Abstract
All known DNA tumor viruses are known to target and inactivate two main cell cycle regulatory proteins, retinoblastoma protein (pRb) and p53. Inactivation of pRb promotes host cell cycle progression into S phase, and inactivation of p53 promotes cell immortalization. The DNA tumor virus Kaposi's sarcoma associated herpesvirus (KSHV)-encoded latency-associated nuclear antigen (LANA) was shown to target and inactivate pRb as well as p53. In this report we provide evidence that these functions are conserved in the homologous protein encoded by the related gammaherpesvirus herpesvirus saimiri (HVS). ORF73, the HVS homologue of LANA, is shown to bind both p53 and pRb in vitro and in vivo, to colocalize with p53 in human T cells infected with HVS, and in cells overexpressing both ORF73 and p53, as well as to adversely influence pRB/E2F and p53 transcriptional regulation. The C terminus of LANA, the region most highly conserved in ORF73, is shown to be responsible for both pRb and p53 interactions, supporting the hypothesis that these functions are conserved in both homologues. Finally, the region of p53 targeted by LANA (and ORF73) maps to the domain required for tetramerization. However, preliminary cross-linking studies do not detect disruption of p53 tetramerization by either LANA or HVS-encoded ORF73, suggesting that p53 inactivation may be by a mechanism independent of tetramer disruption.
The association between viral infection and oncogenesis has been noted since the early 1950s, when viral particles were observed by electron microscopy in tumor tissue but not in normal tissue (19). Since then, much effort has gone into understanding the mechanisms by which viruses promote oncogenesis, particularly with regard to viral deregulation of the cell cycle. Oncogenic viruses influence a key restriction point in the cell cycle, the G1→S checkpoint, by targeting the E2F/retinoblastoma (pRb) protein complex (29). The tumor suppressor protein pRb sequesters E2F, a transcription factor required for entry into S phase. Virally encoded oncoproteins target pRb and therefore release E2F, resulting in the promotion of S-phase entry (14, 15, 23). There are a number of mechanisms by which viral proteins can release E2F from pRb. First, viral proteins can directly bind and sequester pRb, as in the cases of EBNA3C of Epstein-Barr virus, E7 of human papillomavirus, and the large T antigen of simian virus 40 (14, 30, 46, 47). Second, viral proteins can inactivate pRb by phosphorylation, as is seen in the cases of v-cyclin D of Kaposi's sarcoma-associated herpesvirus (KSHV) and Tax protein of human T-cell leukemia virus type 1 (9, 27, 42). Third, viral proteins can block endogenous inhibitors of cyclin-dependent kinases such as p21 and p27, thereby promoting phosphorylation and inactivation of pRb. This is seen with such proteins as E1A of adenovirus and E7 of human papillomavirus (18, 58).
Oncogenic viruses also target and inactivate p53 and have evolved mechanisms to inactivate p53-mediated cell death (4). The E6 protein of human papillomavirus in complex with E6-associated protein acts as a ubiquitin ligase to mediate p53 ubiquitination and degradation (57). The simian virus 40 T antigen prevents p53-mediated apoptosis by physically sequestering and inactivating p53 (33). It has also been demonstrated that HAUSP, a cellular protein which stabilizes p53 by deubiquitination, is physically associated with a herpesviral protein, Vmw130. This has led to speculation that Vmw130 induces p53 degradation by inhibiting HAUSP (41).
KSHV is the most recently identified human herpesvirus (10). It is associated with a number of human cancers, including Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease (8, 10, 16, 45). In most immunocompetent patients, the virus exists in a latent state, with multiple genome copies maintained extrachromosomally and only a small subset of viral genes expressed (6, 17, 36). Evidence suggests that the major latent nuclear antigen, LANA, is critical for the induction of oncogenesis mediated by KSHV due to its ability to antagonize both pRb and p53 functions (20, 49). LANA has been shown to bind and inactivate pRb, thereby upregulating transcription from the E2F promoter, and exogenously expressed LANA overcomes pRb-induced growth arrest in SAOS-2 cells (49). LANA also inhibits both the transcriptional activity of p53 and p53-mediated apoptosis in SAOS-2 and NIH 3T3 cells (20). Furthermore, LANA and p53 have been shown to colocalize in vivo in tissue from Kaposi's sarcoma patients, primary effusion lymphoma cell lines and human herpesvirus 8-associated solid lymphoma, with apoptosis rarely being detected in cells expressing LANA (37). Consistent with its role as an antagonist to pRb and p53, LANA has been shown to transform primary REF cells in vitro when expressed in conjunction with H-Ras, as well as to drive tumorigenesis in nude mice (49). Evidence thus points to LANA as a major destabilizer of cell cycle regulation in infected cells and a contributing factor to cell immortalization.
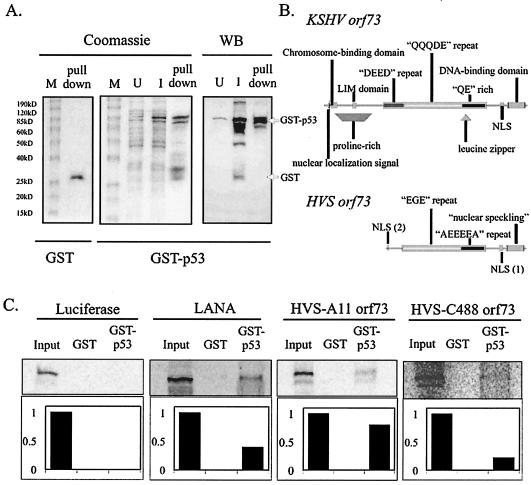
Herpesvirus saimiri (HVS) is another member of the gammaherpesvirus family. HVS naturally infects the squirrel monkey but can drive human T cells to immortalization in vitro (5, 44, 51). The KSHV and HVS genomes show great stretches of colinear homology, and in fact KSHV genes are named according to their positional alignment with their HVS homologues (51). Although the HVS homologue of LANA, called ORF73, only shows 33% amino acid similarity with its KSHV counterpart, some sequence characteristics and functions are conserved (26, 55). These conserved features include nuclear localization signals, internal repetitive acidic residues, DNA-binding regions, and the capacity to tether viral episomes to host chromatin (Fig. 1b) (2, 26, 55).
FIG. 1.
KSHV LANA and HVS ORF73 bind to p53 in vitro. (A) GST (left panel) and GST-p53 (middle and right panels) were purified from bacterial cell lysate with glutathione-Sepharose 4B beads (Amersham Pharmacia, Inc., Piscataway, N.J.). Uninduced lysate, induced lysate, and pulled-down proteins were resolved on a 10% Tris-glycine-SDS gels. GST-p53 protein expression and purification were also confirmed by Western blot analysis (right panel), as described in Materials and Methods. M, markers; U, uninduced lysate; I, induced lysate; WB, Western blot. (B) Side by side comparison of the functional domains and distinct sequence motifs shared by KSHV LANA (top) and HVS ORF73 (bottom). NLS, nuclear localization signal; LIM, the LIM domain which mediates protein-protein interactions via cysteine-mediated coordination of zinc ions. (C) GST and GST-p53 pulldowns of [35S]methionine-labeled, in vitro-translated luciferase, in vitro-translated KSHV LANA, in vitro-translated ORF73 from HVS strain A11, and in vitro-translated ORF73 from HVS strain C488.
Here we demonstrate that two other functions of the LANA protein are conserved by HVS-encoded ORF73, the inhibition of the two major cell cycle regulatory proteins pRb and p53. We demonstrate that ORF73 binds to human p53 in vitro and in vivo and that these two proteins colocalize in human T cells transformed by HVS as well as in 293T cells overexpressing ORF73 and p53. Furthermore, ORF73 is shown to downregulate the transcriptional activity of p53, as has been demonstrated previously with LANA (20). The p53-binding region of LANA is now more finely mapped to the 400 amino acid residues of the carboxy terminus, which is relatively conserved between the KSHV and HVS homologues (26, 55). This region of p53 bound by both KSHV LANA and HVS ORF73 maps primarily to the tetramerization domain.
