Abstract
Between February 2001 and January 2002, an increase in the number of Klebsiella pneumoniae isolates with reduced susceptibility to expanded-spectrum cephalosporins (RSKp) was detected in the neonatal unit of the Juan Canalejo Hospital, and 21 patients were either colonized or infected by the bacterial isolates. The current “gold standard” method for typing K. pneumoniae isolates is pulsed-field gel electrophoresis. However, this technique is expensive and time-consuming. In a search for faster and accurate alternatives to this method, we investigated PCR-based fingerprinting techniques (enterobacterial repetitive intergenic consensus sequence PCR [ERIC-PCR], repetitive extragenic palindromic sequence-based PCR [REP-PCR], and RAPD [randomly amplified polymorphic DNA]) for their ability to characterize K. pneumoniae isolates. The causal agent of the nosocomial outbreak was characterized by these techniques and was found to be a single epidemic strain (RSKp). A multiple regression logistic model was developed to identify potential independent factors associated with colonization and/or infection by RSKp. Logistic regression analysis was applied to all significant variables (P < 0.05) in the univariate analysis, and it was revealed that intubation (odds ratio [OR], 27.0; 95% confidence interval [95%CI], 5.39 to 135.14) and prematurity (OR, 4.4; 95%CI, 0.89 to 21.89) were such independent factors. Moreover, oxime cephalosporins did not appear to be statistically significant. Overall, the results showed that PCR-based techniques are expeditious and useful methods for typing K. pneumoniae isolates. Of the techniques studied, ERIC-PCR showed the highest discriminatory index (D = 0.828), followed by RAPD (D = 0.826) and REP-PCR (D = 0.773)
Klebsiella pneumoniae has previously been reported to be associated with 2 to 5% of nosocomial infections, particularly those involving the lower respiratory and urinary tracts (8, 16, 22, 25). Resistance of this species to expanded-spectrum cephalosporins was first described in the early 1980s, and an increase in resistance has occurred since 1986 (3, 7, 8, 18, 22). Resistant isolates probably acquire their resistance by producing extended-spectrum β-lactamases (ESBLs) (22). The epidemiology of ESBL has previously been investigated, and several studies have described the potential risk factors associated with colonization or infection with multiresistant K. pneumoniae isolates (1, 28). The present study describes a nosocomial outbreak caused by a K. pneumoniae strain that showed reduced sensitivity to expanded-spectrum cephalosporins.
Pulsed-field gel electrophoresis (PFGE) analysis of genome macrorestriction fragments has been shown to be a discriminatory technique for typing different microorganisms (2, 10, 11, 12); however, it is technically demanding and time-consuming and requires specific equipment. Other PCR-based typing techniques, such as randomly amplified polymorphic DNA (RAPD) analysis, are faster and easier to perform. In recent studies, RAPD analysis has been successfully used to type diverse microorganisms (6, 11, 15, 25, 30).
Moreover, repetitive extragenic palindromic sequence-based PCR (REP-PCR) and enterobacterial repetitive intergenic consensus sequence PCR (ERIC-PCR) have also been successfully used for typing K. pneumoniae isolates (4, 7, 8, 13, 16). However, the comparative typing ability, reproducibility, discriminatory power, and efficiency of these methods were not fully investigated in all of these studies. Therefore, although these PCR-based methods are widely used and reported in the medical literature, it remains to be determined which is the most discriminatory and reproducible PCR-based method for typing K. pneumoniae isolates in an outbreak setting. We sought to do this in the present study.
K. pneumoniae is an important hospital-acquired pathogen with the potential to cause severe morbidity and mortality in pediatric patients. Several outbreaks of infection caused by K. pneumoniae isolates that are simultaneously resistant to broad-spectrum cephalosporins and aminoglycosides have been reported (1, 3, 18, 22). The risk factors associated with colonization or infection by epidemic strains harboring ESBL and showing high expanded-spectrum cephalosporin MICs have already been clearly demonstrated (1). However, the risk factors associated with infection or colonization by bacterial strains for which the expanded-spectrum cephalosporin MICs are not high (or low), i.e., strains that are categorized as susceptible according to NCCLS criteria but show reduced susceptibility to expanded-spectrum cephalosporins, remain unknown.
In summary, we report the molecular characterization of a nosocomial outbreak caused by an epidemic strain of K. pneumoniae (RSKp) with reduced susceptibility to expanded-spectrum cephalosporins. In addition, we also report the risk factors associated with infection or colonization by this bacterial strain.
(Part of this study was presented previously [M. Cartelle, A. Beceiro, E. Gil, M. Dominguez, F. Molina, J. M. Eiros, and G. Bou, 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1438, p. 143, 2002].)
MATERIALS AND METHODS
Microbiological studies. (i) PFGE.
Macrorestriction analysis of chromosomal DNA with XbaI (New England Biolabs, Boston, Mass.) was carried out by PFGE according to published procedures (12). PFGE was performed by using a CHEF-DRIII apparatus (Bio-Rad Laboratories, Richmond, Calif.), with pulses ranging from 0.5 to 15 s and at a voltage of 6 V/cm, at 14°C for 20 h. Products were detected after ethidium bromide staining (50 mg/liter) and photographed with Polaroid type 665 film. The interpretation of PFGE patterns was according to the method of Tenover et al. (27).
(ii) PCR typing methods.
Chromosomal DNA of the K. pneumoniae isolates was obtained according to standard protocols (24). For REP-PCR and ERIC-PCR, 500 ng of the chromosomal template was used, and the reactions were carried out with oligonucleotides, as previously reported (29). Aliquots (20 μl) of each sample were subjected to electrophoresis on 1.0% agarose gels.
Isolates belonging to the same genotypes assigned according to the PCR band pattern showed identical or highly similar profiles (up to two bands different). The REP-PCR was performed as follows: denaturation for 10 min at 94°C; amplification for 1 min at 94°C, 1 min at 45°C, and 2 min at 72°C for 30 cycles; and elongation for 16 min at 72°C. The ERIC-PCR was performed as follows: denaturation for 10 min at 94°C; amplification for 1 min at 94°C, 1 min at 52°C, and 2 min at 72°C for 30 cycles; and then elongation for 16 min at 72°C.
