Abstract
Trophoblasts, the specialized cells of the placenta, play a major role in implantation and formation of the maternal-fetal interface. Through an unusual differentiation process examined in this review, these fetal cells acquire properties of leukocytes and endothelial cells that enable many of their specialized functions. In recent years a great deal has been learned about the regulatory mechanisms, from transcriptional networks to oxygen tension, which control trophoblast differentiation. The challenge is to turn this information into clinically useful tests for monitoring placental function and, hence, pregnancy outcome.
In some societies the critical importance of the placenta in determining pregnancy outcome is acknowledged by its special treatment after birth. For example, the Malay people bury placentas in prominent locations near their homes, a symbolic act in recognition of the fact that the placenta is an essential in utero companion of the baby. We now know that this prescient ritual reflects an important reality. During the last decade and a half, numerous studies in transgenic mice have shown that placentation is a critical regulator of embryonic and fetal development. For example, inactivation of the retinoblastoma (Rb) tumor suppressor gene in mice resulted in unscheduled cell proliferation, apoptosis, and widespread developmental defects that eventually led to embryonic death. Careful analysis of the placenta showed numerous abnormalities, including disruption of the transport region where nutrient, waste, and gas exchange occurs and a decrease in vascularization. When conditional knockout and tetraploid rescue strategies were used to supply Rb-null embryos with wild-type placentas, the animals survived to term; the neurological and erythroid abnormalities that were thought to lead to the intrauterine demise of the pups were abrogated (1). Thus, Rb function in the murine extraembryonic lineages is required for normal differentiation of cells within the embryo. The principle that embryonic and placental development are tightly linked has been strongly reinforced by studies in the burgeoning field of life-course epidemiology and has led to the proposal of the “developmental origins hypothesis”: adult medical conditions such as cardiovascular disease and type 2 diabetes originate in response to undernutrition either in utero or during infancy and early childhood (2). Given that the placenta is an important regulator of fetal growth before birth, it is likely that a subset of the initiating events that eventually lead to the aforementioned diseases will be traced to faulty placentation at structural and/or functional levels (S1).
In this context it becomes critically important to understand, at a molecular level, placental development, which determines the organ’s functional capacity. This review focuses on one part of this process: trophoblast differentiation, a component that is integral to implantation and trophoblast invasion of the uterus.
As discussed below, the trophectoderm layer of the blastocyst is the first embryonic cell type to exhibit highly differentiated functions. Approximately 1 week after fertilization, trophoblasts participate in a complex dialogue with maternal cells that enables implantation, a process that quickly sequesters the human embryo within the uterine wall. Further embryonic development requires the rapid assembly of the basic building blocks of the placenta: floating and anchoring chorionic villi. The unique structure of the human maternal-fetal interface is established by differentiation of cytotrophoblasts in anchoring villi. The latter process entails many unusual elements. For example, these fetal cells, which are derived from the placenta, form elaborate connections with maternal vessels, thereby diverting uterine blood flow to the placenta. Moreover, the hemiallogeneic placental cells that reside within the uterine wall coexist with an unusual population of decidual leukocytes, predominantly CD56bright, CD16— NK cells. Due to the sheer size and complexity of the published literature, an in-depth analysis of trophoblast interactions with the maternal immune system is beyond the scope of this review.
In humans, defects in formation of the maternal-fetal interface that are associated with a variety of pregnancy complications are helping to pinpoint critical aspects of this process that are particularly vulnerable to failure. Genomic and proteomic approaches will yield a great deal of information about the mechanisms involved. The challenge is to translate this knowledge into clinical tests of placental function for the purpose of predicting, diagnosing, and/or treating the major diseases associated with pregnancy, such as preeclampsia, with an incidence of 7–8% (3), and preterm labor, with an incidence of 10% (4). The application of these types of tests will revolutionize the practice of maternal-fetal medicine, with lifelong benefits to the health of both the mother and her offspring, and also have a huge impact on the staggering economic costs associated with these pregnancy complications.
Fertilization and the initial stages of development
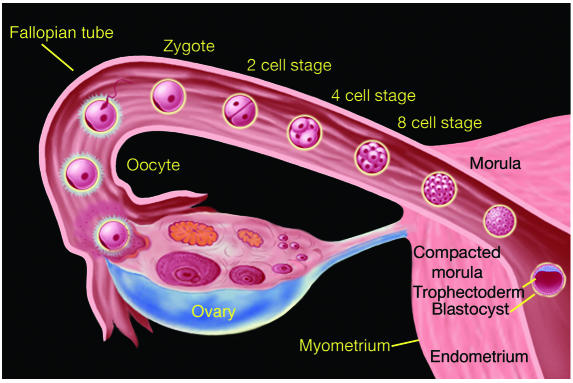
Fertilization occurs in the fallopian tube within 24 to 48 hours of ovulation (Figure 1). The initial stages of development, from fertilized ovum (zygote) to a mass of cells (morula), occur as the embryo passes through the fallopian tube encased within a nonadhesive protective coating known as the zona pellucida. The morula enters the uterine cavity approximately 2 to 3 days after fertilization.
Figure 1.
The early stages of human development from fertilization to blastocyst formation. Fertilization occurs in the fallopian tube within 24 to 48 hours of ovulation. The initial stages of development, from fertilized ovum (zygote) to a solid mass of cells (morula), occur as the embryo passes through the fallopian tube encased within a nonadhesive protective shell (the zona pellucida). The morula enters the uterine cavity approximately two to three days after fertilization. The appearance of a fluid-filled inner cavity marks the transition from morula to blastocyst and is accompanied by cellular differentiation: the surface cells become the trophoblast (and give rise to extraembryonic structures, including the placenta) and the inner cell mass gives rise to the embryo. Within 72 hours of entering the uterine cavity, the embryo hatches from the zona, thereby exposing its outer covering of trophectoderm. Figure kindly provided by S.S. Gambhir and J. Strommer, Stanford University (Stanford, California, USA).
Implantation
Trophectoderm differentiation.
Implantation is dependent on differentiation of the trophoblast lineage (reviewed in ref. 5). In the mouse, the first signs of this process are evident during the morula stage, when cell division creates two distinct cellular populations. The outer cells of the blastocyst, which are polarized, give rise to the trophectoderm, whereas cells in the interior, which are not polarized, give rise to the inner cell mass (Figure 1) (6). Interestingly, these initial events do not irreversibly establish the extraembryonic and embryonic murine lineages: the polarized outer cells can contribute to the inner cell mass (7), and the inner cells retain the ability to differentiate into trophectoderm (S2). Whether human blastomeres exhibit a similar degree of plasticity is unknown. At a molecular level, this cellular polarization requires epithelial-cadherin–mediated homotypic cell adhesion (8) as well as protein kinase C signaling (9). The transcriptional regulators that govern segregation of the extraembryonic and embryonic lineages are also beginning to be identified. The homeobox gene nanog is more strongly expressed in the inner apolar cells of the morula than in the outer cells that are destined to form the trophectoderm. In the blastocyst, nanog expression is confined to the inner cell mass and, in the later embryo, to primordial germ cells. This factor, which is required for embryonic stem cell self-renewal, may play an important role in generation of the intraembryonic lineage (10, 11). Conversely, by the late morula stage, the caudal-related homeobox gene cdx2 is more strongly expressed in the outer layer of polarized blastomeres (5). Overexpression of this transcription factor in embryonic stem cells induces trophoblast formation (S3). Additionally, the domain transcription factor Oct4 is required to generate the inner cell mass (12) by a mechanism that includes suppression of trophoblast-specific genes (13, 14). The high-mobility group transcription factor Sox2 may play a role similar to that of Oct4 (15). However, neither Oct4 nor Sox2 expression is confined to particular subsets of blastomeres, making it unlikely that these transcription factors are involved in initial decisions that govern the fate of these cells. The above findings led Rossant and colleagues (5) to propose that polarization provides molecular cues (e.g., cdx2 expression) that specify the trophoblast lineage, with apolarization delivering different signals (e.g., Nanog, Oct4, and Sox2) to cells that are fated to become components of the inner cell mass. In this scenario it is interesting to note the important contribution of both genetic and positional cues. Recent data that were generated using an RNA interference approach to modulate gene expression in human embryonic stem cells suggest that these transcription factors are playing similar roles in this system (16).