HVS ORF73 is also shown to bind human pRb both in vivo and in vitro as well as to stimulate transcriptional activation from the E2F promoter, like LANA. As shown for LANA, the interaction domain is located at the carboxy terminus of HVS ORF73. The carboxy terminal 240 amino acid residues of LANA and HVS strain A11 ORF73 are 37% similar and 22% identical (55). This is consistent with the hypothesis that the anti-p53 and anti-pRb functions ascribed to this region of LANA are conserved in ORF73 (20, 49). Our results therefore demonstrate that HVS ORF73 can antagonize the two major cell cycle regulatory protein p53 and pRb, similar to KSHV-encoded LANA.
MATERIALS AND METHODS
Plasmids and antibodies.
pA3M-LANA and the pA3M-LANA deletion constructs pA3M-HVS-A11 and pA3M-HVS-C488 carry c-Myc-tagged ORF73 under control of both the T7 and cytomegalovirus promoters and were constructed by PCR amplification of the respective open reading frames followed by EcoRI and BamHI digestion and insertion into the pA3M vector (13). The p53 expression vector pC53-C1N3 carries a wild-type human p53 gene with a proline polymorphism at residue 73 and is controlled by the cytomegalovirus promoter (gift from G. Nabel, National Institutes of Health, Bethesda, Md.). pGEX-p53 expresses an N-terminal glutathione S-transferase (GST)-p53 fusion protein and was derived from pGEX-2T (Amersham Pharmacia, Inc., Piscataway, N.J.) by insertion of human p53 cDNA at the BamHI and EcoRI sites (gift from G. Nabel). GST-p53 deletion constructs were constructed by insertion of PCR fragments into the pGEX-5x-1 backbone (gift from S. Berger, University of Pennsylvania, Philadelphia) (43). The hemagglutinin (HA)-pRb fusion protein was expressed under the control of the cytomegalovirus promoter from pA3M-HA-pRb, and the GST-pRb fusion protein was expressed from pGEX-pRb (gift from N. Gramatikakis, Boston University, Boston, Mass.).
The p53 reporter plasmid pG13 contains 13 p53-binding sites upstream of the luciferase gene (gift from W. El-Deiry, University of Pennsylvania, Philadelphia) (56) and was constructed by EcoRV insertion of p53-binding sequences into the pGL-2 luciferase reporter plasmid (Promega, Inc., Madison, Wis.). The 3x-wt-E2F-luc and 3x-mut-E2F-luc plasmids contain three wild-type E2F binding sites and three mutant E2F binding sites upstream of the luciferase gene, respectively (a gift from S. Gaubatz, Philipps University, Germany) (39).
The DO-1 and Pab 240 anti-p53 monoclonal antibodies were obtained from Santa Cruz Biotechnologies, Inc. (Santa Cruz, Inc., Calif.), and anti-HA antibody was obtained from Babco, Inc. (Princeton, N.J.). The 9E10 anti-Myc monoclonal antibody was obtained from the University of Michigan Hybridoma Core Facility. Rabbit polyclonal anti-A11 antibodies were a gift from Adrian Whitehouse (University of Leeds, Leeds, United Kingdom) (26). MM antiserum was obtained from a Kaposi's sarcoma-positive patient and has cross-reactivity to LANA (7).
In vitro translation and GST pulldown assays.
LANA and LANA deletion constructs were in vitro transcribed and translated with the TNT T7 rabbit reticulocyte extract system (Promega, Inc., Madison, Wis.), with 5 μg of template and 35S-labeled methionine and cysteine (PerkinElmer, Inc., Boston, Mass.). HVS ORF73 constructs were in vitro transcribed and translated with 5 μg of plasmid template and either 35S-labeled methionine or 14C-labeled leucine (PerkinElmer).
For GST pulldown assays, GST, GST-p53, or GST-pRb protein was expressed in Escherichia coli DH5α cells and purified by incubation of sonicated cell lysate with glutathione-Sepharose beads (Amersham Pharmacia, Inc., Uppsala, Sweden) for 6 h at 4°C under constant rotation. Protein yield was quantified by gel resolution and densitometry with bovine serum albumin standards. Equal amounts of GST and GST fusion protein were used in all experiments. To control for nonspecific binding in the GST pulldown experiment, in vitro-translated product was first gently agitated with glutathione-Sepharose beads in NETN buffer (20 mM Tris, pH 8, 1 mM EDTA, 100 mM NaCl, 0.5% NP-40, 2 μg of aprotinin per ml, 1 μg of pepstatin A per ml, 1 mM phenylmethylsulfonyl fluoride, and 2 μg of leupeptin per ml) for 30 min at 4°C, followed by gentle agitation with GST-coupled glutathione-Sepharose beads for 1 h at 4°C. Cleared in vitro-translated protein was then rotated overnight with GST-p53-coupled glutathione-Sepharose beads at 4°C. Precleared pellets and GST-p53 pulldown pellets were washed five times in NETN buffer before resolution on 8% Tris-glycine gels. Gels were processed with AutoFluor (National Diagnostics, Inc., Atlanta, Ga.), dried, and exposed to phosphorimager plates for quantification with a Fuji Storm Phosphoimager (Molecular Dynamics, Inc., Madison, Wis.).
In vivo immunoprecipitation assays.
For immunoprecipitation assays, 10 μg of pC53-C1N3 and 30 μg of pA3M-LANA, pA3M-A11, or pA3M-C488 was cotransfected into 20 million 293T cells and incubated for 18 to 24 h at 37°C in 5% CO2. Cells were harvested, washed in phosphate-buffered saline, and resuspended in 500 μl of radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris, pH 7.5, 2 mM EDTA, 150 mM NaCl, 0.5% NP-40, 1 mM phenylmethylsulfonyl fluoride, 1 μg of aprotinin per ml, 1 μg of pepstatin A per ml, and 1 μg of leupeptin per ml). Lysates were incubated on ice for 1 h with intermittent vortexing, followed by clearing of debris and incubation overnight of supernatant with 1 to 2 μg of anti-Myc antibody 9E10 (from mouse ascites fluid). The antibody complex was precipitated by gentle rotation with 25 μl of a 1:1 protein A-Sepharose-protein G-Sepharose slurry for 90 min at 4°C. Immunoprecipitated protein was washed three times in RIPA buffer before resolution on 8% Tris-glycine gels.
Glutaraldehyde cross-linking analysis.
To measure the degree of p53 tetramerization, 10 million 293T cells were cotransfected with 10 μg of pC53-C1N3 and 10, 20, or 30 μg of the pA3M-LANA expression vector (or empty pA3M vector). Cells were harvested 20 h later, lysed with RIPA buffer, and centrifuged at 15,000 rpm at 4°C for 15 min to clear cellular debris. To the cleared supernatant, glutaraldehyde was added to a final concentration of 0.005 or 0.01%, and the cross-linking reaction was allowed to proceed at room temperature for a set incubation period (32). Reactions were stopped with 2× sodium dodecyl sulfate (SDS) buffer, heated at 100°C for 10 min, and resolved on 8% Tris-glycine gels. Blots were probed with 1:500 anti-p53 antibody as indicated.
Cell culture.
The human embryonic kidney 293T cell line and the p53-null SAOS-2 osteosarcoma cell line were grown in Dulbecco's modified Eagle's medium supplemented with antibiotics, l-glutamine, and 10% fetal bovine serum. Cell lines were transfected by either electroporation (210 V, 975 μF) or Lipofectamine 2000 reagent (Invitrogen, Inc., Bethesda, Md.) as per the manufacturer's instructions. BJAB human B cells were grown in RPMI supplemented with antibiotics, l-glutamine, and 10% fetal bovine serum and transfected by electroporation (220 V, 975 μF).
p53- and E2F-responsive transactivation assays.