The RAPD analysis was performed according to the manufacturer's instructions (Ready-To-Go RAPD analysis beads; Amersham Pharmacia Biotech, ICN). Aliquots (20 μl) of each sample were subjected to electrophoresis on 2.0% agarose gels. Amplified products were detected after ethidium bromide staining (50 mg/liter) and photographed with Polaroid type 665 film. Isolates belonging to the same genotypes were grouped according to the PCR band pattern (up to two bands different).
(iii) Reproducibility and discriminatory index.
Reproducibility was measured as the ability of a technique to yield identical results with testing of the same strain on three different experiments with different DNA preparations, and it was expressed as the percentage of strains that gave the same profile in the three separate experiments (+++, >75%; ++, 50 to 75%; +, <50%).
The discriminatory index was calculated according to the method of Hunter and Gaston (14). We studied a set of K. pneumoniae isolates associated with outbreak of infection that were genetically related; therefore, we modified the method accordingly. For PCR-based methods, we maintained the same number of strains in all cases and evaluated the differences in genotypes. To consider the presence of subtypes among a specific genotype, we introduced a correcting factor in the equation of Hunter and Gaston by adding the number of strains with different subtypes belonging to a specific genotype. This correction may improve, at least in part, some of the limitations of this index.
Fifty isolates of K. pneumoniae (including forty-three clinical and seven American Type Culture Collection [ATCC] isolates) were used to determine the reproducibility and discriminatory index of the different techniques.
(iv) Strains.
Clinical isolates of K. pneumoniae were identified by API 20E (bioMérieux) and MicroScan (Walkaway-96).
In genotypic studies and for comparative purposes, seven different isolates of K. pneumoniae were used as controls (ATCC 7761, ATCC 10031, ATCC 13883, ATCC 27736, ATCC 29995, ATCC 29665, and ATCC 9621).
Forty-three clinical isolates of K. pneumoniae were used for genotypic studies. Of these, 33 were isolated from 21 newborns, and they showed a phenotype of reduced susceptibility to expanded-spectrum cephalosporins and aztreonam (ESBL phenotype). The remaining 10 clinical isolates of K. pneumoniae were isolated from 10 different patients during the outbreak, and they were probably not related to the outbreak-associated strains (non-ESBL phenotype).
For comparative purposes and to facilitate interpretation of the results, the DNA band patterns of 31 K. pneumoniae strains isolated from 31 different patients are shown (Table 1); 21 of these patients were either colonized or infected by strain RSKp (showing an ESBL phenotype), and the remaining 10 were colonized or infected by K. pneumoniae isolates not showing an ESBL phenotype and probably not associated with the outbreak of infection. Also, the interpretation of the band pattern of the seven ATCC K. pneumoniae isolates is shown (Table 1).
TABLE 1.
Patients infected or colonized by the RSKp strains and those included in the genotypic study
| Patient dataa
|
Wardb | Type of infection or colonizationc | Sample typed | Hospital stay (days) | Previous treatmente | ESBL phenotype | Genotype as determined by:
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No.f | Sex | Date of isolation | PFGE | ERIC-PCR | REP-PCR | RAPD | ||||||
| 1 | F | 2.5.01 | P-ICU | CoI | Co.Ex. | 8 | A, To | Yes | I | 1 | A.1 | a.1 |
| 2 | M | 2.7.01 | N-Dep | Ent | Stool | 5 | A, To, Mp | Yes | I | 1 | A.1 | a.1 |
| 3 | M | 2.19.01 | N-Dep | Colonization | End.Tube | 5 | A-Clav, Ce | Yes | I | 1 | A.1 | a.2 |
| 4 | F | 2.19.01 | N-Dep | Ent | Stool | 2 | A, To | Yes | I | 1 | A.1 | a.2 |
| 5 | F | 2.19.01 | N-Dep | Colonization | End.Tube | 78 | Ce, Te, Azt | Yes | I | 1 | A.1 | a.2 |
| 6 | M | 2.20.01 | N-Dep | Col | Co.Ex. | 7 | A, To | Yes | I | 1 | A.1 | a.2 |
| 7 | F | 2.23.01 | N-ICU | Ent | Stool | 10 | A, To, Te | Yes | I | 1 | A.1 | a.2 |
| 8 | M | 3.10.01 | N-ICU | CaI | Cateter | 14 | A, To, Te | Yes | I | 1 | A.1 | a.2 |
| 9 | F | 4.4.01 | P-ICU | Ent | Stool | 44 | A, To, Mz | Yes | I | 1 | A.1 | a.2 |
| 10 | F | 4.10.01 | P-ICU | UTI | Urine | 22 | A, To, Gn | Yes | I | 1 | A.1 | a.2 |
| 11 | F | 4.20.01 | N-Dep | UTI | Urine | 35 | A, To, Te, Caz | Yes | I | 1 | A.1 | a.2 |
| 12 | M | 4.30.01 | N-Dep | Sepsis | Blood | 28 | Te, Ctx, Me, A, To, Mp | Yes | I | 1 | A.1 | a.2 |
| 13 | M | 5.8.01 | N-Dep | Colonization | Stool | 8 | A, To | Yes | I | 1 | A.1 | a.2 |
| 14 | M | 6.8.01 | N-Dep | Colonization | End.Tube | 19 | A, To, Cf, Gn | Yes | I | 1 | A.1 | a.2 |
| 15 | F | 7.2.01 | N-Dep | CoI | Co.Ex | 6 | A, To, Me | Yes | I | 1 | A.1 | a.2 |
| 16 | M | 7.5.01 | N-Dep | Sepsis | Blood | 80 | Tc, Caz, Me | Yes | I | 1 | A.1 | a.2 |