The implantation process.
The initial stages of embryonic and uterine development, which are spatially separated, must be temporally coordinated for pregnancy to occur. Once the blastocyst emerges from the zona pellucida, approximately 6 days after fertilization, the embryonic and maternal cells enter into a complex dialogue that enables implantation, a process that rapidly sequesters the human embryo within the uterine wall (reviewed in refs. 17 and 18). Hormones play very important roles in implantation. For example, studies using transgenic approaches show that uterine expression of progesterone receptor A and estrogen receptor α, but not progesterone receptor B or estrogen receptor β, is required for implantation (19, 20). Nevertheless, the situation is complex, as decidualization, the specialized response of endometrial stromal cells to pregnancy, can be experimentally induced in estrogen receptor α null females (21, 22). In mice, a very narrow range of estrogen concentrations determines the duration of the window of receptivity, i.e., the period of time during which the uterus is able to support implantation (23). Interestingly, estrogen also has effects on the blastocyst, as its 4-hydroxy catechol metabolite mediates blastocyst activation, a requisite step in implantation (24). As for the uterus, a number of cytokines, growth factors, and transcription factors play important roles in this cascade. For example, maternal expression of leukemia inhibitory factor (LIF) is required to initiate implantation (25). IL-11 (26), insulin-like growth factors and binding proteins (27, S4), Hoxa-10 (28, 29), and a forkhead transcription factor (30) are involved in decidualization. The dialogue between the blastocyst and the uterus involves molecular families that might logically be predicted to function during the peri-implantation period (e.g., EGF family members; ref. 31) as well as unexpected participants, such as endocannabinoids and their G-protein–coupled receptors (32). Embryonic signals such as those generated by chorionic gonadotropin also play important roles by acting on the endometrium (33). In keeping with the results of global gene profiling experiments in other systems, the application of this approach to the study of murine implantation (34, 35) and human uterine receptivity (36, 37) graphically demonstrates the myriad of mechanisms that are involved in these processes.
The current challenge is to use the data summarized above to gain entrée into the pathways that control implantation. To date, evidence from many different sources, including studies in mice and humans, suggests that LIF is at the top of the pyramid of molecules whose maternal expression is required for effective implantation. What are the downstream effectors? The long-standing observation that vascular permeability increases at the site of murine implantation prompted investigators to look for the expression of molecules with functions that are relevant to this process. For example, phospholipase A2 generates arachidonic acid, a substrate for the COX-2 enzyme, which produces prostaglandins. In some genetic strains of mice, deleting cox-2 interferes with implantation (38), while in others a compensatory upregulation of COX-1 expression rescues this phenotype, resulting in the birth of live pups, although litter size is reduced (39) because decidual growth is retarded (40). Interestingly, the converse is also true, e.g., COX-2 compensation is observed in the absence of COX-1 (41). With regard to the actions of cox-2 products, evidence to date suggests that the COX-2–derived prostaglandin, prostacyclin, plays a particularly important role in implantation by activating the nuclear hormone receptor PPARδ (42), which is required for normal (murine) placental development (43). Other PPAR family members, such as PPARγ, play important roles in human trophoblast differentiation and function (S5, S6). Finally, an emerging area of research is the investigation of evolutionarily conserved genes, including those encoding FGFs, IGFs, bone morphogenetic proteins, Wnts, Noggin, and Indian hedgehog proteins and their receptors, which appear to have potential roles in implantation and embryo spacing in the uterus (44, 45). It is important to note that other pathways lie downstream from LIF, e.g., Msx-1 and Wnt4, whose expression is also aberrant in Hoxa10–null mice (46).
In humans, the implantation story has an unexpected twist. Recent evidence suggests that the L-selectin system, which mediates rolling and tethering of leukocytes on blood vessels, plays an important role in implantation (47). This mechanism is particularly well suited for initiating implantation because of its many special attributes (48). For example, the rapid kinetics that characterize interactions between these carbohydrate-binding receptors (on leukocytes) and their specialized oligosaccharide ligands (on blood vessel walls) allows for rolling and tethering of leukocytes when they encounter shear stress. Subsequently, integrin-mediated firm adhesion is triggered by exposure to the chemokine- and cytokine-rich milieu at the vessel wall. At a morphological level, analogies can be drawn between key steps in leukocyte emigration from blood and trophoblast attachment to the uterine wall. Implantation begins with apposition; the trophectoderm of the originally free-floating blastocyst lies adjacent to the uterine epithelium, but the blastocyst is easily dislodged (Figure 2) (49). Soon thereafter, blastocyst adhesion to the uterine wall is stabilized, and trophoblasts transmigrate across the uterine epithelium, a process that in humans buries the entire embryo beneath the uterine surface. Subsequent development depends on the ability of trophoblasts to adhere to the uterine epithelium under conditions of shear stress that are created when these fetal cells breach uterine vessels, a process that diverts maternal blood flow to the placenta (Figure 3). At a molecular level, trophoblast adhesion from the stage of implantation onwards is an integrin-dependent process (50, 51) that takes place in a chemokine- and cytokine-rich microenvironment analogous to the blood-vascular interface (52, 53). In this context it is interesting to note that in humans uterine expression of chemokines is hormonally regulated and the blastocyst expresses chemokine receptors (S7).
Figure 2.
Implantation in humans involves a number of the molecular mechanisms that mediate leukocyte emigration from the blood to sites of inflammation or injury. The diagram was made from a combination of images: MECA-79 antibody staining of uterine tissue sections and L-selectin antibody staining of cultured embryos. Recently acquired evidence suggests that an implantation-competent human blastocyst expresses L-selectin on its surface (green). This receptor interacts with specialized carbohydrate ligands, including sulfated species, recognized by the MECA-79 antibody, which stains the uterine luminal and glandular epithelium. The specialized nature of these interactions translates into an unusual form of cell adhesion: rolling and tethering. In the uterus, MECA-79 immunoreactivity peaks during the window of receptivity. This finding suggests that apposition, the first step in implantation, includes L-selectin_mediated tethering of the blastocyst to the upper portion of the posterior wall of the uterine fundus.
Figure 3.
Oxygen tension plays an important role in guiding the differentiation process that leads to cytotrophoblast invasion of the uterus. (A) The early stages of placental development take place in a relatively hypoxic environment that favors cytotrophoblast proliferation rather than differentiation along the invasive pathway. Accordingly, this cell population (light green cells) rapidly increases in number as compared with the embryonic lineages. (B) As development continues, cytotrophoblasts (dark green cells) invade the uterine wall and plug the maternal vessels, a process that helps maintain a state of physiological hypoxia. As indicated by the blunt arrows, cytotrophoblasts migrate farther up arteries than veins. (C) By 10 to 12 weeks of human pregnancy, blood flow to the intervillous space begins. As the endovascular component of cytotrophoblast invasion progresses, the cells migrate along the lumina of spiral arterioles, replacing the maternal endothelial lining. Cytotrophoblasts are also found in the smooth muscle walls of these vessels. In normal pregnancy the process whereby placental cells remodel uterine arterioles involves the decidual and inner third of the myometrial portions of these vessels. As a result, the diameter of the arterioles expands to accommodate the dramatic increase in blood flow that is needed to support rapid fetal growth later in pregnancy. It is likely that failed endovascular invasion leads, in some cases, to abortion, whereas an inability to invade to the appropriate depth is associated with preeclampsia and a subset of pregnancies in which the growth of the fetus is restricted.