We transfected 10 million BJAB cells or 2 million SAOS-2 cells with 1 μg of pG13, 1 μg of pC53-C1N3, and various amounts of pA3M-LANA, pA3M-A11, or pA3M-C488; 3 μg of green fluorescent protein (GFP) expression vector was also transfected per sample to measure transfection efficiencies. Cells were harvested 15 h later, washed with 1× phosphate-buffered saline, and lysed in 1× luciferase cell culture lysis buffer (Promega, Inc., Madison, Wis.). Cellular debris was pelleted, and 1 to 5 μl of supernatant was used for each measurement of luciferase activity with an Opticomp I luminometer (MGM Instruments, Inc., Hamden, Conn.). Aliquots of cell lysate were saved for confirmation of protein expression by Western blot analysis. For E2F luciferase reporter assays, 5 μg of 3x-wt-E2F-luc or 3x-mut-E2F-luc and increasing amounts of LANA or HVS-A11 ORF73 were transfected into 15 million BJAB cells and cultured at 37°C for 15 h before harvesting for luciferase assay (38). Aliquots of each sample were saved for Western blot analysis.
Immunofluorescence assays.
293T cells were transfected with 10 μg of pC53-C1N3 and 30 μg of pA3MA11. Cells were harvested 20 h later, spread onto microscope slides, and fixed with a 1:1 acetone-methanol solution for 10 min at −20°C. Cells were blocked with 20% normal goat serum in phosphate-buffered saline, washed again, and cross-reacted to 1:100 anti-A11 rabbit antiserum and 1:100 anti-p53 mouse antibody. Slides were washed three times in phosphate-buffered saline and cross-reacted with 1:1,000 goat anti-rabbit immunoglobulin-fluorescein isothiocyanate- and goat anti-mouse immunoglobulin-Texas Red-conjugated secondary antibodies. Slides were examined with an Olympus BX60 fluorescence microscope, and images were captured with a PixelFly digital camera (Cooke, Inc., Auburn Hills, Mich.). CJ-1 cells (a gift from J. Jung, Harvard University Primate Center, Southborough, Mass.), a human T-cell line transformed by HVS strain C488, were fixed and analyzed in the same fashion.
Western blot analysis.
Electrophoresed proteins were blotted onto 0.45-μm nitrocellulose paper (Osmonics, Inc., Minnetonka, Minn.) at 100 V for 1 h. Blots were blocked with 5% milk in phosphate-buffered saline and washed three times with TBST buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% Tween 20) before overnight incubation with the 9E10 anti-Myc hybridoma supernatant or 1:500 anti-p53 antibody at 4°C. Blots were washed three times with TBST and incubated with 1:3,300 anti-mouse or anti-rabbit immunoglobulin-horseradish peroxidase-conjugated secondary antibody (Amersham Pharmacia, Inc., Uppsala, Sweden). Cross-reacted proteins were detected by incubation with Luminol (Sigma-Aldrich, Inc., St. Louis, Mo.), and images were captured with a Fuji Dark Box II Station and Fuji ImageReader LAS-1000 Pro software (Molecular Dynamics).
RESULTS
HVS-encoded ORF73 from strains A11 and C488 binds p53 in vitro at the carboxy-terminal domain.
The GST-p53 fusion protein was expressed in bacteria, purified, and confirmed by Western blot analysis (Fig. 1A, right panel). To determine whether HVS ORF73, the homologue of KSHV-encoded LANA (Fig. 1B), was also capable of binding to p53 in vitro, we incubated GST-p53 with [35S]methionine-labeled, in vitro-translated luciferase, in vitro-translated LANA, and in vitro-translated ORF73 proteins, washed complexes precipitated by GST and GST-p53, then resolved bound fractions on SDS-polyacrylamide gel electrophoresis (PAGE) gels (Fig. 1C). The results of our assay demonstrated that LANA was retained by GST-p53 fusion protein but not by GST protein alone, as expected (20). Similarly, ORF73 from the A11 and C488 strains of HVS were also retained by GST-p53 protein, in contrast to GST protein alone, demonstrating that ORF73 from HVS is able to specifically bind p53 in vitro. Additionally, the binding was relatively stronger than that seen for KSHV-encoded LANA for both A11 and C488 ORF73 (Fig. 1C). In vitro-translated luciferase, used as a negative control, was not retained by either GST or GST-p53-coupled glutathione-Sepharose beads (Fig. 1C).
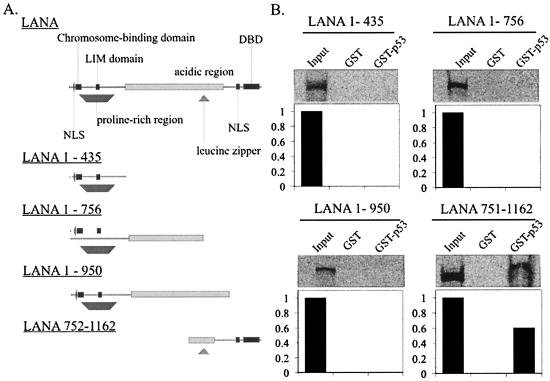
In previous studies, the domain of LANA that bound to p53 was not identified. Therefore, we wanted to determine the domain of LANA responsible for binding to p53. A series of LANA deletion constructs were tested for their ability to bind to the GST-p53 fusion protein in vitro (Fig. 2A). The results of the in vitro binding assay show that the carboxy-terminal domain located within amino acids 752 to 1162 was sufficient for binding p53. Importantly, little or no binding was seen with the amino-terminal truncations by the GST-p53 fusion protein (Fig. 2B). Also of interest, this same domain has previously been shown to bind to the KSHV terminal repeat and is critical for maintenance of KSHV episomes (24, 31).
FIG. 2.
C-terminal 400 amino acids of KSHV LANA bind p53 in vitro. (A) LANA deletion constructs spanning residues 1 to 435, 1 to 756, 1 to 950, and 752 to 1162 were in vitro transcribed and translated in the presence of [35S]methionine and then incubated with equal amounts of either GST or GST-p53 glutathione-Sepharose beads for 4 h at 4°C. (B) Retained radiolabeled protein was resolved on 7% Tris-glycine gels. Bands were quantified by phophorimager analysis (Amersham Pharmacia, Inc., Uppsala, Sweden).
HVS-encoded ORF73 protein associates with p53 in human embryonic kidney epithelial cells.
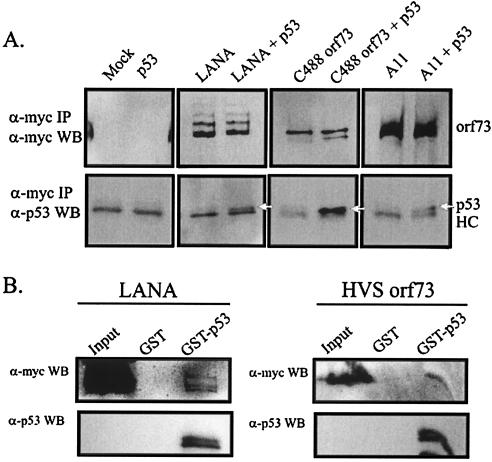
In vivo coimmunoprecipitation experiments were conducted to determine whether HVS ORF73 from strains A11 and C488 was able to associate with p53 in vivo, as was previously demonstrated with LANA. Our results show that p53 complexed with KSHV-encoded LANA as well as with HVS strain C488- and HVS strain A11-encoded ORF73 proteins. The immunoprecipitation assays with the monoclonal antibody against c-Myc, which targets the Myc tag of the LANA and ORF73 proteins, bound and precipitated LANA and ORF73 from A11 and C488 (Fig. 3A, lanes 3 through 8) as well as p53 exogenously expressed from a heterologous promoter (Fig. 3A, bottom panels, white arrows in lanes 4, 6, and 8). The p53 signal runs slightly higher than that of the heavy chain (labeled HC) (Fig. 3A, bottom panels). All lysates for assays were collected 18 h posttransfection, as overexpression of p53 for longer periods resulted in cell death in our hands (data not shown).