| 17 | M | 7.12.01 | N-Dep | UTI | Urine | 7 | A, To | Yes | I | 1 | A.1 | a.2 |
| 18 | M | 12.24.01 | N-Dep | UTI | Urine | 45 | Me, Te, Caz, A, To | Yes | I | 1 | A.1 | a.2 |
| 19 | F | 12.28.01 | N-Dep | Sepsis | Blood | 171 | Te, Ctx, Me, Co, To, Azt, Ce, Ak | Yes | I | 1 | A.1 | a.2 |
| 20 | M | 2.12.02 | N-Dep | Sepsis | Blood | 6 | A | Yes | I | 1 | A.1 | a.2 |
| 21 | M | 2.16.02 | N-Dep | Sepsis | Blood | 14 | A, To, Te | Yes | I | 1 | A.1 | a.2 |
| 22 | M | 1.16.01 | PHC-C | UTI | Urine | 0 | No | II | 2 | A.2 | b | |
| 23 | F | 12.6.01 | P-Dep | Sepsis | Blood | 270 | A, To, Im, Mp, Va | No | III | 3 | A.1 | c |
| 24 | M | 2.8.01 | M-ICU | Wound infection | Wo.Ex. | 58 | No | IV | 4 | B | d | |
| 25 | F | 2.8.01 | M-ICU | Wound infection | Wo.Ex. | 18 | No | III | 3 | A.1 | c | |
| 26 | F | 2.20.01 | PHC-C | UTI | Urine | 0 | No | V | 5 | C | e | |
| 27 | F | 3.5.01 | PHC-C | UTI | Urine | 0 | No | VI | 6 | D | f | |
| 28 | F | 4.9.01 | M-ICU | UTI | Urine | 240 | No | VII | 7 | E | g | |
| 29 | M | 5.1.01 | M-ICU | UTI | Urine | 150 | No | VIII | 8 | F | h | |
| 30 | F | 5.2.01 | SCI-ICU | UTI | Urine | 130 | A-Clav | No | IX | 9 | G | i |
| 31 | M | 6.1.01 | O-Dep | Hearing infection | Hear.Ex. | 0 | No | X | 10 | H | j | |
| 32 (7761) | XI | 11 | I.1 | k | ||||||||
| 33 (10031) | XII | 12 | I.2 | l | ||||||||
| 34 (13883) | XIII | 13 | J | ll | ||||||||
| 35 (27736) | XIV | 14 | K | m | ||||||||
| 36 (29995) | XV | 15 | L | n | ||||||||
| 37 (29665) | XVI | 16 | LL | ñ | ||||||||
| 38 (9621) | XVII | 17 | M | o | ||||||||
M, male; F, female. Date of isolation: month.day.year.
Wards: N-Dep, neonatal unit; N-ICU, neonatal intensive care unit; P-Dep, pediatric department; P-ICU, pediatric ICU; M-ICU, medical ICU other than those for neonatal and pediatric patients; PHC-C, primary health care center; SCI-ICU, spinal cord injury ICU; O-Dep, otolaryngology department.
Col, conjunctival infection; Ent, enterocolitis; UTI, urinary tract infection; CaI, catheter infection.
Co.Ex., conjunctival exudate; Wo.Ex., wound exudate; End.Tube, endotracheal tube; Hear.Ex., hearing exudate.
A, ampicillin; To, tobramycin; Tc, ticarcillin; Caz, ceftazidime; Gn, gentamicin; Me, metronidazole; Mp, meropenem; A-Clav, amoxicillin-clavulanate; Ce, cephazoline; Co, colistin; Azt, aztreonam; Ctx, cefotaxime; Te, teicoplanin; Ak, amikacin; Im, imipenem; Va, vancomycin.
ATCC numbers are given in parentheses for nonclinical isolates.
To localize the source of the RSKp strain that caused the nosocomial outbreak of infection or colonization, samples were collected from different areas of the neonatal unit. Samples were analyzed by ERIC-PCR to test for any genetic relationship with the RSKp epidemic strain.
(v) Antimicrobial susceptibility pattern.
The pattern of antibiotic susceptibility was determined by disk diffusion according to NCCLS criteria (20). MICs were confirmed by E-test (AB Biodisk, Solna, Sweden) according to the manufacturer's directions.
Subjects and methods used in the clinical epidemiological study: risk factors involved in acquiring strain RSKp. (i) Setting.
The present study was carried out in the neonatal unit of the Juan Canalejo Hospital Complex in La Coruña, Spain. This is a 1,200-bed university tertiary-level medical center that serves a population of 516,000 people.
(ii) Patients.
In February 2001, the Department of Microbiology in the hospital complex reported the occurrence of a cluster of K. pneumoniae isolates with reduced susceptibility to expanded-spectrum cephalosporins (RSKp) in the neonatal unit. The infection control personnel were immediately engaged in investigation of the outbreak. In addition to clinical samples, screening samples were obtained twice a week from all patients admitted to the neonatal unit. Screening samples were obtained from pharynx and rectum. A total of 21 newborns were infected or colonized by RSKp.
Several patients were selected to take part in case-control studies aimed at detecting the risk factors associated with colonization or infection by RSKp strains.
A case study patient was defined as a patient who was admitted to the neonatal unit during the period from 1 February 2001 to 29 February 2002 and then became either clinically infected or colonized by RSKp. A control patient was defined as a patient admitted to the neonatal unit between 1 February 2001 and 29 February 2002 whose screening cultures were all negative for RSKp. These patients were retrospectively selected. A total of 21 case patients and 44 control patients were thus considered in the study.
Clinical and epidemiological data were collected from the medical records of all patients admitted to the unit during the period under study. The potential risk factors studied are discussed below (see Tables 4 and 5).
TABLE 4.