Together, these findings raised the possibility that implantation and placentation utilize other components of the leukocyte emigration system, such as selectins and their ligands (Figure 2). Immunolocalization experiments showed that the trophectoderm, which covers the surface of implantation-competent human embryos, stains brightly for L-selectin but does not express the other members of the selectin family. As the luminal epithelium becomes receptive during the luteal phase of the menstrual cycle, these uterine cells display a dramatic upregulation of the expression of the specialized sulfated oligosaccharides that function as high-affinity receptors for L-selectin. A variety of assays show that the L-selectin system functions by tethering human trophoblasts under conditions of shear stress. It will be very interesting to identify points of intersection between this pathway and the other molecular cascades, summarized above, that are known to regulate implantation.
Finally, there are interesting data from both human and murine systems that highlight the importance of achieving implantation during the period of optimal uterine receptivity. For example, female mice with a null mutation for the gene encoding cytosolic phospholipase A2α have small litters and often exhibit pregnancy failure (54). Interestingly, the cause is a shifting forward in time of the window of receptivity. The consequences of delaying implantation include retarded fetal-placental growth and abnormal uterine spacing of embryos. Similarly, mice that lack expression of the LDL receptor–related protein, which was originally thought to be required for implantation (55), exhibit delayed development. It is likely that this same effect or a related variant also occurs in humans, as the risk of early pregnancy loss increases as a function of delaying implantation (56).
Trophoblast invasion and maturation of the maternal-fetal interface
Trophoblast stem cell self-renewal.
Once the embryo is anchored within the uterine wall, the next major hurdle is rapid formation of the extraembryonic lineages, a necessary prelude to assembly of the maternal-fetal interface. In recent years a great deal of information about trophoblast differentiation has come from a variety of experimental approaches. The generation of mouse trophoblast stem cell lines from early-stage mouse embryos (i.e., prior to embryonic day [E] 7.5) has proved to be an important experimental tool for studying this process as well as the mechanisms that promote self-renewal of the trophoblast stem cell population (57, 58). The actions of FGF family members are crucial, as trophoblast stem cells are derived by plating disaggregated extraembryonic cells on mouse embryonic fibroblasts in the presence of FGF4, which binds Fgfr2 IIIc, ultimately leading to MAPK signaling (5). To date, attempts to use an FGF-based strategy to derive human trophoblast stem cells have failed, which suggests that different factors are required for self-renewal of this population. This is not unexpected, as despite the many similarities at a molecular level between murine and human placentation, there are also dramatic morphological differences. Although treatment of human embryonic stem cells with bone morphogenic protein–4 results in the expression of syncytial trophoblast markers, the cells do not continue to divide (59). Thus, the majority of what we know about human trophoblast differentiation has come from studies of early-gestation placental cells using in situ and in vitro approaches (17).
Trophoblast differentiation.
Additionally, gene deletion studies in mice have either advertently or inadvertently yielded interesting insights into the molecular requirements of normal placentation (60). Development of the tetraploid rescue technique for providing mutant embryos with wild-type placentas has allowed a careful separation of the embryonic and extraembryonic phenotypes (61, 62). As a result, many aspects of murine trophoblast differentiation can now be explained in terms of specific transcriptional regulators (5, 58, 63). As for those regulators that control early events, deletion of the T-box gene Eomes results in embryonic death soon after implantation due to an arrest in trophectoderm development (64). Embryos that lack expression of activating protein–2γ, a member of a family of transcription factors that controls cell proliferation, die at E7.5 due to malformation of the maternal-fetal interface. The defect is likely attributable to negative effects on the stem cell population, which has reduced expression of Eomes and cdx2, two genes that are upregulated in response to FGF treatment of trophoblast stem cells (65). Deletion of an orphan receptor, estrogen-receptor–related receptor protein β, results in death at E9.5. Demise is attributable to depletion of the trophoblast stem/progenitor population, which is shunted to the giant cell lineage (66), the murine equivalent of the human cytotrophoblast population that carries out interstitial and endovascular invasion (58, 67). Interestingly, deletion of the TGF-β family member Nodal also leads to expansion of the trophoblast giant cell population at the expense of the other subtypes (68).
Basic helix-loop-helix (bHLH) transcription factors play important roles during the later stages of trophoblast differentiation. Mash2, a paternally imprinted gene, is required for the production of the precursor population that gives rise to the spongiotrophoblast layer (69). Interestingly, there is evidence that the polycomb group protein, extraembryonic ectoderm development (known as Eed), may be required for lineage-specific Mash2 repression during differentiation (70). Since bHLH transcription factors function as dimers, their binding partners also need to be expressed at the proper time and location. Finally, their activity can be inhibited by interactions with dominant-negative HLH factors that lack DNA-binding sequences, e.g., Id1 and Id2, which also localize to the extraembryonic ectoderm (S8). Human trophoblast progenitors also express Mash2 and Id2, which are downregulated as the cells differentiate along the invasive pathway (71). In keeping with the known functions of these factors, forced expression of Id2 inhibits cytotrophoblast differentiation in vitro (72). This is one example of the many similarities between murine and human placentation at the molecular level. In contrast, another bHLH factor, Hand1, is required for the differentiation of murine primary and secondary giant cells (73, 74). That human trophoblasts beyond the blastocyst stage do not express Hand1 is one of the few examples of divergent placental evolution in the two species (75, S9).
A number of transcription factors also regulate formation of the labyrinth zone, the area of the murine placenta that corresponds to the floating villi of the human placenta, i.e., where the transport of nutrients, wastes, and gasses takes place (58). A screen of human tissues revealed the surprising result that glial cells missing-1, which controls the neuronal to glial transition in Drosophila (76), is solely and constitutively expressed in placental cytotrophoblasts (71). Mice that lack expression of this transcription factor die at E10 because of a block in the branching of the chorioallantoic interface and an absence of the placental labyrinth (77). Other known regulators of labyrinth development include retinoic acid receptors (78, 79) and PPARγ (80). Wnt2 (81) and growth factor, e.g., hepatocyte growth factor (82), signals are also required.
Placental function regulates many aspects of embryonic and fetal development.
When tetraploid (wild-type) and diploid (mutant) cells are aggregated, the hyperdiploid cells are allocated to the placenta. This method is a powerful technique for supplying mutant embryos with normal placentas, thereby separating a molecule’s embryonic and extraembryonic effects (61, 62). The widespread application of this technology has revealed the critical importance of normal placental function to embryonic and fetal development. A startling array of effects is propagated downstream from abnormal placentation. For example, targeted mutation of the DNA-binding domain of the Ets2 transcription factor produces numerous defects in the extraembryonic compartment, including a substantial decrease in MMP-9 production, an attendant failure in extracellular matrix remodeling, and a proliferation defect that involves the ectoplacental cone. Ets2-null embryos, which are growth restricted, die before E8.5. Subsequent tetraploid rescue experiments resulted in the birth of viable, fertile mice with hair defects — a dramatically different embryonic phenotype from that observed when the entire conceptus lacks Ets2 expression (83). The general phenomenon of abnormal placental function affecting embryonic and fetal development has been documented in association with the deletion of many other genes from very diverse molecular families. For example, deletion of JunB, an immediate early gene product and member of the AP-1 transcription factor family, also causes fetal growth restriction. This ultimately leads to embryonic death between E8.5 and E10 due to defects in both placental and decidual vessels, the latter receiving improper signals from invading trophoblasts. Again, tetraploid rescue resulted in fetuses that were normally grown but in this case osteopenic (84). Likewise, fetal growth restriction secondary to defects in the placental labyrinth of the extracellular signal regulated kinase-2–null mice is rescued by tetraploid aggregation (85). Similarly, tetraploid rescue results in the birth of mice lacking the HGF receptor c-Met (86) or SOCS-3 (87).