FIG. 3.
p53 coimmunoprecipitates in vivo with KSHV LANA and HVS ORF73. (A) We transfected 20 million 293T cells with: lane 1, empty vector alone (mock); lane 2, p53 expression vector alone; lane 3, Myc-tagged LANA alone; lane 4, Myc-tagged LANA and p53 expression vector together; lane 5, Myc-tagged HVS C488 ORF73 alone; lane 6, Myc-tagged HVS C488 ORF73 and p53 expression vector together; lane 7, Myc-tagged HVS A11 ORF73 alone; or lane 8, Myc-tagged HVS A11 ORF73 and p53 expression vector together. Cells were harvested 20 h later, lysed with RIPA buffer, and incubated with anti-Myc mouse ascites fluid overnight, which targeted the tagged LANA/ORF73 proteins. Antibody-bound LANA/ORF73 proteins and associated protein complexes were immunoprecipitated the next day with a protein A-protein G-Sepharose bead slurry (Amersham Pharmacia, Inc., Piscataway, N.J.) and resolved on 12% Tris-glycine gels, which were blotted with either anti-Myc antibody (top panels) or anti-human p53 antibody (bottom panels) (Santa Cruz Biotechnologies, Inc.). The mouse immunoglobulin G heavy chain used to immunoprecipitate Myc-tagged proteins migrates slightly below the p53 protein and is indicated HC. p53 is marked by white arrows. (B) An inverse pulldown strategy was also employed by immunoprecipitating LANA and ORF73 with bacterially prepared GST-p53; 10 million 293T cells were transfected with either KSHV LANA (left panels) or HVS A11 ORF73 (right panels). Cells were lysed with RIPA buffer and incubated with either glutathione-Sepharose-bound GST or GST-p53. Washed beads were resuspended in 2× SDS buffer. Input, GST-pulled-down and GST-p53-pulled-down fractions were resolved on an 8% Tris-glycine gel. LANA and ORF73 were detected by blotting with an anti-Myc antibody (α-Myc WB), and p53 was detected by blotting with anti-p53 antibody (α-p53 WB).
To corroborate this association in cells, we decided to use bacterially expressed GST-p53 to pull down heterologously expressed LANA and ORF73. Glutathione-Sepharose-bound GST or GST-p53 was incubated with lysates from cells expressing either LANA (Fig. 3B, left panels) or HVS ORF73 (Fig. 3B, right panels). GST- and GST-p53-bound proteins were then fractionated on SDS-PAGE. LANA (Fig. 3B, top left panel) as well as ORF73 (Fig. 3B, top right panel) were found to complex with the GST-p53 fusion protein but not with GST protein alone. The LANA/ORF73 signal was seen consistently, but prolonged incubation resulted in degradation of the signal (data not shown). The relative amounts of GST-p53 used for the immunoprecipitation of LANA and ORF73 are shown in the lower panels of each set, labeled anti-p53 WB, indicating the same blot stripped and reprobed with an anti-p53 antibody.
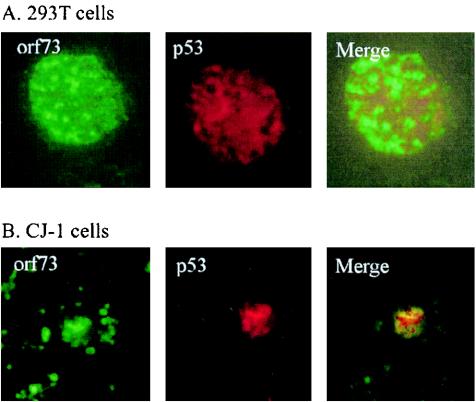
LANA was previously shown to colocalize with p53 in Kaposi's sarcoma tissue, primary effusion lymphoma cells, and SAOS-2 cells expressing exogenous p53 (20, 37). To determine if ORF73 encoded by HVS similarly colocalized with p53, we incubated specific antibodies for ORF73 and p53 to determine colocalization in vivo. We show that the p53 and ORF73 fluorescence signals overlap, indicating nuclear colocalization in vivo (Fig. 4A). We then wanted to determine if colocalization of ORF73 and p53 occurred in CJ-1 cells, a human T-cell line transformed with HVS. In CJ-1 cells, p53 and ORF73 colocalized, as expected, suggesting an in vivo association of these two proteins in HVS-transformed human primary T cells (Fig. 4B).
FIG. 4.
ORF73 of HVS and human p53 colocalize in 293T and CJ-1 cells. (A) 293T cells were cotransfected with 10 μg of p53 and 30 μg of ORF73, fixed onto slides, and sequentially incubated with mouse anti-p53 antibody, rabbit anti-Myc antibody, and a mixture of Texas Red-conjugated goat anti-mouse and fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin antibodies. (B) Localization of endogenous p53 and ORF73 in CJ-1 human T cells was visualized by fixing and treating cells in the same manner.
HVS LANA homologues repress p53-mediated transcription activation.
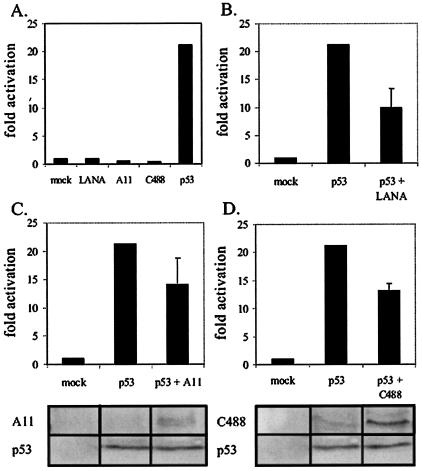
The pG13 plasmid contains 13 tandem repeats of the p53 consensus binding site and the simian virus 40 basal promoter upstream of the luciferase gene (56). Our results show that KSHV LANA and HVS ORF73 had negligible activation of the multimerized p53 promoter element. However, exogenously transfected p53 activated the promoter greater than 20-fold in this transient reporter assay (Fig. 5A). We then wanted to determine how LANA and ORF73 affected p53 activation of its promoter in this assay. We transfected LANA and ORF73 from HVS along with p53 and the reporter plasmid. All transfectants were incubated for no longer than 18 h, as we observed efficient apoptosis of cells when incubated for longer periods. Exogenously expressed LANA repressed p53-mediated activation greater than 50% (Fig. 5B). Similarly, exogenous expression of HVS strain A11 ORF73 and HVS C488 ORF73 inhibited the p53-mediated activation by 33 and 39%, respectively (Fig. 5C and D). This inhibitory activity was dose dependent and reproducible in SAOS-2 cells, which lack endogenous p53 (data not shown). Similar transfection efficiencies among samples was confirmed by the cotransfection of 3 μg of GFP expression vector as well as by Bradford analysis of total protein content in lysed samples.
FIG. 5.
HVS ORF73 represses p53 transactivation. (A) We transfected 10 million BJAB cells with the pG13-Luc reporter alone (mock) or in conjunction with LANA, A11 ORF73, C488 ORF73, or p53 expression vectors (as indicated). (B, C, and D) BJAB cells were transfected with the pG13-Luc reporter vector in the absence or presence of (B) LANA, (C) HVS A11 ORF73, or (D) HVS C488 ORF73. Cells were harvested 18 h posttransfection, and luciferase expression was measured as described. Equal transfection efficiencies across samples were confirmed by cotransfection of 3 μg of GFP expression vector as well as by Bradford analysis of total protein content in lysed samples. Expression of HVS ORF73 from strains A11 (panel C, bottom) and C488 (panel D, bottom) and p53 (panels C and D, bottom) was confirmed by electrophoresis of the lysate of 1 million transfected cells and by Western blotting with anti-Myc or anti-p53 antibodies, as indicated.
LANA and HVS homologues preferentially bind to the tetramerization domain of p53.