Comparison of several qualitative variables with case-control patients prior to isolation
| Variable | Cases (n = 21)
|
Controls (n = 44)
|
Pb | OR (95%CI) | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Mother colonized with S. agalactiae | 2 | 9.5 | 11 | 25 | 0.194 | 0.31 (0.06-1.58) |
| Rupture of amniotic sac more than 24 h before birth | 7 | 33.3 | 5 | 11.4 | 0.045 | 3.90 (1.06-14.31) |
| Cesarean | 8 | 38.1 | 9 | 20.5 | 0.130 | 2.39 (0.76-7.52) |
| Sex | ||||||
| Male | 13 | 61.9 | 29 | 65.9 | 0.752 | 1.19 (0.40-3.50) |
| Female | 29 | 65.9 | 15 | 34.1 | ||
| Birth weight (<1,500 g) | 7 | 33.3 | 3 | 6.8 | 0.010 | 6.83 (1.55-30.09) |
| Underlying disease | 11 | 52.4 | 6 | 13.6 | 0.001 | 6.96 (2.07-23.46) |
| Previous surgery (7 days) | 3 | 14.3 | 0 | 0 | 0.030 | NCa |
| High prematurity (28-30 wk) | 15 | 71.4 | 7 | 15.9 | <0.001 | 13.21 (3.81-45.87) |
| Presence of urinary catheter | 1 | 4.8 | 0 | 0 | 0.323 | NC |
| Presence of nasogastric tube | 2 | 9.5 | 0 | 0 | 0.101 | NC |
| Presence of intravascular catheter | 15 | 71.4 | 6 | 13.6 | <0.001 | 15.83 (4.40-56.93) |
| Parenteral nutrition | 15 | 71.4 | 7 | 15.9 | <0.001 | 13.21 (3.81-45.87) |
| Intubation | 18 | 85.7 | 5 | 11.4 | <0.001 | 46.80 (10.07-217.53) |
| Administration of β-lactam antibiotics | 21 | 100 | 22 | 50 | <0.001 | NC |
| Administration of aminoglycosides | 17 | 81 | 18 | 40.9 | 0.002 | 6.14 (1.77-21.30) |
| Administration of glycopeptides | 9 | 42.9 | 2 | 4.5 | <0.001 | 15.75 (2.99-82.92) |
NC, not calculable.
P values in boldface are statistically significant.
TABLE 5.
Comparison of several quantitative variables with case-control patients prior to isolation
| Variable | Cases (n = 21)
|
Controls (n = 44)
|
Pa | ||
|---|---|---|---|---|---|
| Mean (SD) | Median | Mean (SD) | Median | ||
| Duration (days) of ICU stay | 28.24 (39.44) | 14.00 | 3.02 (6.55) | 1.00 | <0.001 |
| Prior no. of days with bladder catheter | 1.05 (4.80) | 0 | 0.00 | 0 | 0.148 |
| Prior no. of days with nasogastric tube | 0.14 (0.48) | 0 | 0.00 | 0 | 0.039 |
| Prior no. of days with vascular catheter | 17.90 (36.86) | 8.00 | 1.39 (4.09) | 0 | <0.001 |
| Prior no. of days with parenteral nutrition | 10.09 (17.59) | 5.00 | 1.45 (4.99) | 0 | <0.001 |
| Prior no. of days with intubation or tracheostomy | 10.81 (18.14) | 5.00 | 0.48 (1.62) | 0 | <0.001 |
| Prior no. of days of administration with β-lactams | 21.28 (36.94) | 9.00 | 2.11 (5.19) | 0.50 | <0.001 |
| Prior no. of days of administration with aminoglycosides | 10.90 (18.99) | 7.00 | 1.34 (2.54) | 0 | <0.001 |
| Prior no. of days of administration with glycopeptides | 11.38 (23.14) | 0 | 0.68 (3.50) | 0 | <0.001 |
P values in boldface are statistically significant.
(iii) Study design.
This case-control study compared the frequency of exposure and the features of case patients with those of control patients in order to identify and quantify potential independent risk factors associated with colonization and/or infection by RSKp.
(iv) Statistical analysis.
Univariate analysis was carried out for determination of variables significantly associated with colonization or infection by RSKp. Contingency tables were analyzed by two-tailed χ2 test or by Fisher exact test. Quantitative variable differences between case and control patients were compared by using the Student t test or Mann-Whitney test when appropriate. The Kolmogorov-Smirnov test was used to assess normality.
A multiple-regression logistic model was developed to identify the potential independent factors associated with colonization and/or infection by RSKp. We followed a forward stepwise strategy, adjusting for all variables that were statistically significant in the univariate analysis or that were clinically relevant. Ninety-five percent confidence intervals (95%CIs) were calculated as estimators. Two-sided tests were used for all analyses. The results were considered statistically significant at a P of <0.05. The data were stored and analyzed by using SPSS 11.5.
Description of the outbreak and control measures taken.
The strain of RSKp index case of the outbreak was first isolated in cultures of a conjunctival infection from a 7-day-old infant on 5 February 2001. The diagnosis in this case was conjunctival infection, and this patient was considered the index case of the outbreak. This patient remained in the neonatal unit until her health status improved and left the hospital 8 days after admission.
During the following year (February 2001 to February 2002), 20 newborns admitted to the same neonatal unit were infected or colonized by a strain of K. pneumoniae (Table 1) showing the same phenotype of reduced susceptibility (RSKp) to expanded-spectrum cephalosporins. The MICs for the RSKp strain are shown in Table 2. Environmental samples were collected and analyzed for the presence of the RSKp strain. Cultures yielded a positive result for RSKp isolates in some cases, suggesting the involvement of an environment reservoir in the dissemination of the epidemic strains.
TABLE 2.
Antibiotic MICs for the epidemic strain RSKp
| Antibiotic | MIC (mg/liter) for strain RSKp |
|---|---|
| Amoxicillin | >256 |
| Amoxicillin-clavulanic acid | 12 |
| Cephalothin | 128 |
| Cefuroxime | 24 |
| Cefoxitin | 2 |
| Cefotaxime | 0.5 |
| Cefotaxime-clavulanic acid | 0.047 |
| Ceftazidime | 2 |
| Ceftazidime-clavulanic acid | 0.125 |
| Aztreonam | 2 |
| Cefepime | 0.094 |
| Imipenem | 0.19 |
| Meropenem | 0.023 |
| Amikacin | 16 |
| Tobramycin | 8 |
| Ciprofloxacin | 0.023 |
The outbreak was controlled by implementation of certain measures: the relevant patients were either isolated in single cots (whenever possible) or grouped together, and the standard and contact isolation precautions were strengthened. All surfaces of the different intensive care units and departments were also cleaned. Health care personnel were reminded to wash their hands carefully before and after contact with patients and to wear disposable gloves and gowns. Moreover, doctors were advised to use antibiotics with caution, particularly expanded-spectrum cephalosporins.