In other cases, supplying mutant embryos with normal extraembryonic derivatives allows development to progress further than was previously possible. Mice that lack expression of thrombomodulin (88) and desmoplakin (89) fall into this category, as do homozygous null mutants that lack expression of Alk2, which encodes a type I TGF-β family receptor for activins and bone morphogenic protein-7 (90). The latter case provides direct evidence that signals transmitted through this receptor, which is expressed in the extraembryonic region, are required at the time of gastrulation in order to achieve normal mesoderm formation.
Interestingly, the impact of aneuploidy on the conceptus in terms of embryonic development can also be alleviated to varying degrees by tetraploid rescue. For example, the development of mice with a t-complex variant of chromosome 17 is sustained for several additional days by wild-type placental cells (91), and tetraploid aggregation allows the birth of mice that carry an additional maternal X chromosome (92). It is important to note that, while in some cases the effects on the embryo of placental malfunction are likely to be directly attributable to defects in the organs’ transport functions, in many other instances the connection is likely to involve a higher order of complexity. For example, tetraploid aggregation shows that keratin 8 is a necessary component of the barrier that prevents TNF-mediated apoptosis of trophoblast giant cells (93).
The extent to which extraembryonic and embryonic development are linked in humans is an interesting, unresolved question that has gained additional importance due to the realization that the foundation of many aspects of adult health is laid down in utero (i.e., the developmental origins hypothesis [2]). In this context, normal placental function is critical for normal fetal development. At a biochemical level, a great deal of evidence suggests that alterations in placental transport functions are associated with growth restriction (94, S10). However, the actual cause-and-effect relationship is likely to be much more complicated. For example, uterine blood flow is a critical regulator of placental function and, hence, fetal growth (S11). Additionally, impaired placental transport is linked to reduced umbilical blood flow and attendant changes in the fetal circulation (S12). Thus, in humans, it is difficult to sort out the primary defect from the ripple effects. This problem is further complicated by the fact that many commonly used drugs (e.g., nicotine) negatively affect trophoblast differentiation and formation of the maternal-fetal interface (95, 96) as well as placental transport of amino acids and fetal growth (S13). Additionally, subclinical viral (e.g., cytomegalovirus) and bacterial infections, which are surprisingly common, can inhibit cytotrophoblast differentiation and/or invasion (97, 98). Despite the obvious complexity of these interrelationships it is possible to envision a deterioration in placental function that translates into alterations, at the molecular level, in the fetal circulation that are maintained throughout life, one possible explanation for why a restriction in intrauterine growth is linked to adult cardiovascular disease.
At a genetic level, fetal aneuploidies allow an evaluation of the impact of specific changes in chromosome number, deletion, and/or translocation on placental development. For example, in the case of trisomy 21, the ability of cytotrophoblasts to fuse into syncytiotrophoblasts is impaired (Figure 3) (S14). Additionally, cytotrophoblast differentiation along the pathway that leads to uterine invasion is dysregulated, as shown by abnormal expression of stage-specific antigens that are modulated during this process as well as a high rate of apoptosis among this subpopulation of cells (99). We speculate that the latter observations explain the high rate of fetal loss in these pregnancies (see Figure 3). In other cases, such as confined placental mosaicism, which occurs in approximately 2% of viable pregnancies, the consequences often include unexplained fetal growth restriction (S15). In this context, exploring the effects of specific aneuploidies on placental development could yield new insights into mechanisms of both normal and abnormal placentation.
Oxygen regulates trophoblast differentiation and proliferation.
Physiological factors also play an important role in formation of the maternal-fetal interface and, consequently, fetal growth. Oxygen tension, a function of uterine blood flow, is a prime example (S11). In recent years a great deal has been learned about the fundamental mechanisms that couple the trophoblasts’ ability to sense oxygen levels with their differentiative and metabolic status. Important clues about oxygen’s effects on the placenta have come from several lines of evidence that suggest that the early stages of placental (and embryonic) development take place in an environment that is hypoxic relative to the uterus. Specifically, direct measurement of uterine oxygen tension demonstrates physiological hypoxia (2–5% O2; reviewed in ref. 100). Furthermore, blood flow to the human intervillous space does not begin until 10 to 12 weeks of pregnancy (101). Studies in both nonhuman primates (S16) and humans (102) suggest that trophoblasts actively limit their access to uterine blood by plugging the lumina of the decidual vessels. Why? Our work shows that cytotrophoblasts proliferate in vitro under hypoxic conditions that are comparable to those found during early pregnancy in the uterine cavity and the superficial decidua. As trophoblast invasion of the uterus proceeds, the placental cells encounter increasingly higher oxygen levels, which trigger their exit from the cell cycle and subsequent differentiation (103, 104). Hypoxia also regulates cell fate in the murine placenta (105). We speculate that the paradoxical effects of oxygen in controlling the balance between cytotrophoblast proliferation and differentiation explain in part why the mass of the placenta increases much more rapidly than that of the embryo. Histological sections of early-stage pregnant human uteri show bilaminar embryos surrounded by thousands of trophoblast cells (S17). The fact that hypoxia stimulates cytotrophoblasts, but not most other cells, to undergo mitosis (106) could help account for the difference in size between the embryo and the placenta, a discrepancy that continues well into the second trimester of pregnancy (S18). Thus, the structure of the mature placenta is established in advance of the period of rapid fetal growth that occurs during the latter half of pregnancy.
What are the molecular underpinnings of this unusual relationship? Many different lines of evidence suggest that the hypoxia-inducible factor (HIF) system that controls cellular responses to oxygen deprivation is involved (reviewed in refs. 107–109). The three HIF-α family members are bHLH transcription factors that also contain a Per/Arnt/Sim domain that facilitates their dimerization with HIF1-β (the aryl hydrocarbon receptor nuclear translocator). The heterodimers activate the transcription of numerous downstream targets by binding to a hypoxia-responsive promoter element (5′-TACGTG-3′) that is present in a variety of relevant genes, including VEGF, glucose transporter–1, and Stra13, the latter a regulator of murine placental development (63, 110). Because these responses need to be extremely rapid, an important element of control occurs at the protein level. Specifically, enzymatic hydroxylation of certain HIF-α proline residues is required for interactions with the von Hippel–Lindau (VHL) tumor suppressor protein, which under normoxic conditions targets these proteins for polyubiquitination and degradation in the proteosome. Additionally, hydroxylation of specific HIF-α asparagine residues prevents the recruitment of transcriptional coactivators. Interestingly, these enzymatic reactions, which depend on molecular oxygen, do not occur in a low-oxygen environment, providing a direct link between hypoxia, HIF stabilization, and the transcription of downstream target genes. In keeping with the concept that oxygen plays an important role in placental development, deletion of many of the individual components of the cell’s machinery for sensing and responding to changes in oxygen tension leads to prenatal lethality secondary to placental defects. For example, mice that lack expression of either VHL (111) or HIF1-β (105) die in utero as a result of faulty placentation. The lack of heat shock protein 90, which also controls HIF availability (112), is associated with placental defects that lead to embryonic death at E9.0/9.5 (113). VHL and HIFs also play important roles in human cytotrophoblast differentiation and/or invasion in vitro (114). The latter data are in accord with studies of normal and abnormal placentation in vivo. For example, trophoblast remodeling of spiral arterioles is restricted at high altitude, suggesting that hypoxia affects placentation in utero (S19). Interestingly, the incidence of preeclampsia (the new and rapid onset of maternal hypertension, proteinuria, and edema), which is associated with reduced blood flow to the placenta and deficient arterial invasion (S20, S21), increases several-fold at high elevations (S22).
