Abstract
Using the plasmid DNA pSV2-neo (which, when integrated into the cellular genome confers resistance to the antibiotic G418 for selection), we examined and compared the transfection efficiency on NIH 3T3 cells electropermeabilized by applying a sequence of high-frequency unipolar or bipolar square waves or a single square pulse. Results show that a bipolar square wave is, at least, 1.7- and 5.5-fold more efficient than the unipolar square wave and single square pulse, respectively. In the range of electric field strength used for optimum transfection, the survivability of electropermeabilized cells was comparable between the unipolar and bipolar square waves but fell considerably with the single square pulse. Qualitative comparison of cell permeabilization induced by the three types of wave forms and monitored by ethidium bromide uptake revealed that only the bipolar square wave permeabilizes the cell membrane symmetrically at the two hemispheres facing the electrodes. With unipolar square wave or single square pulse, the membrane is permeabilized either on one side or asymmetrically. Taken together, our result suggests that permeabilization of the membrane at multiple sites without affecting cell survivability may account for the improvements in transfection efficiency observed with bipolar oscillating electric fields.
Full text
PDF

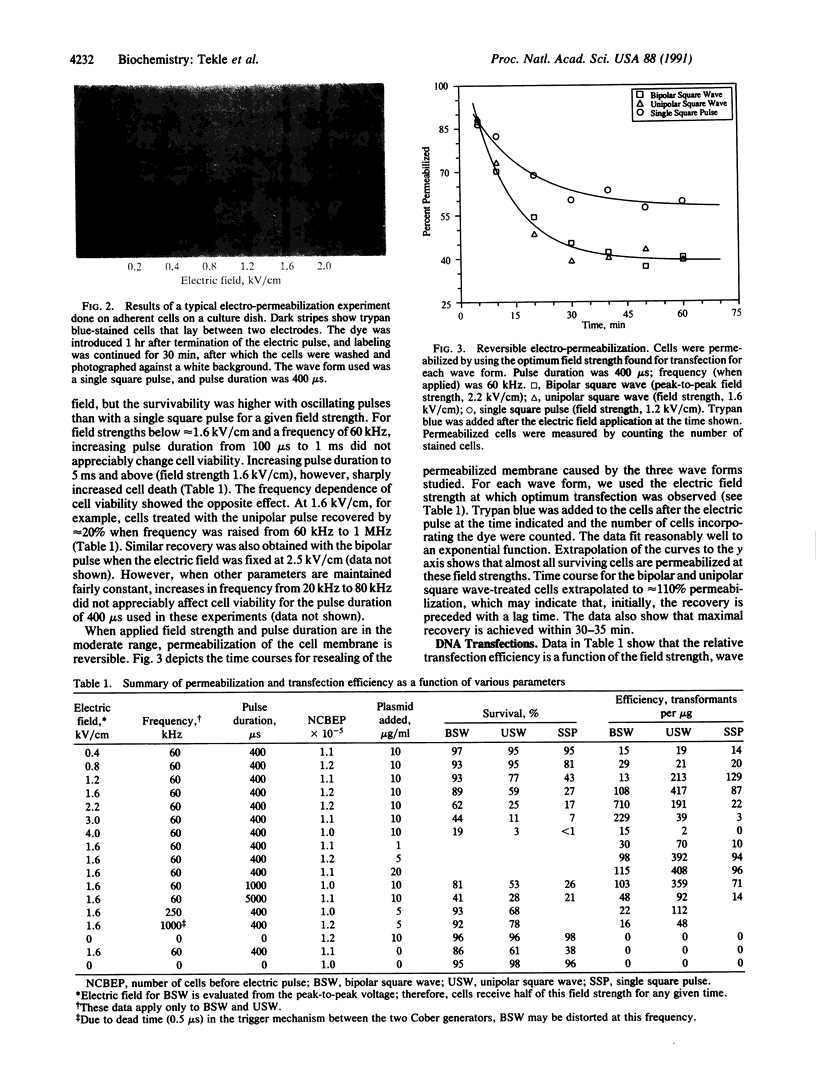

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calvin N. M., Hanawalt P. C. High-efficiency transformation of bacterial cells by electroporation. J Bacteriol. 1988 Jun;170(6):2796–2801. doi: 10.1128/jb.170.6.2796-2801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. C. Cell poration and cell fusion using an oscillating electric field. Biophys J. 1989 Oct;56(4):641–652. doi: 10.1016/S0006-3495(89)82711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D. S., Sowers A. E. Membrane electroporation--fast molecular exchange by electroosmosis. Biochim Biophys Acta. 1990 Mar;1022(3):381–392. doi: 10.1016/0005-2736(90)90289-z. [DOI] [PubMed] [Google Scholar]
- Fromm M. E., Taylor L. P., Walbot V. Stable transformation of maize after gene transfer by electroporation. 1986 Feb 27-Mar 5Nature. 319(6056):791–793. doi: 10.1038/319791a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Scrutton M. C. Gaining access to the cytosol: the technique and some applications of electropermeabilization. Biochem J. 1986 Mar 15;234(3):497–506. doi: 10.1042/bj2340497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson J. C., Yee D. Electroporation: parameters affecting transfer of DNA into mammalian cells. Anal Biochem. 1987 Jul;164(1):44–52. doi: 10.1016/0003-2697(87)90365-4. [DOI] [PubMed] [Google Scholar]