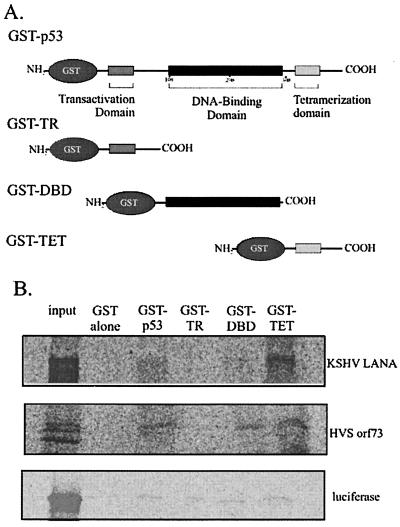
The p53 protein contains three functionally distinct domains, an amino-terminal catalytic domain, a central DNA-binding domain, and a carboxy-terminal tetramerization domain (Fig. 6A) (11, 34). The crystal structures of the DNA-binding domain and the tetramerization domain have been solved and demonstrate that these domains are capable of retaining structure and function when expressed independently of other portions of the protein (11, 34). To test whether LANA and its HVS homologue preferentially interacted with any of these domains, we expressed and purified each of these domains as bacterially prepared GST fusion proteins (Fig. 6A). Equivalent amounts of p53 fusion proteins from the specific domains were incubated with equivalent amounts of in vitro-translated LANA or ORF73 from HVS strain A11 as determined by Coomassie staining and autoradiography. Both LANA and HVS ORF73 preferentially bound the tetramerization domain, while weaker binding was also noted with the DNA-binding domain. No significant binding was observed with the transactivation domain or with a control in vitro-translated luciferase protein (Fig. 6B).
FIG. 6.
LANA preferentially binds to the tetramerization domain of p53 in vitro. (A) Full-length p53 and the p53 transactivation domain (TR), DNA-binding domain (DBD), and tetramerization domain (TET) fused to GST were expressed in E. coli DH5α cells and purified from sonicated cell lysate with glutathione-Sepharose 4B beads (Amersham-Pharmacia). (B) Luciferase and LANA protein were in vitro transcribed and translated in the presence of [35S]methionine and cysteine and incubated with equal amounts of purified p53 constructs for 4 h at 4°C. Pulled-down, in vitro-translated product was resolved on an 8% Tris-glycine gel.
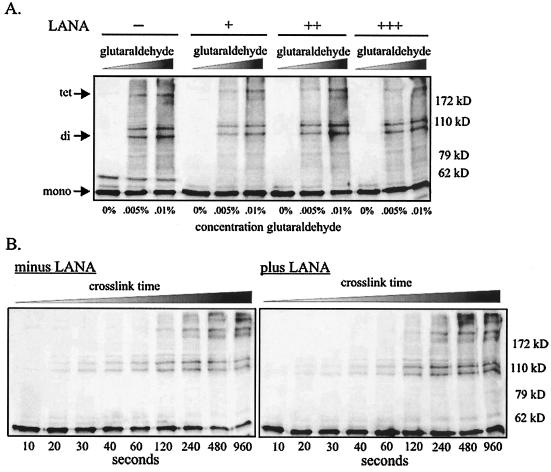
The data demonstrating preferential binding by LANA and ORF73 to the tetramerization domain of p53 immediately suggested a potential mechanism by which these viral proteins inactivate cellular p53. Tetramerization of p53 is essential for both transactivation and tumor suppressive activity in carcinoma cell lines (48). Therefore, a virally encoded protein like LANA which bound the tetramerization domain might disrupt p53 oligomerization, resulting in inactivation. To determine whether LANA is capable of inhibiting oligomerization of p53, we exogenously expressed p53 by transient transfection in 293T cells in the presence and absence of increasing amounts of LANA. Cells were disrupted with a mild lysis buffer, and p53 monomers were cross-linked with either increasing concentrations of glutaraldehyde (Fig. 7A) or 0.005% glutaraldehyde for increasing amounts of time (Fig. 7B) (32). Lysates were then resolved on an 8% denaturing gel. The un-cross-linked monomer (53 kDa), cross-linked dimers (106 kDa), and cross-linked tetramers (212 kDa) were readily detected with an anti-p53 antibody in Western blot assays. The presence of KSHV LANA had negligible effects on the dimerization and tetramerization levels of p53, as determined by this in vitro assay (Fig. 7A and B), suggesting a mechanism of p53 inactivation independent of disruption of its ability for tetramerization. Similar experiments conducted with 293T cells stably expressing LANA and ORF73 produced similar results (data not shown).
FIG. 7.
LANA does not inhibit oligomerization levels of p53. (A) We lysed 10 million 293T cells expressing p53 alone or p53 and LANA in RIPA buffer, and lysates were incubated with 0, 0.005, or 0.01% glutaraldehyde for 20 min at room temperature. Reactions were stopped with 2× SDS loading buffer (100 mM Tris, pH 6.8, 3.6% SDS, 18% glycerol, 9% 2-mercaptoethanol, and 0.05% bromophenol blue), heated at 95°C for 10 min, and resolved on 8% Tris-glycine gels. p53 monomers, dimers, and tetramers were detected with an anti-p53 antibody. (B) 293T cells expressing p53 in the absence (left panel) or presence (right panel) of LANA were lysed and treated with 0.005% glutaraldehyde for increasing periods of time at room temperature before stopping the reaction with 2× SDS loading buffer and resolving on 8% gels.
HVS-encoded LANA homologues bind to pRb in vivo and in vitro.
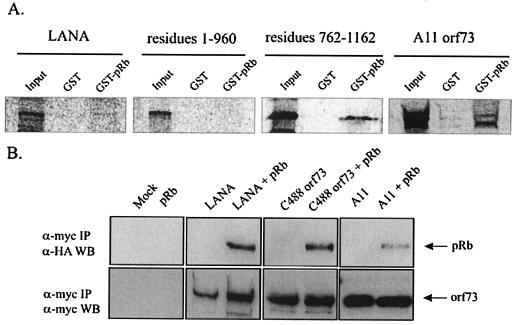
Additional studies have shown that LANA binds to pRb, modulating its activity. To determine if HVS ORF73 associates with pRb and to identify the region required for mediating this association, full-length, amino-terminally truncated and carboxy-terminally truncated LANA were in vitro translated in the presence of [35S]methionine. These in vitro-translated proteins were then incubated with GST-pRb, and complexes were resolved on SDS-PAGE. Full-length LANA and amino acid residues 762 to 1162 were capable of binding to GST-pRb in vitro. However, the amino-terminal 960 amino acids had no significant binding activity (Fig. 8A). These results demonstrated that the region responsible for binding pRb is located in the carboxy terminus of LANA and confirm previous studies (49). Furthermore, the homologue from HVS strain A11 was also complexed with GST-pRb fusion protein but not by GST. This indicates that, like KSHV LANA, ORF73 encoded by HVS was also able to bind pRb in vitro.
FIG. 8.
pRb coimmunoprecipitates with LANA and HVS ORF73 in vivo and in vitro. (A) Full-length LANA and deletion constructs were in vitro translated in the presence of [35S]methionine (TNT-T7 rabbit reticulate lysate) and incubated with either GST or GST-pRb glutathione-Sepharose beads for 4 h at 4°C. Immunoprecipitated proteins were resolved on a 7% Tris-glycine-SDS gel. (B) We transfected 20 million 293T cells with: lane 1, vector alone; lane 2, pRb alone; lane 3, LANA alone; lane 4, LANA and pRb together; lane 5, HVS C488 ORF73 alone; lane 6, HVS C488 ORF73 and pRb together; lane 7, HVS A11 ORF73 alone; or lane 8, HVS A11 ORF73 and pRb together. Cells were harvested 20 h later and lysed with RIPA buffer. Protein complexes were immunoprecipitated with anti-Myc ascites fluid and resolved on a 7% Tris-glycine gel.