Angiogenic and/or vasculogenic factors regulate trophoblast differentiation and proliferation.
Angiogenesis is one well-known consequence of hypoxia (115). In the special case of placentation, the combined actions of the aforementioned transcriptional and physiological regulators result in the differentiation of an unusual subpopulation of cytotrophoblasts with many vascular-type attributes. The progenitors, which are components of anchoring chorionic villi, leave the placenta and attach to the uterine wall, the first step in both the interstitial and endovascular components of uterine invasion (Figure 3). A great deal about these processes has been learned by constructing in vitro models that allow functional perturbation of molecules that are regulated during human uterine invasion (Figure 4). For example, as cytotrophoblasts transition from the fetal to the maternal compartment, they modulate expression of a broad repertoire of growth factors and receptors with diverse roles in vasculogenesis, angiogenesis, and lymphangiogenesis: VEGFR1–3, soluble VEGFR-1 (also known as sFlt1), VEGF-A, VEGF-C, and placental growth factor (PlGF). Functional perturbation experiments show that these ligand-receptor interactions promote cytotrophoblast differentiation and/or invasion and survival (116). We speculate that the expression of regulators of lymphangiogenesis, e.g., VEGFR-3 and VEGF-C (117), could contribute to the specialized nature of trophoblast-lined uterine vessels, which are able to expand greatly as fetal requirements for maternal blood increase in the latter half of pregnancy. Additionally, these cytotrophoblasts express Ang2, a ligand that is also involved in lymphangiogenesis (118, 119). Since cytotrophoblasts lack expression of Tie receptors (which bind Ang2), maternal cells are the likely targets (118).
Figure 4.
Illustration of two in vitro models for studying human cytotrophoblast invasion. (A) When human cytotrophoblasts (light green cells encircled in red) are isolated from early-gestation placentas and plated on an extracellular matrix (ECM) substrate (Matrigel), they differentiate along the pathway that leads to uterine invasion. By 12 hours in culture these cells form aggregates that resemble cell columns of anchoring villi, and by 48 hours they switch on expression of a repertoire of stage-specific antigens that are expressed in cytotrophoblasts within the uterine wall in situ (dark green cells). These molecules facilitate uterine invasion, vascular mimicry, and evasion of the maternal immune response. (B) When anchoring villi are dissected from the surfaces of early-gestation human placentas (blue box) and plated on an ECM substrate, cytotrophoblasts in cell columns continue to differentiate. By 48 hours many cytotrophoblasts have left the columns and invaded the substrate (green box). During this process they execute the same phenotypic switch that isolated cells carry out.
Defects in cytotrophoblast differentiation are associated with preeclampsia.
As cytotrophoblasts invade the uterine wall they also acquire a vascular-like repertoire of adhesion molecules. The onset of cytotrophoblast differentiation and/or invasion is characterized by reduced staining for receptors characteristic of polarized cytotrophoblast epithelial progenitors —integrin α6β4 and epithelial cadherin— and the onset of expression of adhesion receptors characteristic of endothelium — vascular-endothelial cadherin, IgG family members VCAM-1 and PECAM-1, and integrins αVβ3 and α1β1 (reviewed in ref. 120). Thus, as cytotrophoblasts from anchoring villi invade and remodel the wall of the uterus, these epithelial cells of ectodermal origin acquire an adhesion receptor repertoire characteristic of endothelial cells. We theorize that this switch permits the heterotypic adhesive interactions that allow fetal and maternal cells to cohabit in the uterine vasculature during normal pregnancy.
The preeclampsia syndrome reveals the significance of the differentiation program in which invasive cytotrophoblasts acquire vascular-like properties. Preeclampsia, a serious complication, is the leading cause of maternal mortality in developed countries (reviewed in ref. 121). The mother shows signs of widespread alterations in endothelial function, such as high blood pressure, proteinuria, and edema. In some cases the fetus stops growing, resulting in fetal growth restriction. Compounding the dangers of this condition is the fact that the maternal and fetal signs can appear suddenly at any time from mid–second trimester until term, hence the name preeclampsia (derived from the Greek eklampsis, meaning sudden flash or development).
Specific placental defects are associated with preeclampsia, especially the most severe cases that occur during the second and early third trimesters of pregnancy. The anchoring villi that give rise to invasive cytotrophoblasts are most severely affected. The extent of interstitial invasion of the uterine parenchyma is variable but frequently shallow (Figure 3). Endovascular invasion of the blood vessels is consistently rudimentary, making it extremely difficult to find any maternal vessels that contain cytotrophoblasts (S21, S23, 122). These anatomical defects suggested to us that during preeclampsia, cytotrophoblast differentiation along the invasive pathway is abnormal. Biopsies of the uterine wall of women with this syndrome showed that invasive cytotrophoblasts retain expression of adhesion receptors characteristic of the progenitor population and fail to turn on receptors that promote invasion and/or assumption of an endothelial phenotype (122). It is interesting to note that these deficits do not occur in isolation. In the most severely affected patients, immunolocalization on tissue sections of the placenta showed that cytotrophoblast VEGF-A and VEGFR-1 staining decreased; however, staining for PlGF was unaffected. Cytotrophoblast secretion of the soluble form of VEGFR-1 (sFlt-1) in vitro also increased (116), an observation that gains additional importance in light of the recent discovery that excess sFlt-1 produces a preeclampsia-like syndrome in rats (123). However, it is important to note that preeclampsia has a very complex etiology and an equally complex constellation of placental effects. For example, in macaques (S16) and humans, endovascular cytotrophoblasts express the neural cell adhesion molecule, which is significantly downregulated in preeclampsia (Figure 5). Data such as these reinforce the concept that this pregnancy complication is associated with global deficits in cytotrophoblast differentiation and/or invasion.
Figure 5.
In severe preeclampsia as compared to normal pregnancy, cytotrophoblast invasion is shallow and the cells fail to switch on the expression of stage-specific antigens that are normally upregulated as they penetrate the uterine wall and blood vessels (BVs). (A and C) Cytokeratin (CK) staining of tissue sections of the maternal-fetal interface allows visualization of cytotrophoblasts that invade the uterine wall. (B) In normal pregnancy, the subpopulation of human cytotrophoblasts that carries out endovascular invasion upregulates expression of the neural cell adhesion molecule (NCAM). (D) In cases of severe preeclampsia (SPE), NCAM immunoreactivity is either absent or very weak (indicated by the arrows). AV, anchoring villus; COL, cell column of anchoring villus.
Conclusions and future directions
In recent years a great deal of progress has been made toward identifying the factors that govern trophoblast differentiation and, consequently, implantation and formation of the maternal-fetal interface. One important source of information has been the surprising number of transgenic mice, produced for other purposes, that have primary placental defects, a trend that was noted a decade ago (60). These analyses have also revealed the critical importance of normal placental function to embryonic development, as there are many examples in which tetraploid aggregation, which supplies a mutant embryo with a normal placenta, either rescues or lessens the severity of the embryonic defects. This principle likely applies to humans, as some aspects of adult health appear to be programmed in utero. Another important source of information has been studies of normal human placental development, which have led in turn to a better understanding of the defects that are associated with common pregnancy complications such as preeclampsia. In this context, the future challenge is to translate our basic knowledge of factors that govern trophoblast differentiation into clinically useful tests of placental function, a process that will inevitably revolutionize the practice of maternal-fetal medicine. Recently, interesting examples of this type of translational research have been published. For example, increased circulating levels of soluble VEGFR-1 and reduced levels of PlGF predict the subsequent development of preeclampsia (124, 125). By building on these types of studies, prenatal tests of placental function will eventually become as common as those tests already in place to assess the health of other important organs. Along the way we will also learn a great deal about possible therapeutic strategies for preventing and/or treating pregnancy complications.