- Marszalek P., Liu D. S., Tsong T. Y. Schwan equation and transmembrane potential induced by alternating electric field. Biophys J. 1990 Oct;58(4):1053–1058. doi: 10.1016/S0006-3495(90)82447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Meilhoc E., Masson J. M., Teissié J. High efficiency transformation of intact yeast cells by electric field pulses. Biotechnology (N Y) 1990 Mar;8(3):223–227. doi: 10.1038/nbt0390-223. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Dower W. J., Tompkins L. S. High-voltage electroporation of bacteria: genetic transformation of Campylobacter jejuni with plasmid DNA. Proc Natl Acad Sci U S A. 1988 Feb;85(3):856–860. doi: 10.1073/pnas.85.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P. H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou-Lee T. M., Turgeon R., Wu R. Expression of a foreign gene linked to either a plant-virus or a Drosophila promoter, after electroporation of protoplasts of rice, wheat, and sorghum. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6815–6819. doi: 10.1073/pnas.83.18.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H. Electroporation in biology: methods, applications, and instrumentation. Anal Biochem. 1988 Nov 1;174(2):361–373. doi: 10.1016/0003-2697(88)90035-8. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers A. E. Fusion events and nonfusion contents mixing events induced in erythrocyte ghosts by an electric pulse. Biophys J. 1988 Oct;54(4):619–626. doi: 10.1016/S0006-3495(88)82997-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper H., Jones H., Zimmermann U. Large scale transfection of mouse L-cells by electropermeabilization. Biochim Biophys Acta. 1987 Jun 12;900(1):38–44. doi: 10.1016/0005-2736(87)90275-6. [DOI] [PubMed] [Google Scholar]
- Tatsuka M., Orita S., Yagi T., Kakunaga T. An improved method of electroporation for introducing biologically active foreign genes into cultured mammalian cells. Exp Cell Res. 1988 Sep;178(1):154–162. doi: 10.1016/0014-4827(88)90386-2. [DOI] [PubMed] [Google Scholar]
- Tekle E., Astumian R. D., Chock P. B. Electro-permeabilization of cell membranes: effect of the resting membrane potential. Biochem Biophys Res Commun. 1990 Oct 15;172(1):282–287. doi: 10.1016/s0006-291x(05)80206-2. [DOI] [PubMed] [Google Scholar]
- Weaver J. C., Harrison G. I., Bliss J. G., Mourant J. R., Powell K. T. Electroporation: high frequency of occurrence of a transient high-permeability state in erythrocytes and intact yeast. FEBS Lett. 1988 Feb 29;229(1):30–34. doi: 10.1016/0014-5793(88)80791-9. [DOI] [PubMed] [Google Scholar]
- Wong T. K., Neumann E. Electric field mediated gene transfer. Biochem Biophys Res Commun. 1982 Jul 30;107(2):584–587. doi: 10.1016/0006-291x(82)91531-5. [DOI] [PubMed] [Google Scholar]
- Xie T. D., Sun L., Tsong T. Y. Study of mechanisms of electric field-induced DNA transfection. I. DNA entry by surface binding and diffusion through membrane pores. Biophys J. 1990 Jul;58(1):13–19. doi: 10.1016/S0006-3495(90)82349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T., Tsuruta S., Mikawa H. The effect of cell synchronization on the efficiency of stable gene transfer by electroporation. FEBS Lett. 1989 Mar 13;245(1-2):201–203. doi: 10.1016/0014-5793(89)80221-2. [DOI] [PubMed] [Google Scholar]
- Zimmermann U. Electrical breakdown, electropermeabilization and electrofusion. Rev Physiol Biochem Pharmacol. 1986;105:176–256. [PubMed] [Google Scholar]