To test whether this interaction occurs in vivo, HA-tagged pRb was coexpressed in 293T cells along with empty vector, Myc-tagged LANA, or Myc-tagged ORF73 from HVS strain C488 or Myc-tagged ORF73 from HVS strain A11. In immunoprecipitation assays, antibodies against the Myc tag (9E10) were able to precipitate complexes of all three viral proteins which included pRb, as detected by the HA signal from the HA-tagged pRb expression construct (Fig. 8B). However, in the absence of these viral proteins, pRb was not detected, suggesting the specificity of the association (Fig. 8B).
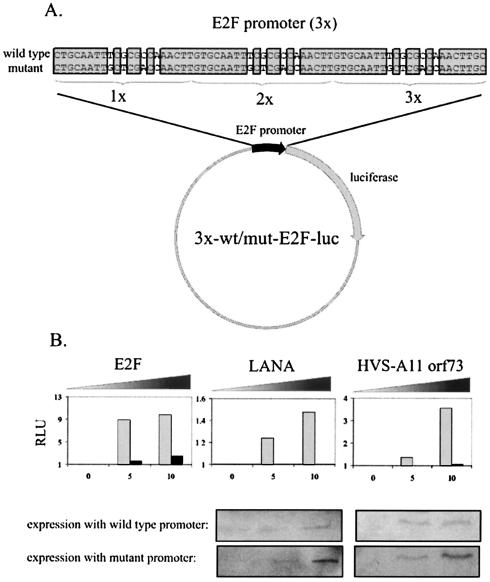
ORF73 encoded by HVS strain A11 transactivates the E2F promoter but not a mutant E2F promoter in reporter assays.
3x-wt-E2F-luc and 3x-mut-E2F-luc have three copies of the wild-type and mutant E2F promoter sequence, respectively, upstream of the luciferase gene (Fig. 9A). Cotransfection with E2F increased expression of luciferase (Fig. 9B). The addition of LANA, as expected, resulted in an increase of luciferase activity, and addition of HVS-A11 ORF73 also increased activity of luciferase in this assay. Importantly, each of these viral proteins had no significant effect in terms of increased luciferase activity when cotransfected with a mutant E2F promoter (Fig. 9B). The increased activation seen was consistent in multiple experiments, although somewhat less robust than that seen with other promoters (49). HVS ORF73 from strain A11 was, moreover, more potent in its ability to activate the E2F promoter in this assay (Fig. 9B). These results suggest that the HVS homologue of LANA is also capable of stimulating activation of E2F transcriptional activity in a similar manner to that seen with KSHV-encoded LANA. Similar transfection efficiencies among samples was confirmed by the cotransfection of 3 μg of GFP expression vector as well as by Bradford analysis of total protein content in lysed samples.
FIG. 9.
LANA and HVS A11 ORF73 transactivate the E2F promoter but not a mutant E2F promoter. (A) The 3x-wt-E2F-Luc and 3x-mut-E2F-Luc vectors have three wild-type and three mutant copies of the luciferase gene, respectively. (B) We transfected 10 million BJAB cells with 5 μg of 3x-wt-E2F-Luc (light bars) or 3x-mut-E2F-Luc (dark bars) and 0, 5, or 10 μg of E2F, LANA, or HVS A11 ORF73 expression vector. Cells were harvested 18 h later and lysed in reporter buffer (Promega), and luciferase activity was measured as described in Materials and Methods. Transfection efficiencies were measured by cotransfecting 3 μg of GFP expression vector. LANA and A11 expression was confirmed by Western blotting.
DISCUSSION
A large body of evidence suggests that LANA encoded by KSHV is a multifunctional protein (2, 12, 20, 21, 38, 40, 49, 50). First, LANA ensures that both daughter cells inherit copies of the viral genome by tethering viral episomes to chromatin proteins during mitosis (2, 12). LANA has been shown to physically interact with the chromosome binding protein histone H1 and may associate with the DEK protein and methyl-CpG binding protein (12, 40). A LANA mutant deleted of residues 1 to 22 neither associates with chromosomes nor supports episome maintenance. A histone H1-LANA(Δ1-22) fusion protein, however, retains both of these functions (53).
It is thought that the amino terminus of LANA mediates chromatin binding, while the carboxy terminus mediates binding to a 13-bp sequence in the terminal repeat region of the viral episome, anchoring the viral episome to the host chromosome (3, 13, 24, 53). Reverse transcription-PCR microarray assays and specific promoter-reporter assays demonstrated that LANA can activate expression from a number of promoters, such as the human telomerase reverse transcriptase promoter and the E2F-responsive promoter, while repressing expression from other promoters, such as the human immunodeficiency virus long terminal repeat promoter and the p53-responsive promoter (20, 38, 49, 50). LANA also appears to promote progression through the cell cycle (20, 21, 49) and redistributes the cellular pool of glycogen synthase kinase-3 beta to the nucleus, inhibiting degradation of β-catenin in the cytoplasm (21, 22). Increased levels of β-catenin, in turn, upregulate the Lef-Tcf transcription factors, which drive cell cycle progression by enhancing transcription of S-phase entry genes such as myc, jun, and ccnd1 (cyclin D1) (28, 54).
Second, LANA is thought to inhibit two major regulators of cell cycle control, pRb and p53. Similar to known DNA tumor viruses, KSHV encodes a protein (LANA) that deregulates p53 and pRb (29). LANA-expressing cells are resistant to p53-dependent apoptosis (as measured by sensitivity to gamma irradiation) but not p53-independent apoptosis (as measured by sensitivity to actinomycin D) (20). Also, expression of LANA has been shown to overcome the flat-cell phenotype in pRb-null cells (49). SAOS-2 cells enter cell cycle arrest upon exogenous pRb expression, but coexpression with LANA overcomes this phenotype, indicating a reentry into the cell cycle (49). Inhibition of pRb and p53 enables LANA-expressing cells to circumvent the G1/S checkpoint and the apoptotic pathway, respectively, leading to immortalization and tumorigenesis (20, 49). Primary rat embryo fibroblast cells expressing both LANA and the oncogene H-ras injected into mice resulted in tumors within 3 to 4 days and continued to grow for 7 additional days postinjection. Importantly, cells expressing only H-ras induced very small tumors which failed to grow after 5 days (49). Like simian virus 40 large T antigen and human papillomavirus E6 and E7 proteins, LANA is an important KSHV protein for deregulation of cell cycle control.
Recently, increased attention has been given to a LANA homologue from HVS, a gammaherpesvirus closely related to KSHV (25, 52, 55). The ORF73 locus is poorly conserved among those gammaherpesviruses whose genomes have been completely sequenced (less than 20% conservation between the ORF73 encoded by KSHV, rhesus rhadinovirus, murine herpesvirus, ateles herpesvirus, and HVS) (1). Moreover, ORF73 is completely absent in some members of the gammaherpesvirus family (alcelaphine herpesvirus 1, bovine herpesvirus 4, callitichine herpesvirus 3, equine herpesvirus 2, Epstein-Barr virus, and cercopithicine herpesvirus 15) (1, 55).
However, a number of secondary structural motifs as well as functions appear to be conserved between KSHV LANA and HVS ORF73. For example, the distinct internal acid residue repeat region is apparent in the HVS homologue, as it is in LANA (26, 55). Also, residues responsible for the nuclear localization of the protein and for tethering of the protein to host metaphase chromosomes and viral terminal repeat regions have been identified (26, 55). Here we demonstrate that two more functions are conserved between these herpesvirus homologues, antagonism of the oncoprotein p53 and antagonism of the oncoprotein pRb. HVS ORF73 from two separate viral strains was able to bind human p53 in vitro and in vivo. ORF73 from strain A11 was shown to colocalize with p53 when coexpressed in 293T cells. Additionally, ORF73 from strain C488 colocalized with p53 in CJ-1 cells (which naturally express p53 and ORF73). Furthermore, ORF73 from both strains A11 and C488 was able to repress p53-mediated transcriptional activation in vivo. Likewise, ORF73 was demonstrated to bind human pRb in vivo and in vitro and to upregulate transcriptional activity from the wild type but not a mutant E2F promoter. These results suggest that the p53- and pRb-antagonizing activities of LANA are conserved in its HVS ORF73 homologue.
