Frontiers in Human Neuroscience. 2013;7:647. doi: 10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Cohen Kadosh K, Blakemore SJ. The social brain in adolescence: evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience and Biobehavioral Reviews. 2011;35:1654–64. doi: 10.1016/j.neubiorev.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends in Cognitive Sciences. 2007;11:219–27. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Current Opinion in Neurobiology. 2005;15:239–44. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–57. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Conway MA. Memory and the self. Journal of Memory and Language. 2005;53:594–628. doi: 10.1016/j.jml.2005.08.005. [DOI] [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychological Review. 2000;107:261–88. doi: 10.1037//0033-295X. [DOI] [PubMed] [Google Scholar]
- Conway MA, Singer JA, Tagini A. The Self and Autobiographical Memory: Correspondence and Coherence. Social Cognition. 2004;22:491–529. doi: 10.1521/soco.22.5.491.50768. [DOI] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cunningham SJ, Brebner JL, Quinn F, Turk DJ. The self-reference effect on memory in early childhood. Child Development. 2013;85:808–23. doi: 10.1111/cdev.12144. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2012;24:1742–52. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval C, Desgranges B, Eustache F, Piolino P. Looking at the self under the microscope of cognitive neurosciences: from self-consciousness to consciousness of others. Psychologie & Neuropsychiatrie Du Vieillissement. 2009;7:7–19. doi: 10.1684/pnv.2009.0163. [DOI] [PubMed] [Google Scholar]
- Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54:313–27. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London. B, Biological Sciences. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H. In search of the emotional self: an fMRI study using positive and negative emotional words. The American Journal of Psychiatry. 2003;160:1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Lepage M, Graham SJ, Grady C, Keightley ML, … Mayberg H. Distributed self in episodic memory: neural correlates of successful retrieval of self-encoded positive and negative personality traits. NeuroImage. 2004;22:1596–604. doi: 10.1016/j.neuroimage.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Functional neuroimaging of self-referential encoding with age. Neuropsychologia. 2010;48:211–9. doi: 10.1016/j.neuropsychologia.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin JA, Puff CR, Mason HF, Marston SP. Self-reference encoding and incidental recall by children. Bulletin of the Psychonomic Society. 1984;22:87–89. doi: 10.3758/BF03333770. [DOI] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience. 2006;1:18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–61. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Jankowski KF, Moore WE, Merchant JS, Kahn LE, Pfeifer JH. But do you think I’m cool? Developmental differences in striatal recruitment during direct and reflected social self-evaluations. Developmental Cognitive Neuroscience. 2014;8:40–54. doi: 10.1016/j.dcn.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–14. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends in Cognitive Sciences. 2007;11:194–6. doi: 10.1016/j.tics.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Klein SB. Self, memory, and the self-reference effect: an examination of conceptual and methodological issues. Personality and Social Psychology Review : An Official Journal of the Society for Personality and Social Psychology, Inc. 2012;16:283–300. doi: 10.1177/1088868311434214. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex (New York, NY : 1991) 2004;14:647–54. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Martinelli P, Sperduti M, Piolino P. Neural substrates of the self-memory system: new insights from a meta-analysis. Human Brain Mapping. 2013;34:1515–29. doi: 10.1002/hbm.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings A-L, Clasen LS, Giedd JN, Blakemore S-J. The Developmental Mismatch in Structural Brain Maturation during Adolescence. Developmental Neuroscience. 2014:1–14. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- Mills KL, Lalonde F, Clasen LS, Giedd JN, Blakemore SJ. Developmental changes in the structure of the social brain in late childhood and adolescence. Social Cognitive and Affective Neuroscience. 2014;9:123–31. doi: 10.1093/scan/nss113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17:1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Encoding-specific effects of social cognition on the neural correlates of subsequent memory. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2004;24:4912–7. doi: 10.1523/JNEUROSCI.0481-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18:1586–94. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Morel N, Villain N, Rauchs G, Gaubert M, Piolino P, Landeau, … Chételat G. Brain activity and functional coupling changes associated with self-reference effect during both encoding and retrieval. PloS One. 2014;9:e90488. doi: 10.1371/journal.pone.0090488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RJ, Schaer M, Debbané M. Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neuroscience and Biobehavioral Reviews. 2012;36:1043–59. doi: 10.1016/j.neubiorev.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain-a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Northoff G, Qin P, Feinberg TE. Brain imaging of the self-conceptual, anatomical and methodological issues. Consciousness and Cognition. 2011;20:52–63. doi: 10.1016/j.concog.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–31. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN. The role of social cognition in emotion. Trends in Cognitive Sciences. 2008;12:65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Blakemore SJ. Adolescent social cognitive and affective neuroscience: past, present, and future. Social Cognitive and Affective Neuroscience. 