Supplementary Material
Acknowledgments
We thank the past and present members of our laboratory who have made important contributions to this work and S.K. Dey for his thoughtful comments. We also thank Harbindar Singh for information about birth practices in Malaysia and Mary McKenney for excellent editorial support. Funding sources include The University of California Tobacco-Related Disease Program (8DT-0176), The National Institute of General Medical Sciences Minority Biomedical Research Support Research Initiative for Scientific Enhancement (R25 GM59298 and R25 GM56847), and NIH Grants U01 HD 42283 (part of the Cooperative Program on Trophoblast-Maternal Tissue Interactions), HL 64597, and HD 30367.
Due to space constraints, a number of important references could not be included in this article. Interested readers can find a supplementary reading list at http://www.jci.org/cgi/content/full/114/6/744/DC1.
Footnotes
Nonstandard abbreviations used: bHLH, basic helix-loop-helix; E, embryonic day; HIF, hypoxia-inducible factor; LIF, leukemia inhibitory factor; PlGF, placental growth factor; Rb, retinoblastoma; VHL, von Hippel–Lindau.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Wu L, et al. Extra-embryonic function of Rb is essential for embryonic development and viability. Nature. 2003;421:942–947. doi: 10.1038/nature01417. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ. Developmental origins of adult health and disease. J. Epidemiol. Community Health. 2004;58:114–115. doi: 10.1136/jech.58.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sibai BM, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am. J. Obstet. Gynecol. 1997;177:1003–1010. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 5.Kunath T, Strumpf D, Rossant J. Early trophoblast determination and stem cell maintenance in the mouse-a review. Placenta. 2004;25(Suppl.):S32–S38. doi: 10.1016/j.placenta.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Johnson MH, Ziomek CA. The foundation of two distinct cell lineages within the mouse morula. Cell. 1981;24:71–80. doi: 10.1016/0092-8674(81)90502-x. [DOI] [PubMed] [Google Scholar]
- 7.Rossant J, Vijh KM. Ability of outside cells from preimplantation mouse embryos to form inner cell mass derivatives. Dev. Biol. 1980;76:475–482. doi: 10.1016/0012-1606(80)90395-4. [DOI] [PubMed] [Google Scholar]
- 8.Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom TL. The effects of phorbol ester on mouse blastomeres: a role for protein kinase C in compaction? Development. 1989;106:159–171. doi: 10.1242/dev.106.1.159. [DOI] [PubMed] [Google Scholar]
- 10.Chambers I, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 11.Mitsui K, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 12.Nichols J, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Roberts RM. Silencing of the gene for the beta subunit of human chorionic gonadotropin by the embryonic transcription factor Oct-3/4. J. Biol. Chem. 1996;271:16683–16689. doi: 10.1074/jbc.271.28.16683. [DOI] [PubMed] [Google Scholar]
- 14.Ezashi T, Ghosh D, Roberts RM. Repression of Ets-2-induced transactivation of the tau interferon promoter by Oct-4. Mol. Cell. Biol. 2001;21:7883–7891. doi: 10.1128/MCB.21.23.7883-7891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avilion AA, et al. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hay DC, Sutherland L, Clark J, Burdon T. Oct-4 knockdown induces similar patterns of endoderm and trophoblast differentiation markers in human and mouse embryonic stem cells. Stem Cells. 2004;22:225–235. doi: 10.1634/stemcells.22-2-225. [DOI] [PubMed] [Google Scholar]
- 17.Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N. Engl. J. Med. 2001;345:1400–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 18.Paria BC, Reese J, Das SK, Dey SK. Deciphering the cross-talk of implantation: advances and challenges. Science. 2002;296:2185–2188. doi: 10.1126/science.1071601. [DOI] [PubMed] [Google Scholar]
- 19.Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O’Malley BW. Reproductive functions of progesterone receptors. Recent Prog. Horm. Res. 2002;57:339–355. doi: 10.1210/rp.57.1.339. [DOI] [PubMed] [Google Scholar]
- 20.Hewitt SC, Korach KS. Estrogen receptors: structure, mechanisms and function. Rev. Endocr. Metab. Disord. 2002;3:193–200. doi: 10.1023/a:1020068224909. [DOI] [PubMed] [Google Scholar]
- 21.Paria BC, Tan J, Lubahn DB, Dey SK, Das SK. Uterine decidual response occurs in estrogen receptor-alpha-deficient mice. Endocrinology. 1999;140:2704–2710. doi: 10.1210/endo.140.6.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis Hewitt S, Goulding EH, Eddy EM, Korach KS. Studies using the estrogen receptor alpha knockout uterus demonstrate that implantation but not decidualization-associated signaling is estrogen dependent. Biol. Reprod. 2002;67:1268–1277. doi: 10.1095/biolreprod67.4.1268. [DOI] [PubMed] [Google Scholar]
- 23.Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2963–2968. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paria BC, et al. Coordination of differential effects of primary estrogen and catecholestrogen on two distinct targets mediates embryo implantation in the mouse. Endocrinology. 1998;139:5235–5246. doi: 10.1210/endo.139.12.6386. [DOI] [PubMed] [Google Scholar]
- 25.Stewart CL, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 26.Robb L, et al. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat. Med. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- 27.Crossey PA, Pillai CC, Miell JP. Altered placental development and intrauterine growth restriction in IGF binding protein-1 transgenic mice. J. Clin. Invest. 2002;110:411–418. doi:10.1172/JCI200210077. doi: 10.1172/JCI10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benson GV, et al. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- 29.Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J. Clin. Invest. 1998;101:1379–1384. doi: 10.1172/JCI1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christian M, et al. Cyclic AMP-induced forkhead transcription factor, FKHR, cooperates with CCAAT/enhancer-binding protein beta in differentiating human endometrial stromal cells. J. Biol. Chem. 2002;277:20825–20832. doi: 10.1074/jbc.M201018200. [DOI] [PubMed] [Google Scholar]
- 31.Paria BC, Elenius K, Klagsbrun M, Dey SK. Heparin-binding EGF-like growth factor interacts with mouse blastocysts independently of ErbB1: a possible role for heparin sulfate proteoglycans and ErbB4 in blastocyst implantation. Development. 1999;126:1997–2005. doi: 10.1242/dev.126.9.1997. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, et al. Differential G protein-coupled cannabinoid receptor signaling by anandamide directs blastocyst activation for implantation. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14914–14919. doi: 10.1073/pnas.2436379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fazleabas AT, Donnelly KM, Srinivasan S, Fortman JD, Miller JB. Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2543–2548. doi: 10.1073/pnas.96.5.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemberger M, et al. UniGene cDNA array-based monitoring of transcriptome changes during mouse placental development. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13126–13131. doi: 10.1073/pnas.231396598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reese J, et al. Global gene expression analysis to identify molecular markers of uterine receptivity and embryo implantation. J. Biol. Chem. 2001;276:44137–44145. doi: 10.1074/jbc.M107563200. [DOI] [PubMed] [Google Scholar]
- 36.Kao LC, et al. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143:2119–2138. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- 37.Carson DD, et al. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol. Hum. Reprod. 2002;8:871–879. doi: 10.1093/molehr/8.9.871. [DOI] [PubMed] [Google Scholar]