The region of the LANA protein responsible for interacting with both p53 and pRb was more finely mapped with a series of deletion constructs. The C terminal-most amino acid residues of LANA (762 to 1162) retained the ability to bind both p53 and pRb in vitro. These results corroborate previous studies which demonstrated that the amino terminus of LANA does not bind p53 and that this region of LANA is incapable of antagonizing the ability of p53 to function as a transcriptional activator or cell cycle regulator (20). The carboxy-terminal domain of LANA binds to pRb in vitro (amino acids 735 to 990) (20, 49). Indeed, it is the C terminus of LANA that shares the greatest number of conserved residues with ORF73 from HVS; 56 amino acid residues within the carboxy-terminal 400 amino acids are identical (55). This relatively high degree of conservation (compared to sequence from other portions of these molecules) is consistent with the hypothesis that the p53/pRb regulatory functions attributed to the LANA C terminus is conserved in the HVS ORF73 homologue.
In this report we also provide evidence that the LANA and ORF73 molecules specifically bind the tetramerization domain of p53. This suggests a mechanism by which LANA and its homologues could counteract p53 activity, by disruption of p53 tetramerization. Tetramerization of the oncoprotein is absolutely required for function, as monomer and dimer forms of p53 are inactive in vivo (34). In our studies, we were unable to detect disruption of p53 oligomerization by LANA or HVS ORF73 (data not shown). However, more sensitive methods such as fluorescence resonance energy transfer, small-angle X-ray diffraction, and fluorescence anisotropy may be able to detect such interference. It is also possible that LANA and HVS ORF73 inactivate p53 by an alternative mechanism, such as by simply sequestering the molecule, thereby inhibiting function. Studies are under way to determine the other possible mechanisms by which LANA mediates inactivation of p53 function.
Acknowledgments
We thank Wafik El-Deiry (University of Pennsylvania) for E2F-responsive reporter vectors and Shelly Berger (University of Pennsylvania) for p53 and GST-p53 expression vectors. We also thank Paul M. Lieberman (The Wistar Institute) and Yan Yuan (Dental School of the University of Pennsylvania) for suggestions.
This work was supported by grants from the Leukemia and Lymphoma Society of America and Public Health Service grants CA072510 and CA 091792 from the NCI and DE01436 from NIDCR (to E.S.R.). E.S.R. is a scholar of the Leukemia and Lymphoma Society of America.
REFERENCES
- 1.Alba, M. M., D. Lee, F. M. Pearl, A. J. Shepherd, N. Martin, C. A. Orengo, and P. Kellam. 2001. VIDA: a virus database system for the organization of animal virus genome open reading frames. Nucleic Acids Res. 29:133-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. [DOI] [PubMed] [Google Scholar]
- 3.Ballestas, M. E., and K. M. Kaye. 2001. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol. 75:3250-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates, S., and K. H. Vousden. 1996. p53 in signaling checkpoint arrest or apoptosis. Curr. Opin. Genet. Dev. 6:12-18. [DOI] [PubMed] [Google Scholar]
- 5.Biesinger, B., I. Muller-Fleckenstein, B. Simmer, G. Lang, S. Wittmann, E. Platzer, R. C. Desrosiers, and B. Fleckenstein. 1992. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc. Natl. Acad. Sci. USA 89:3116-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274-1278. [DOI] [PubMed] [Google Scholar]
- 7.Callahan, J., S. Pai, M. Cotter, and E. S. Robertson. 1999. Distinct patterns of viral antigen expression in Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus coinfected body-cavity-based lymphoma cell lines: potential switches in latent gene expression due to coinfection. Virology 262:18-30. [DOI] [PubMed] [Google Scholar]
- 8.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 9.Cesarman, E., R. G. Nador, F. Bai, R. A. Bohenzky, J. J. Russo, P. S. Moore, Y. Chang, and D. M. Knowles. 1996. Kaposi's sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J. Virol. 70:8218-8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 265:1865-1869. [DOI] [PubMed] [Google Scholar]
- 11.Cho, Y., S. Gorina, P. D. Jeffrey, and N. P. Pavletich. 1994. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science 265:346-355. [DOI] [PubMed] [Google Scholar]
- 12.Cotter, M. A., 2nd, and E. S. Robertson. 1999. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology 264:254-264. [DOI] [PubMed] [Google Scholar]
- 13.Cotter, M. A., 2nd, C. Subramanian, and E. S. Robertson. 2001. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen binds to specific sequences at the left end of the viral genome through its carboxy-terminus. Virology 291:241-259. [DOI] [PubMed] [Google Scholar]
- 14.DeCaprio, J. A., J. W. Ludlow, J. Figge, J. Y. Shew, C. M. Huang, W. H. Lee, E. Marsilio, E. Paucha, and D. M. Livingston. 1988. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell 54:275-283. [DOI] [PubMed] [Google Scholar]
- 15.DeCaprio, J. A., J. W. Ludlow, D. Lynch, Y. Furukawa, J. Griffin, H. Piwnica-Worms, C. M. Huang, and D. M. Livingston. 1989. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell 58:1085-1095. [DOI] [PubMed] [Google Scholar]
- 16.Dupin, N., T. L. Diss, P. Kellam, M. Tulliez, M. Q. Du, D. Sicard, R. A. Weiss, P. G. Isaacson, and C. Boshoff. 2000. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood 95:1406-1412. [PubMed] [Google Scholar]
- 17.Dupin, N., C. Fisher, P. Kellam, S. Ariad, M. Tulliez, N. Franck, E. van Marck, D. Salmon, I. Gorin, J. P. Escande, R. A. Weiss, K. Alitalo, and C. Boshoff. 1999. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 96:4546-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandes, E. R., J. Y. Zhang, and R. J. Rooney. 1998. Adenovirus E1A-regulated transcription factor p120E4F inhibits cell growth and induces the stabilization of the cdk inhibitor p21WAF1. Mol. Cell. Biol. 18:459-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox, J. D. 1952. Virus-like bodies in human cancer; a electron-microscope study of the filtrable components of normal and neoplastic tissue. Med. Arts Sci. 6:91-100. [PubMed] [Google Scholar]
- 20.Friborg, J., Jr., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889-894. [DOI] [PubMed] [Google Scholar]
- 21.Fujimuro, M., and S. D. Hayward. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus manipulates the activity of glycogen synthase kinase-3beta. J. Virol. 77:8019-8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujimuro, M., F. Y. Wu, C. ApRhys, H. Kajumbula, D. B. Young, G. S. Hayward, and S. D. Hayward. 2003. A novel viral mechanism for dysregulation of beta-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat. Med. 9:300-306. [DOI] [PubMed] [Google Scholar]
- 23.Furukawa, Y., J. A. DeCaprio, A. Freedman, Y. Kanakura, M. Nakamura, T. J. Ernst, D. M. Livingston, and J. D. Griffin. 1990. Expression and state of phosphorylation of the retinoblastoma susceptibility gene product in cycling and noncycling human hematopoietic cells. Proc. Natl. Acad. Sci. USA 87:2770-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garber, A. C., M. A. Shu, J. Hu, and R. Renne. 2001. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:7882-7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giles, M. S., P. G. Smith, P. L. Coletta, K. T. Hall, and A. Whitehouse. 2003. The herpesvirus saimiri ORF 73 regulatory region provides long-term transgene expression in human carcinoma cell lines. Cancer Gene Ther. 10:49-56. [DOI] [PubMed] [Google Scholar]