RESULTS
REP-PCR, ERIC-PCR, and RAPD analysis of K. pneumoniae ATCC strains.
K. pneumoniae strains ATCC 7761, ATCC 10031, ATCC 13883, ATCC 27736, ATCC 29995, ATCC 29665, and ATCC 9621 were used to test the capacity of typing of the PCR-based DNA fingerprinting techniques (strains 32 to 38 in Table 1). Analysis by PFGE of these strains showed seven different genotypes according to the criteria of Tenover et al. (27 and data not shown).
Using PCR-DNA techniques, seven different genotypes were obtained by ERIC-PCR and RAPD techniques, whereas six genotypes (one genotype had two subtypes) were detected by REP-PCR (data not shown).
Overall, the results suggested that the PCR-based techniques were useful, particularly RAPD and ERIC-PCR. The next step was to extend the study to a set of clinical strains of K. pneumoniae putatively involved in a nosocomial outbreak of infection or colonization.
Study of the nosocomial outbreak. (i) PFGE of the clinical strains.
A total of 43 clinical strains of K. pneumoniae isolated from patients between February 2001 and February 2002 were used with two objectives: (i) to confirm the usefulness of the PCR-based typing methods and (ii) to characterize the nosocomial outbreak of infection.
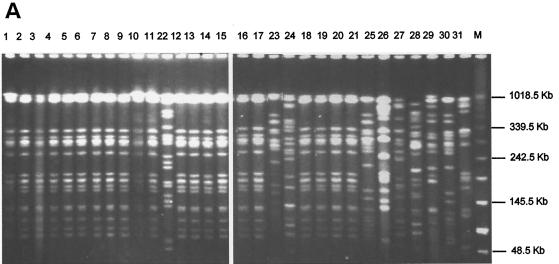
Thirty-three RSKp strains (isolated from 21 newborns) with the ESBL phenotype and probably related to the nosocomial outbreak of infection were tested by PFGE for genetic relationship. For comparative purposes, 10 clinical strains of K. pneumoniae not showing any ESBL phenotype and isolated at outbreak were used as controls. The results of PFGE of 31 representative strains of K. pneumoniae described in Table 1 are shown in Fig. 1A. Analysis by PFGE is considered the “gold standard” method for typing K. pneumoniae isolates, and therefore we used it first in our attempt to identify the genotypes of the RSKp strains. All strains with ESBL phenotype (MICs in Table 2) were not distinguished in epidemiological terms, and they were assigned to genotype I. Overall, 17 different genotypes, classified according to the criteria of Tenover et al. (27), were obtained by applying this technique to the representative K. pneumoniae strains (Table 1).
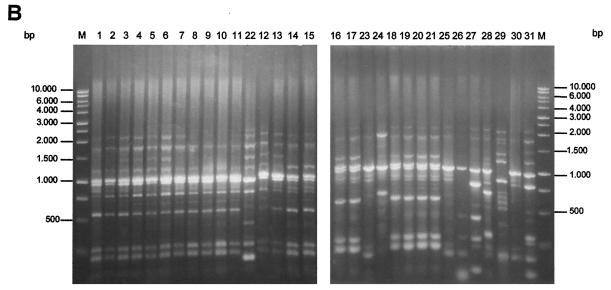
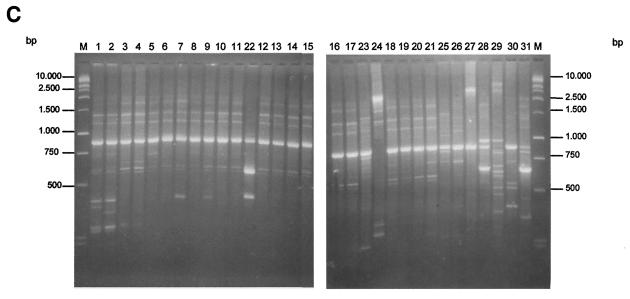
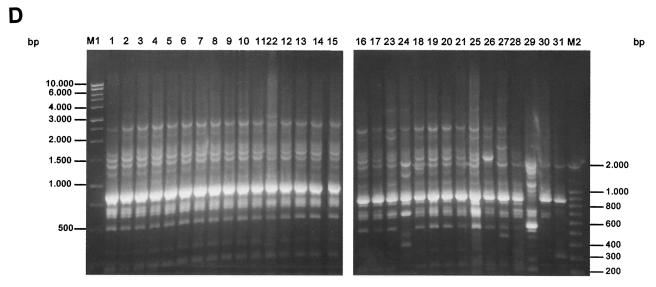
FIG. 1.
PFGE (A), ERIC-PCR (B), RAPD (C), and REP-PCR (D) band patterns obtained with the strain indicated above the gel and described in Table 1.
(ii) ERIC-PCR, RAPD, and REP-PCR analysis of clinical strains.
Strains identical to those analyzed by PFGE were tested by ERIC-PCR, RAPD, and REP-PCR. Using the ERIC-PCR technique, we detected a band pattern ranging from 0.2 to 3 kb (Fig. 1B). All of the RSKp strains showed the same band pattern, and they were assigned to genotype 1. Control strains 22 to 31 showed different band profiles, and they were assigned to genotypes 2 to 10. From all of the strains tested, a total of 17 different genotypes were obtained by ERIC-PCR (Table 1). The ERIC-PCR and PFGE techniques therefore showed similar discriminatory capacities.
Using RAPD-PCR, we also obtained a band pattern from 0.2 to 3 kb (Fig. 1C). On the gels, a major DNA band was obtained with each different strain; with the epidemic RSKp strains; this band migrated to ca. 800 bp. Moreover, a few minor bands were obtained at lower molecular weights. The minor bands showed some degree of variability in different experiments in contrast to the main amplified bands, which remained quite stable in different experiments. All of the RSKp strains yielded an identical genotype (a.2 in Table 1). There was some degree of variability associated with this technique, as seen with strains 1 and 2 (Fig. 1C). For this reason these strains were categorized as subtypes of the main genotype. Overall, RAPD yielded 17 different genotypes with the K. pneumoniae strains studied.