2012;7:1–10. doi: 10.1093/scan/nsr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Kahn LE, Merchant JS, Peake SJ, Veroude K, Masten, … Dapretto M. Longitudinal change in the neural bases of adolescent social self-evaluations: effects of age and pubertal development. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2013;33:7415–9. doi: 10.1523/JNEUROSCI.4074-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. “I know you are but what am I?!”: neural bases of self- and social knowledge retrieval in children and adults. Journal of Cognitive Neuroscience. 2007;19:1323–37. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, Lieberman MD. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Development. 2009;80:1016–38. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Merchant JS, Colich NL, Hernandez LM, Rudie JD, Dapretto M. Neural and behavioral responses during self-evaluative processes differ in youth with and without autism. Journal of Autism and Developmental Disorders. 2013;43:272–85. doi: 10.1007/s10803-012-1563-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Peake SJ. Self-development: integrating cognitive, socioemotional, and neuroimaging perspectives. Developmental Cognitive Neuroscience. 2012;2:55–69. doi: 10.1016/j.dcn.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi CL, Duff MC, Denburg NL, Tranel D, Rudrauf D. Medial PFC damage abolishes the self-reference effect. Journal of Cognitive Neuroscience. 2012;24:475–81. doi: 10.1162/jocn_a_00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Liu Y, Shi J, Wang Y, Duncan N, Gong Q, … Northoff G. Dissociation between anterior and posterior cortical regions during self-specificity and familiarity: a combined fMRI-meta-analytic study. Human Brain Mapping. 2012;33:154–64. doi: 10.1002/hbm.21201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default mode network? NeuroImage. 2011;57:1221–33. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Ray RD, Shelton AL, Hollon NG, Michel BD, Frankel CB, Gross JJ, Gabrieli JDE. Cognitive and neural development of individuated self-representation in children. Child Development. 2009;80:1232–42. doi: 10.1111/j.1467-8624.2009.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. Journal of Personality and Social Psychology. 1977;35:677–88. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. What you believe versus what you think they believe: a neuroimaging study of conceptual perspective-taking. The European Journal of Neuroscience. 2003;17:2475–80. doi: 10.1046/j.1460-9568.2003.02673.x. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. Journal of Cognitive Neuroscience. 2004;16:988–99. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Sajonz B, Kahnt T, Margulies DS, Park SQ, Wittmann A, Stoy M, … Bermpohl F. Delineating self-referential processing from episodic memory retrieval: common and dissociable networks. NeuroImage. 2010;50:1606–17. doi: 10.1016/j.neuroimage.2010.01.087. [DOI] [PubMed] [Google Scholar]
- Saxe R, Moran JM, Scholz J, Gabrieli J. Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Social Cognitive and Affective Neuroscience. 2006;1:229–34. doi: 10.1093/scan/nsl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Debbané M, Lagioia A, Salomon R, D’Argembeau A, Eliez S. Comparing the neural bases of self-referential processing in typically developing and 22q11.2 adolescents. Developmental Cognitive Neuroscience. 2012;2:277–89. doi: 10.1016/j.dcn.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C, Burnett S, Blakemore SJ. Development of the self-concept during adolescence. Trends in Cognitive Sciences. 2008;12:441–6. doi: 10.1016/j.tics.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Serbun SJ, Shih JY, Gutchess AH. Memory for details with self-referencing. Memory (Hove, England) 2011;19:1004–14. doi: 10.1080/09658211.2011.626429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons CS, Johnson BT. The self-reference effect in memory: a metaanalysis. Psychological Bulletin. 1997;121:371–94. doi: 10.1037/0033-2909.121.3.371. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9136641. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Walhovd KB, Dale AM, Østby Y, Grydeland H, Richardson, … Fjell AM. Brain development and aging: overlapping and unique patterns of change. NeuroImage. 2013;68:63–74. doi: 10.1016/j.neuroimage.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk DJ, Cunningham SJ, Macrae CN. Self-memory biases in explicit and incidental encoding of trait adjectives. Consciousness and Cognition. 2008;17:1040–5. doi: 10.1016/j.concog.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience and Biobehavioral Reviews. 2010;34:935–46. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Viard A, Lebreton K, Chételat G, Desgranges B, Landeau B, Young A, … Piolino P. Patterns of hippocampal-neocortical interactions in the retrieval of episodic autobiographical memories across the entire life-span of aged adults. Hippocampus. 2010;20:153–65. doi: 10.1002/hipo.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard A, Piolino P, Desgranges B, Chételat G, Lebreton K, Landeau B, … Eustache F. Hippocampal activation for autobiographical memories over the entire lifetime in healthy aged subjects: an fMRI study. Cerebral Cortex (New York, NY : 1991) 2007;17:2453–67. doi: 10.1093/cercor/bhl153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villain N, Landeau B, Groussard M, Mevel K, Fouquet M, Dayan J, … Chételat G. A simple way to improve anatomical mapping of functional brain imaging. Journal of Neuroimaging : Official Journal of the American Society of Neuroimaging. 2010;20:324–33. doi: 10.1111/j.1552-6569.2010.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Individual Achievement Test 2nd Edition (WIAT II) London: The Psychological Corp; 2005. [Google Scholar]
- Zhu L, Guo X, Li J, Zheng L, Wang Q, Yang Z. Hippocampal activity is associated with self-descriptiveness effect in memory, whereas self-reference effect in memory depends on medial prefrontal activity. Hippocampus. 2012;22:1540–52. doi: 10.1002/hipo.20994. [DOI] [PubMed] [Google Scholar]