- 26.Hall, K. T., M. S. Giles, D. J. Goodwin, M. A. Calderwood, A. F. Markham, and A. Whitehouse. 2000. Characterization of the herpesvirus saimiri ORF73 gene product. J. Gen. Virol. 81:2653-2658. [DOI] [PubMed] [Google Scholar]
- 27.Haller, K., Y. Wu, E. Derow, I. Schmitt, K. T. Jeang, and R. Grassmann. 2002. Physical interaction of human T-cell leukemia virus type 1 Tax with cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol. Cell. Biol. 22:3327-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He, T. C., A. B. Sparks, C. Rago, H. Hermeking, L. Zawel, L. T. da Costa, P. J. Morin, B. Vogelstein, and K. W. Kinzler. 1998. Identification of c-MYC as a target of the APC pathway. Science 281:1509-1512. [DOI] [PubMed] [Google Scholar]
- 29.Helt, A. M., and D. A. Galloway. 2003. Mechanisms by which DNA tumor virus oncoproteins target the Rb family of pocket proteins. Carcinogenesis 24:159-169. [DOI] [PubMed] [Google Scholar]
- 30.Howley, P. M., K. Munger, B. A. Werness, W. C. Phelps, and R. Schlegel. 1989. Molecular mechanisms of transformation by the human papillomaviruses. Princess Takamatsu Symp. 20:199-206. [PubMed] [Google Scholar]
- 31.Hu, J., A. C. Garber, and R. Renne. 2002. The Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus supports latent DNA replication in dividing cells. J. Virol. 76:11677-11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishioka, C., H. Shimodaira, C. Englert, A. Shimada, M. Osada, L. Q. Jia, T. Suzuki, M. Gamo, and R. Kanamaru. 1997. Oligomerization is not essential for growth suppression by p53 in p53-deficient osteosarcoma Saos-2 cells. Biochem. Biophys. Res. Commun. 232:54-60. [DOI] [PubMed] [Google Scholar]
- 33.Jay, G., G. Khoury, A. B. DeLeo, W. G. Dippold, and L. J. Old. 1981. p53 transformation-related protein: detection of an associated phosphotransferase activity. Proc. Natl. Acad. Sci. USA 78:2932-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeffrey, P. D., S. Gorina, and N. P. Pavletich. 1995. Crystal structure of the tetramerization domain of the p53 tumor suppressor at 1.7 angstroms. Science 267:1498-1502. [DOI] [PubMed] [Google Scholar]
- 35.Kaelin, W. G., Jr., D. C. Pallas, J. A. DeCaprio, F. J. Kaye, and D. M. Livingston. 1991. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell 64:521-532. [DOI] [PubMed] [Google Scholar]
- 36.Katano, H., T. Sata, T. Suda, T. Nakamura, N. Tachikawa, H. Nishizumi, S. Sakurada, Y. Hayashi, M. Koike, A. Iwamoto, T. Kurata, and S. Mori. 1999. Expression and antigenicity of human herpesvirus 8 encoded ORF59 protein in AIDS-associated Kaposi's sarcoma. J. Med. Virol. 59:346-355. [DOI] [PubMed] [Google Scholar]
- 37.Katano, H., Y. Sato, and T. Sata. 2001. Expression of p53 and human herpesvirus-8 (HHV-8)-encoded latency-associated nuclear antigen with inhibition of apoptosis in HHV-8-associated malignancies. Cancer 92:3076-3084. [DOI] [PubMed] [Google Scholar]
- 38.Knight, J. S., M. A. Cotter 2nd, and E. S. Robertson. 2001. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus transactivates the telomerase reverse transcriptase promoter. J. Biol. Chem. 276:22971-22978. [DOI] [PubMed] [Google Scholar]
- 39.Krek, W., D. M. Livingston, and S. Shirodkar. 1993. Binding to DNA and the retinoblastoma gene product promoted by complex formation of different E2F family members. Science 262:1557-1560. [DOI] [PubMed] [Google Scholar]
- 40.Krithivas, A., M. Fujimuro, M. Weidner, D. B. Young, and S. D. Hayward. 2002. Protein interactions targeting the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus to cell chromosomes. J. Virol. 76:11596-11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, M., D. Chen, A. Shiloh, J. Luo, A. Y. Nikolaev, J. Qin, and W. Gu. 2002. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416:648-653. [DOI] [PubMed] [Google Scholar]
- 42.Li, M., H. Lee, D. W. Yoon, J. C. Albrecht, B. Fleckenstein, F. Neipel, and J. U. Jung. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional cyclin. J. Virol. 71:1984-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu, L., D. M. Scolnick, R. C. Trievel, H. B. Zhang, R. Marmorstein, T. D. Halazonetis, and S. L. Berger. 1999. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol. Cell. Biol. 19:1202-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore, P. S., S. J. Gao, G. Dominguez, E. Cesarman, O. Lungu, D. M. Knowles, R. Garber, P. E. Pellett, D. J. McGeoch, and Y. Chang. 1996. Primary characterization of a herpesvirus agent associated with Kaposi's sarcoma. J. Virol. 70:549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nador, R. G., E. Cesarman, A. Chadburn, D. B. Dawson, M. Q. Ansari, J. Sald, and D. M. Knowles. 1996. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood 88:645-656. [PubMed] [Google Scholar]
- 46.Parker, G. A., T. Crook, M. Bain, E. A. Sara, P. J. Farrell, and M. J. Allday. 1996. Epstein-Barr virus nuclear antigen (EBNA)3C is an immortalizing oncoprotein with similar properties to adenovirus E1A and papillomavirus E7. Oncogene 13:2541-2549. [PubMed] [Google Scholar]
- 47.Pietenpol, J. A., R. W. Stein, E. Moran, P. Yaciuk, R. Schlegel, R. M. Lyons, M. R. Pittelkow, K. Munger, P. M. Howley, and H. L. Moses. 1990. TGF-beta 1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell 61:777-785. [DOI] [PubMed] [Google Scholar]
- 48.Pietenpol, J. A., T. Tokino, S. Thiagalingam, W. S. el-Deiry, K. W. Kinzler, and B. Vogelstein. 1994. Sequence-specific transcriptional activation is essential for growth suppression by p53. Proc. Natl. Acad. Sci. USA 91:1998-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radkov, S. A., P. Kellam, and C. Boshoff. 2000. The latent nuclear antigen of kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene hras transforms primary rat cells. Nat. Med. 6:1121-1127. [DOI] [PubMed] [Google Scholar]
- 50.Renne, R., C. Barry, D. Dittmer, N. Compitello, P. O. Brown, and D. Ganem. 2001. Modulation of cellular and viral gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schafer, A., D. Lengenfelder, C. Grillhosl, C. Wieser, B. Fleckenstein, and A. Ensser. 2003. The latency-associated nuclear antigen homolog of herpesvirus saimiri inhibits lytic virus replication. J. Virol. 77:5911-5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shinohara, H., M. Fukushi, M. Higuchi, M. Oie, O. Hoshi, T. Ushiki, J. Hayashi, and M. Fujii. 2002. Chromosome binding site of latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus is essential for persistent episome maintenance and is functionally replaced by histone H1. J. Virol. 76:12917-12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tetsu, O., and F. McCormick. 1999. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422-426. [DOI] [PubMed] [Google Scholar]
- 55.Verma, S. C., and E. S. Robertson. 2003. ORF73 of Herpesvirus saimiri strain C488 tethers the viral genome to metaphase chromosomes and binds to cis-acting DNA sequences in the terminal repeats. J. Virol. 77:12494-12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, W., and W. S. El-Deiry. 2003. Bioluminescent molecular imaging of endogenous and exogenous p53-mediated transcription in vitro and in vivo using an HCT116 human colon carcinoma xenograft model. Cancer Biol. Ther. 2:196-202. [DOI] [PubMed] [Google Scholar]
- 57.Werness, B. A., A. J. Levine, and P. M. Howley. 1990. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248:76-79. [DOI] [PubMed] [Google Scholar]
- 58.Zerfass-Thome, K., W. Zwerschke, B. Mannhardt, R. Tindle, J. W. Botz, and P. Jansen-Durr. 1996. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene 13:2323-2330. [PubMed] [Google Scholar]