Using REP-PCR, we again observed a band profile ranging from 0.2 to 3 kb (Fig. 1D). All strains with the ESBL phenotype were also grouped with an identical genotype (pattern A.1 in Table 1). With the control strains of K. pneumoniae tested, 14 different genotypes and one different subtype were obtained; strain 22 showed only one band different from those of the strains associated with the nosocomial infection and was categorized with this technique as a subtype of the main genotype. Moreover, although the genotype of strain 23 was clearly differentiated from that of the RSKp strains by PFGE, ERIC-PCR, and RAPD, the results of the REP-PCR technique showed the genotype to be practically indistinguishable from that of the epidemic RSKp strain (only one minor band differed), thus revealing some limitations of this technique. Overall, REP-PCR yielded 14 genotypes among the strains analyzed.
(iii) Reproducibility and discriminatory index of the PCR-based methods.
The discriminatory index was calculated with the 43 clinical strains of K. pneumoniae and the 7 ATCC strains of K. pneumoniae as described in Materials and Methods. The overall results are shown in Table 3. The ERIC-PCR showed the best discriminatory capacity, followed by RAPD and REP-PCR, respectively. As previously reported, RAPD showed the lowest degree of reproducibility.
TABLE 3.
Discriminatory index (D) and reproducibility of the PCR typing methods studied
| Typing method | D | Reproducibility |
|---|---|---|
| ERIC-PCR | 0.828 | +++ |
| REP-PCR | 0.773 | +++ |
| RAPD | 0.826 | ++ |
(iv) Risk factors for clinical colonization or infection by strain RSKp.
From 1 February 2001 to 29 February 2002, 21 patients (cases) admitted to the neonatal unit became clinically colonized (n = 4) or infected (n = 17) by the RSKp strain. In the same period, 44 patients admitted to the unit were either infected or colonized by non-RSKp strains (controls).
The clinical presentations in the infected newborns were as follows: sepsis, n = 5; urinary tract infection, n = 4; enterocolitis, n = 4; conjunctival infection, n = 3; and catheter infection, n = 1. Samples were taken from an endotracheal tube (n = 3) and stool (n = 1) of colonized patients (Table 1).
In the univariate analysis, the differences between the 21 cases and the 44 controls are shown in Tables 4 and 5. The following factors were associated with strain RSKp: number of additional hours >24 h before birth with rupture of amniotic sac (33.3% in cases versus 11.4% in controls), low birth weight (33.3% versus 6.8%), and prematurity (71.4% versus 15.9%). Parenteral nutrition (71.4% versus 15.9%), intubation (85.7% versus 11.4%), underlying disease (52.4% versus 13.6%), previous surgery (14.3% versus 0%), and the presence of intravascular catheter (71.4% versus 13.6%) were also more frequent in the infected or colonized group, as well as administration of antibiotics: β-lactam, aminoglycosides, or glycopeptides. There were also statistically significant differences between the number of days of stay in neonatal unit. The controls had lower numbers of days of intensive care unit stays than those infected or colonized by the RSKp strain (28.24 versus 3.02 days). Similarly, the number of days with nasogastric tube was higher for the case group (0.14 versus 0), as well as the number of days with vascular catheter (17.9 versus 1.39), parenteral nutrition (10.09 versus 1.45), intubation (10.81 versus 0.48), and administration of antibiotics.
The multiple-logistic-regression model (Table 6) revealed that the presence of intubation (odds ratio [OR], 27.0; 95%CI, 5.39 to 135.14) and prematurity (OR, 4.41; 95%CI, 0.89 to 21.89) were factors independently associated with RSKp infection and/or colonization.
TABLE 6.
Logistic regression model for predicting infection or colonization by K. pneumoniae
| Factor | P | OR | 95%CI |
|---|---|---|---|
| Presence of intubation | <0.001 | 27.00 | 5.39-135.14 |
| Prematurity | 0.070 | 4.41 | 0.89-21.89 |
DISCUSSION
In the past two decades, a significant number of nosocomial outbreaks of infection by K. pneumoniae have been reported (1, 18, 25), causing increasing concern in hospitals. In order to investigate the origin of the infection, the route of dissemination, and the prevalence of isolates in a bacterial population, several phenotypic and molecular typing methods have been described. Rapid and accurate identification of the strains involved in an outbreak is important, both for limiting the severity of the outbreak and for tracing the source of the infecting organism. Although antibiotyping may sometimes be used to distinguish isolates with different antibiotic susceptibility patterns, distinction between strains with slight differences in resistance profiles may be difficult. Therefore, genotypic methods, including plasmid typing, ribotyping, PFGE of chromosomal DNA restriction fragments, and PCR fingerprinting have been used to investigate nosocomial K. pneumoniae strains (2, 4-6, 8, 9, 11-13, 15, 16, 30). The relative advantages and disadvantages of these methods have previously been reviewed (21).
The REP-PCR, ERIC-PCR, and RAPD fingerprinting techniques have previously been used for typing K. pneumoniae strains (4, 5, 7, 8, 9, 13, 16, 30). However, these studies were performed with a small number of strains, and it was probably assumed that these techniques worked well for typing K. pneumoniae strains, without confirmation of this by detailed comparison of the techniques for typing K. pneumoniae isolates. Indeed, there are no reports in the medical literature of a comparative study of the four methods used in the present study for typing K. pneumoniae isolates. Therefore, although the lack of reproducibility of the RAPD method has been demonstrated several times, the power of discrimination and reproducibility of the ERIC-PCR, REP-PCR, and RAPD techniques remain unknown. Therefore, to assess the degree of usefulness of the PCR-based fingerprinting techniques (ERIC-PCR, RAPD, and REP-PCR) we carried out the present study with a set of control K. pneumoniae ATCC strains and also 43 K. pneumoniae clinical strains. All of the strains were previously tested with PFGE as the gold standard method.