- 38.Lim H, et al. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, et al. Rescue of female infertility from the loss of cyclooxygenase-2 by compensatory up-regulation of cyclooxygenase-1 is a function of genetic makeup. J. Biol. Chem. 2004;279:10649–10658. doi: 10.1074/jbc.M312203200. [DOI] [PubMed] [Google Scholar]
- 40.Cheng JG, Stewart CL. Loss of cyclooxygenase-2 retards decidual growth but does not inhibit embryo implantation or development to term. Biol. Reprod. 2003;68:401–404. doi: 10.1095/biolreprod.102.009589. [DOI] [PubMed] [Google Scholar]
- 41.Reese J, Brown N, Paria BC, Morrow J, Dey SK. COX-2 compensation in the uterus of COX-1 deficient mice during the pre-implantation period. Mol. Cell. Endocrinol. 1999;150:23–31. doi: 10.1016/s0303-7207(99)00033-7. [DOI] [PubMed] [Google Scholar]
- 42.Lim H, et al. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev. 1999;13:1561–1574. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barak Y, et al. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc. Natl. Acad. Sci. U. S. A. 2002;99:303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paria BC, et al. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1047–1052. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev. Biol. 2002;245:280–290. doi: 10.1006/dbio.2002.0645. [DOI] [PubMed] [Google Scholar]
- 46.Daikoku T, et al. Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: Evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol. Endocrinol. 2004;18:1238–1250. doi: 10.1210/me.2003-0403. [DOI] [PubMed] [Google Scholar]
- 47.Genbacev OD, et al. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299:405–408. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- 48.Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 49.Carson DD, et al. Embryo implantation. Dev. Biol. 2000;223:217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- 50.Damsky CH, Fitzgerald ML, Fisher SJ. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J. Clin. Invest. 1992;89:210–222. doi: 10.1172/JCI115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J. Clin. Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guleria I, Pollard JW. The trophoblast is a component of the innate immune system during pregnancy. Nat. Med. 2000;6:589–593. doi: 10.1038/75074. [DOI] [PubMed] [Google Scholar]
- 53.Red-Horse K, Drake PM, Gunn MD, Fisher SJ. Chemokine ligand and receptor expression in the pregnant uterus: reciprocal patterns in complementary cell subsets suggest functional roles. Am. J. Pathol. 2001;159:2199–2213. doi: 10.1016/S0002-9440(10)63071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song H, et al. Cytosolic phospholipase A2alpha is crucial for ‘on-time’ embryo implantation that directs subsequent development. Development. 2002;129:2879–2889. doi: 10.1242/dev.129.12.2879. [DOI] [PubMed] [Google Scholar]
- 55.Herz J, Couthier DE, Hammer RE. Correction: LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1993;73:428. doi: 10.1016/0092-8674(93)90130-i. [DOI] [PubMed] [Google Scholar]
- 56.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N. Engl. J. Med. 1999;340:1796–1799. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 58.Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat. Rev. Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 59.Xu RH, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat. Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 60.Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- 61.Nagy A, et al. Embryonic stem cells alone are able to support fetal development in the mouse. Development. 1990;110:815–821. doi: 10.1242/dev.110.3.815. [DOI] [PubMed] [Google Scholar]
- 62.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cross JC, et al. Genes, development and evolution of the placenta. Placenta. 2003;24:123–130. doi: 10.1053/plac.2002.0887. [DOI] [PubMed] [Google Scholar]
- 64.Russ AP, et al. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature. 2000;404:95–99. doi: 10.1038/35003601. [DOI] [PubMed] [Google Scholar]
- 65.Auman HJ, et al. Transcription factor AP-2gamma is essential in the extra-embryonic lineages for early postimplantation development. Development. 2002;129:2733–2747. doi: 10.1242/dev.129.11.2733. [DOI] [PubMed] [Google Scholar]
- 66.Luo J, et al. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature. 1997;388:778–782. doi: 10.1038/42022. [DOI] [PubMed] [Google Scholar]
- 67.Adamson SL, et al. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev. Biol. 2002;250:358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- 68.Ma GT, et al. Nodal regulates trophoblast differentiation and placental development. Dev. Biol. 2001;236:124–135. doi: 10.1006/dbio.2001.0334. [DOI] [PubMed] [Google Scholar]
- 69.Guillemot F, Nagy A, Auerbach A, Rossant J, Joyner AL. Essential role of Mash-2 in extraembryonic development. Nature. 1994;371:333–336. doi: 10.1038/371333a0. [DOI] [PubMed] [Google Scholar]
- 70.Wang J, Mager J, Schnedier E, Magnuson T. The mouse PcG gene eed is required for Hox gene repression and extraembryonic development. Mamm. Genome. 2002;13:493–503. doi: 10.1007/s00335-002-2182-7. [DOI] [PubMed] [Google Scholar]
- 71.Janatpour MJ, et al. A repertoire of differentially expressed transcription factors that offers insight into mechanisms of human cytotrophoblast differentiation. Dev. Genet. 1999;25:146–157. doi: 10.1002/(SICI)1520-6408(1999)25:2<146::AID-DVG9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 72.Janatpour MJ, et al. Id-2 regulates critical aspects of human cytotrophoblast differentiation, invasion and migration. Development. 2000;127:549–558. doi: 10.1242/dev.127.3.549. [DOI] [PubMed] [Google Scholar]
- 73.Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat. Genet. 1998;18:271–275. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- 74.Firulli AB, McFadden DG, Lin Q, Srivastava D, Olson EN. Heart and extra-embryonic mesodermal defects in mouse embryos lacking the bHLH transcription factor Hand1. Nat. Genet. 1998;18:266–270. doi: 10.1038/ng0398-266. [DOI] [PubMed] [Google Scholar]
- 75.Knofler M, Meinhardt G, Vasicek R, Husslein P, Egarter C. Molecular cloning of the human Hand1 gene/cDNA and its tissue-restricted expression in cytotrophoblastic cells and heart. Gene. 1998;224:77–86. doi: 10.1016/s0378-1119(98)00511-3. [DOI] [PubMed] [Google Scholar]
- 76.Jones BW, Fetter RD, Tear G, Goodman CS. glial cells missing: a genetic switch that controls glial versus neuronal fate. Cell. 1995;82:1013–1023. doi: 10.1016/0092-8674(95)90280-5. [DOI] [PubMed] [Google Scholar]
- 77.Anson-Cartwright L, et al. The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat. Genet. 2000;25:311–314. doi: 10.1038/77076. [DOI] [PubMed] [Google Scholar]
- 78.Sapin V, Dolle P, Hindelang C, Kastner P, Chambon P. Defects of the chorioallantoic placenta in mouse RXRalpha null fetuses. Dev. Biol. 1997;191:29–41. doi: 10.1006/dbio.1997.8687. [DOI] [PubMed] [Google Scholar]
- 79.Wendling O, Chambon P, Mark M. Retinoid X receptors are essential for early mouse development and placentogenesis. Proc. Natl. Acad. Sci. U. S. A. 1999;96:547–551. doi: 10.1073/pnas.96.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barak Y, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 81.Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122:3343–3353. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- 82.Schmidt C, et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 83.Yamamoto H, et al. Defective trophoblast function in mice with a targeted mutation of Ets2. Genes Dev. 1998;12:1315–1326. doi: 10.1101/gad.12.9.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kenner L, et al. Mice lacking JunB are osteopenic due to cell-autonomous osteoblast and osteoclast defects. J. Cell Biol. 2004;164:613–623. doi: 10.1083/jcb.200308155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hatano N, et al. Essential role for ERK2 mitogen-activated protein kinase in placental development. Genes Cells. 2003;8:847–856. doi: 10.1046/j.1365-2443.2003.00680.x. [DOI] [PubMed] [Google Scholar]