With the strains of K. pneumoniae shown in Table 1, 17 different genotypes were obtained by ERIC-PCR that were identical to those obtained by PFGE and RAPD, whereas only 14 genotypes were obtained by REP-PCR. These results revealed differences in the discrimination capacities of the PCR methods assayed.
Regarding reproducibility, similar results were obtained with REP-PCR and ERIC-PCR. To determine reproducibility, all of the PCR fingerprinting experiments were repeated three times. Use of ERIC-PCR and REP-PCR yielded practically identical results in three different experiments. Only the faint bands showed small differences, and the major DNA band pattern structure was identical in all experiments. With RAPD, some differences were obtained in different experiments; therefore, the reproducibility of this technique was not as good as that of the ERIC-PCR and REP-PCR techniques. This is a clear disadvantage when experiments are performed at different times or by different groups of investigators.
Overall, the results showed that ERIC-PCR was the most discriminatory of the techniques (D = 0.828), followed by RAPD (D = 0.826) and REP-PCR (D = 0.773), and ERIC-PCR may therefore be the best PCR-based method for typing K. pneumoniae isolates.
It is important to note that ERIC-PCR and RAPD yielded numbers of genotypes identical to those obtained by PFGE, thus showing a good discrimination capacity. However, the index of discrimination (14) may be affected by some limitations related to intrinsic features of this index, i.e., that no correcting factor for small populations has been made and that no correcting factor for the presence of subtypes among a main genotype has been made. With this goal, we introduced a correcting factor in the index of discrimination of Hunter and Gaston (14) considering the presence of subtypes in the genotypes of the bacterial populations.
It is interesting that for other bacterial species, such as Acinetobacter baumannii, the REP-PCR technique was the most discriminatory (17, 26, 29). Thus, the validity of a method in molecular epidemiology depends on the microorganism studied.
The attempt to detect the RSKp strain in environmental samples provided interesting results that strongly suggest that the hospital environment may act as a reservoir of bacterial strains (data not shown), thereby facilitating the dissemination of an epidemic strain, although the possibility of accidental contamination cannot be ruled out. Moreover, these results show the capacity of K. pneumoniae to survive in dry environments such as hospital surfaces.
Regarding the risk factors for acquisition of the RSKp strain the multiple-logistic-regression model (Table 6) revealed the presence of intubation as the most influential factor. It is interesting that β-lactams (oxime cephalosporins) did not appear to be statistically significant in the multiple-logistic-regression model. Probably, the reduced susceptibility of the RSKp strain to oxime cephalosporins did not reach the level sufficient to confer selective advantage, thus precluding its selection. Regarding the mechanism associated with reduced susceptibility to expanded-spectrum cephalosporins, it is important to remark that no ESBLs, porin alterations, or efflux (19) resulting from the use of specific inhibitors (23) was detected in the RSKp strain.
In summary, an outbreak caused by a K. pneumoniae strain with reduced susceptibility to expanded-spectrum cephalosporins was investigated. We found that PCR-based fingerprinting techniques (ERIC-PCR, RAPD, and REP-PCR) are useful and expeditious methods for typing strains of K. pneumoniae when up to two band differences are used as the cutoff, with ERIC-PCR being the most reliable of the methods studied for typing K. pneumoniae isolates.
Acknowledgments
This study was financially supported by the Consejeria de Sanidad y Asuntos Sociales, Xunta de Galicia (PGIDT01SAN00004PR). M.C. received a scholarship from the Spanish Society of Clinical Microbiology and Infectious Diseases.
REFERENCES
- 1.Asensio, A, A. Oliver, P. Gonzalez-Diego, F. Baquero, J. C. Perez-Diaz, P. Ros, J. Cobo, M. Palacios, D. Lasheras, and R. Canton. 2000. Outbreak of a multiresistant Klebsiella pneumoniae strain in an intensive care unit: antibiotic use as risk factor for colonization and infection. Clin. Infect. Dis. 30:55-60. [DOI] [PubMed] [Google Scholar]
- 2.Bidet, P., V. Lalande, B. Salauze, B. Burghoffer, V. Avesani, M. Delmee, A. Rossier, F. Barbut, and J. C. Petit. 2000. Comparison of PCR-ribotyping, arbitrarily primed PCR, and pulsed-field gel electrophoresis for typing Clostridium difficile. J. Clin. Microbiol. 38:2484-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bingen, E. H., P. Desjardins, G. Arlet, F. Bourgeois, P. Mariani-Kurkdjian, N. Y. Lambert-Zechovsky, E. Denamur, A. Philippon, and J. Elion. 1993. Molecular epidemiology of plasmid spread among extended broad-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates in a pediatric hospital. J. Clin. Microbiol. 31:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao, V., T. Lambert, D. Q. Nhu, H. K. Loan, N. K. Hoang, G. Arlet, and P. Courvalin. 2002. Distribution of extended-spectrum beta-lactamases in clinical isolates of Enterobacteriaceae in Vietnam. Antimicrob. Agents Chemother. 46:3739-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davin-Regli, A., D. Monnet, P. Saux, C. Bosi, R. Charrel, A. Barthelemy, and C. Bollet. 1996. Molecular epidemiology of Enterobacter aerogenes acquisition: one-year prospective study in two intensive care units. J. Clin. Microbiol. 34:1474-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisen, D., E. G. Russell, M. Tymms, E. J. Roper, M. L. Grayson, and J. Turnidge. 1995. Random amplified polymorphic DNA and plasmid analyses used in investigation of an outbreak of multiresistant Klebsiella pneumoniae. J. Clin. Microbiol. 33:713-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galani, I., E. Xirouchaki, K. Kanellakopoulou, G. Petrikkos, and H. Giamarellou. 2002. Transferable plasmid mediating resistance to multiple antimicrobial agents in Klebsiella pneumoniae isolates in Greece. Clin. Microbiol. Infect. 8:579-588. [DOI] [PubMed] [Google Scholar]
- 8.Gazouli, M., M. E. Kaufmann, E. Tzelepi, H. Dimopoulou, O. Paniara, and L. S. Tzouvelekis. 1997. Study of an outbreak of cefoxitin-resistant Klebsiella pneumoniae in a general hospital. J. Clin. Microbiol. 35:508-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georghiou, P. R., R. J. Hamill, C. E. Wright, J. Versalovic, T. Koeuth, D. A. Watson, and J. R. Lupski. 1995. Molecular epidemiology of infections due to Enterobacter aerogenes: identification of hospital outbreak-associated strains by molecular techniques. Clin. Infect. Dis. 20:84-94. [DOI] [PubMed] [Google Scholar]
- 10.Giammanco, G. M., S. Pignato, F. Grimont, P. A. Grimont, A. Caprioli, S. Morabito, and G. Giammanco. 2002. Characterization of Shiga toxin-producing Escherichia coli O157:H7 isolated in Italy and in France. J. Clin. Microbiol. 40:4619-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gori A., F. Espinasse, A. Deplano, C. Nonhoff, M. H. Nicolas, and M. J. Struelens. 1996. Comparison of pulsed-field gel electrophoresis and randomly amplified DNA polymorphism analysis for typing extended-spectrum-β-lactamase-producing Klebsiella pneumoniae. J. Clin. Microbiol. 34:2448-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gouby, A., C. Neuwirth, G. Bourg, N. Bouziges, M. J. Carles-Nurit, E. Despaux, and M. Ramuz. 1994. Epidemiological study by pulsed-field gel electrophoresis of an outbreak of extended spectrum beta-lactamase-producing Klebsiella pneumoniae in a geriatric hospital. J. Clin. Microbiol. 32:301-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoy, C. M., C. M. Wood, P. M. Hawkey, and J. W. Puntis. 2000. Duodenal microflora in very-low-birth-weight neonates and relation to necrotizing enterocolitis. J. Clin. Microbiol. 38:4539-4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurley, J. C., E. G. Russell, G. Harrington, and W. J. Spicer. 1996. Investigation of an apparent cluster of Klebsiella pneumoniae bacteremias using random amplified polymorphic DNA analysis. Infect. Control Hosp. Epidemiol. 17:743-745. [DOI] [PubMed] [Google Scholar]
- 16.Kil, K. S., R. O. Darouiche, R. A. Hull, M. D. Mansouri, and D. M. Musher. 1997. Identification of a Klebsiella pneumoniae strain associated with nosocomial urinary tract infection. J. Clin. Microbiol. 35:2370-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koeleman, J. G., J. Stoof, D. J. Biesmans, P. H. Savelkoul, and C. M. Vandenbroucke-Grauls. 1998. Comparison of amplified ribosomal DNA restriction analysis, random amplified polymorphic DNA analysis, and amplified fragment length polymorphism fingerprinting for identification of Acinetobacter genomic species and typing of Acinetobacter baumannii. J. Clin. Microbiol. 36:2522-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macrae, M. B., K. P. Shannon, D. M. Rayner, A. M. Kaiser, P. N. Hoffman, and G. L. French. 2001. A simultaneous outbreak on a neonatal unit of two strains of multiply antibiotic-resistant Klebsiella pneumoniae controllable only by ward closure. J. Hosp. Infect. 49:183-192. [DOI] [PubMed] [Google Scholar]
- 19.Mazzariol, A., J. Zuliani, G. Cornaglia, G. M. Rossolini, and R. Fontana. 2002. AcrAB efflux system: expression and contribution to fluoroquinolone resistance in Klebsiella spp. Antimicrob. Agents Chemother. 46:3984-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 21.Olive, M., and P. Bean. 1999. Principles and applications of methods for DNA-based typing of microbial organisms. J. Clin. Microbiol. 37:1661-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paniara, E., H. Platsouka, E. Dimopoulou, V. Tzelepi, Miriagou, and L. S. Tzouvelekis. 2000. Diversity of beta-lactam resistance levels among related Klebsiella pneumoniae strains isolated in an intensive care unit. J. Chemother. 12:204-207. [DOI] [PubMed] [Google Scholar]
- 23.Ribera A., J. Ruiz, M. T. Jimenez de Anta, and J. Vila. 2002. Effect of an efflux pump inhibitor on the MIC of nalidixic acid for Acinetobacter baumannii and Stenotrophomonas maltophilia clinical isolates. J. Antimicrob. Chemother. 49:697-698. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Silva, J., R. Gatica, C. Aguilar, Z. Becerra, U. Garza-Ramos, M. Velazquez, G. Miranda, B. Leanos, F. Solorzano, and G. Echaniz. 2001. Outbreak of infection with extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a Mexican hospital. J. Clin. Microbiol. 39:3193-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snelling, A. M., P. Gerner-Smidt, P. M. Hawkey, J. Heritage, P. Parnell, C. Porter, A. R. Bodenham, and T. Inglis. 1996. Validation of use of whole-cell repetitive extragenic palindromic sequence-based PCR (REP-PCR) for typing strains belonging to the Acinetobacter calcoaceticus-Acinetobacter baumannii complex and application of the method to the investigation of a hospital outbreak. J. Clin. Microbiol. 34:1193-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsay, R. W., L. K. Siu, C. P. Fung, and F. Y. Chang. 2002. Characteristics of bacteremia between community-acquired and nosocomial Klebsiella pneumoniae infection: risk factor for mortality and the impact of capsular serotypes as a herald for community-acquired infection. Arch. Intern. Med. 162:1021-1027. [DOI] [PubMed] [Google Scholar]
- 29.Vila, J., M. A. Marcos, and M. T. Jimenez de Anta. 1996. A comparative study of different PCR-based DNA fingerprinting techniques for typing of the Acinetobacter calcoaceticus-A. baumannii complex. J. Med. Microbiol. 44:482-489. [DOI] [PubMed] [Google Scholar]
- 30.Vogel, L., G. Jones, S. Triep, A. Koek, and L. Dijkshoorn. 1999. RAPD typing of Klebsiella pneumoniae, Klebsiella oxytoca, Serratia marcescens, and Pseudomonas aeruginosa isolates using standardized reagents. Clin. Microbiol. Infect. 5:270-276. [DOI] [PubMed] [Google Scholar]