- 86.Dietrich S, et al. The role of SF/HGF and c-Met in the development of skeletal muscle. Development. 1999;126:1621–1629. doi: 10.1242/dev.126.8.1621. [DOI] [PubMed] [Google Scholar]
- 87.Takahashi Y, et al. SOCS3: an essential regulator of LIF receptor signaling in trophoblast giant cell differentiation. EMBO J. 2003;22:372–384. doi: 10.1093/emboj/cdg057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Isermann B, Hendrickson SB, Hutley K, Wing M, Weiler H. Tissue-restricted expression of thrombomodulin in the placenta rescues thrombomodulin-deficient mice from early lethality and reveals a secondary developmental block. Development. 2001;128:827–838. doi: 10.1242/dev.128.6.827. [DOI] [PubMed] [Google Scholar]
- 89.Gallicano GI, Bauer C, Fuchs E. Rescuing desmoplakin function in extra-embryonic ectoderm reveals the importance of this protein in embryonic heart, neuroepithelium, skin and vasculature. Development. 2001;128:929–941. doi: 10.1242/dev.128.6.929. [DOI] [PubMed] [Google Scholar]
- 90.Mishina Y, Crombie R, Bradley A, Behringer RR. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev. Biol. 1999;213:314–326. doi: 10.1006/dbio.1999.9378. [DOI] [PubMed] [Google Scholar]
- 91.Sugimoto M, Karashima Y, Abe K, Tan SS, Takagi N. Tetraploid embryos rescue the early defects of tw5/tw5 mouse embryos. Genesis. 2003;37:162–171. doi: 10.1002/gene.10238. [DOI] [PubMed] [Google Scholar]
- 92.Goto Y, Takagi N. Tetraploid embryos rescue embryonic lethality caused by an additional maternally inherited X chromosome in the mouse. Development. 1998;125:3353–3363. doi: 10.1242/dev.125.17.3353. [DOI] [PubMed] [Google Scholar]
- 93.Jaquemar D, et al. Keratin 8 protection of placental barrier function. J. Cell Biol. 2003;161:749–756. doi: 10.1083/jcb.200210004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bajoria R, Sooranna SR, Ward S, D’Souza S, Hancock M. Placental transport rather than maternal concentration of amino acids regulates fetal growth in monochorionic twins: implications for fetal origin hypothesis. Am. J. Obstet. Gynecol. 2001;185:1239–1246. doi: 10.1067/mob.2001.118269. [DOI] [PubMed] [Google Scholar]
- 95.Genbacev O, Bass KE, Joslin RJ, Fisher SJ. Maternal smoking inhibits early human cytotrophoblast differentiation. Reprod. Toxicol. 1995;9:245–255. doi: 10.1016/0890-6238(95)00006-v. [DOI] [PubMed] [Google Scholar]
- 96.Genbacev O, et al. Concordant in situ and in vitro data show that maternal cigarette smoking negatively regulates placental cytotrophoblast passage through the cell cycle. Reprod. Toxicol. 2000;14:495–506. doi: 10.1016/s0890-6238(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 97.Pereira L, Maidji E, McDonagh S, Genbacev O, Fisher S. Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity. J. Virol. 2003;77:13301–13314. doi: 10.1128/JVI.77.24.13301-13314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamamoto-Tabata T, McDonagh S, Chang HT, Fisher S, Pereira L. Human cytomegalovirus interleukin-10 downregulates metalloproteinase activity and impairs endothelial cell migration and placental cytotrophoblast invasiveness in vitro. J. Virol. 2004;78:2831–2840. doi: 10.1128/JVI.78.6.2831-2840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.A., Wright., et al. Trisomy 21 is associated with variable defects in cytotrophoblast differentiation along the invasive pathway. Am. J. Med. Genet. In press. [DOI] [PubMed]
- 100.Maltepe E, Simon MC. Oxygen, genes, and development: an analysis of the role of hypoxic gene regulation during murine vascular development. J. Mol. Med. 1998;76:391–401. doi: 10.1007/s001090050231. [DOI] [PubMed] [Google Scholar]
- 101.Jauniaux E, et al. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jauniaux E, Gulbis B, Burton GJ. The human first trimester gestational sac limits rather than facilitates oxygen transfer to the foetus--a review. Placenta. 2003;24(Suppl. A):S86–S93. doi: 10.1053/plac.2002.0932. [DOI] [PubMed] [Google Scholar]
- 103.Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J. Clin. Invest. 1996;97:540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 105.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Douglas RM, Haddad GG. Genetic models in applied physiology: invited review: effect of oxygen deprivation on cell cycle activity: a profile of delay and arrest. J. Appl. Physiol. 2003;94:2068–2083; discussion 2084. doi: 10.1152/japplphysiol.01029.2002. [DOI] [PubMed] [Google Scholar]
- 107.Semenza GL. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 108.Safran M, Kaelin WG., Jr HIF hydroxylation and the mammalian oxygen-sensing pathway. J. Clin. Invest. 2003;111:779–783. doi:10.1172/JCI200318181. doi: 10.1172/JCI18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Masson N, Ratcliffe PJ. HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O(2) levels. J. Cell. Sci. 2003;116:3041–3049. doi: 10.1242/jcs.00655. [DOI] [PubMed] [Google Scholar]
- 110.Wykoff CC, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Identification of novel hypoxia dependent and independent target genes of the von Hippel-Lindau (VHL) tumour suppressor by mRNA differential expression profiling. Oncogene. 2000;19:6297–6305. doi: 10.1038/sj.onc.1204012. [DOI] [PubMed] [Google Scholar]
- 111.Gnarra JR, et al. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 1997;94:9102–9107. doi: 10.1073/pnas.94.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Katschinski DM, et al. Heat induction of the unphosphorylated form of hypoxia-inducible factor-1alpha is dependent on heat shock protein-90 activity. J. Biol. Chem. 2002;277:9262–9267. doi: 10.1074/jbc.M110377200. [DOI] [PubMed] [Google Scholar]
- 113.Voss AK, Thomas T, Gruss P. Mice lacking HSP90beta fail to develop a placental labyrinth. Development. 2000;127:1–11. doi: 10.1242/dev.127.1.1. [DOI] [PubMed] [Google Scholar]
- 114.Genbacev O, Krtolica A, Kaelin W, Fisher SJ. Human cytotrophoblast expression of the von Hippel-Lindau protein is downregulated during uterine invasion in situ and upregulated by hypoxia in vitro. Dev. Biol. 2001;233:526–536. doi: 10.1006/dbio.2001.0231. [DOI] [PubMed] [Google Scholar]
- 115.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat. Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 116.Zhou Y, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am. J. Pathol. 2002;160:1405–1423. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Makinen T, Alitalo K. Molecular mechanisms of lymphangiogenesis. Cold Spring Harb. Symp. Quant. Biol. 2002;67:189–196. doi: 10.1101/sqb.2002.67.189. [DOI] [PubMed] [Google Scholar]
- 118.Zhou Y, Bellingard V, Feng KT, McMaster M, Fisher SJ. Human cytotrophoblasts promote endothelial survival and vascular remodeling through secretion of Ang2, PlGF, and VEGF-C. Dev. Biol. 2003;263:114–125. doi: 10.1016/s0012-1606(03)00449-4. [DOI] [PubMed] [Google Scholar]
- 119.Gale NW, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev. Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 120.Damsky CH, Fisher SJ. Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr. Opin. Cell Biol. 1998;10:660–666. doi: 10.1016/s0955-0674(98)80043-4. [DOI] [PubMed] [Google Scholar]
- 121.Lain KY, Roberts JM. Contemporary concepts of the pathogenesis and management of preeclampsia. JAMA. 2002;287:3183–3186. doi: 10.1001/jama.287.24.3183. [DOI] [PubMed] [Google Scholar]
- 122.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J. Clin. Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maynard SE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 2003;111:649–658. doi:10.1172/JCI200317189. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Levine RJ, et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 125.Thadhani R, et al. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J. Clin. Endocrinol. Metab. 2004;89:770–775. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.