Abstract
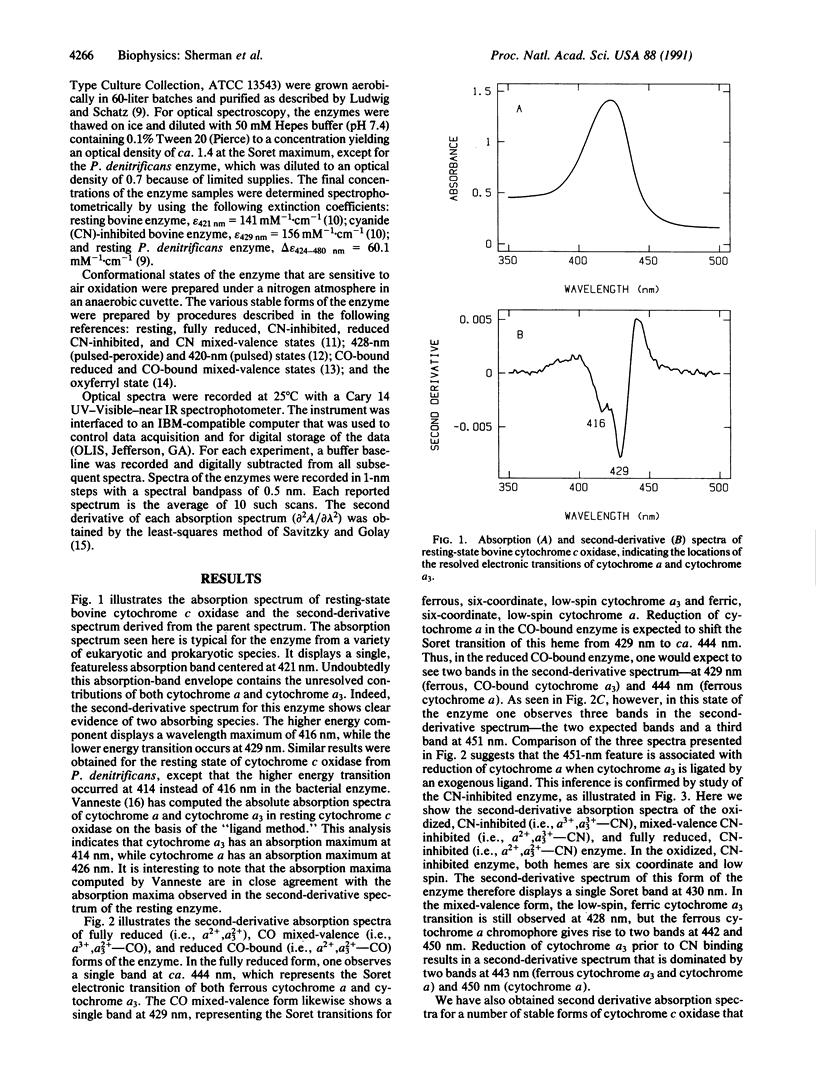
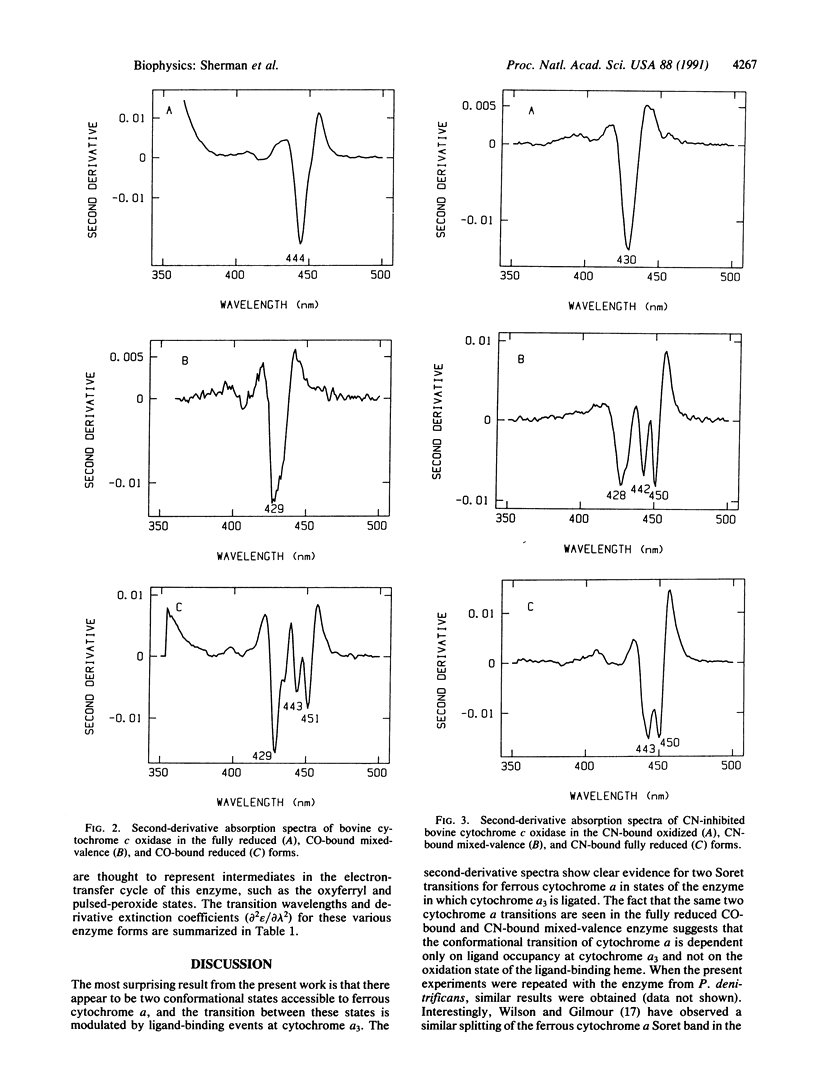
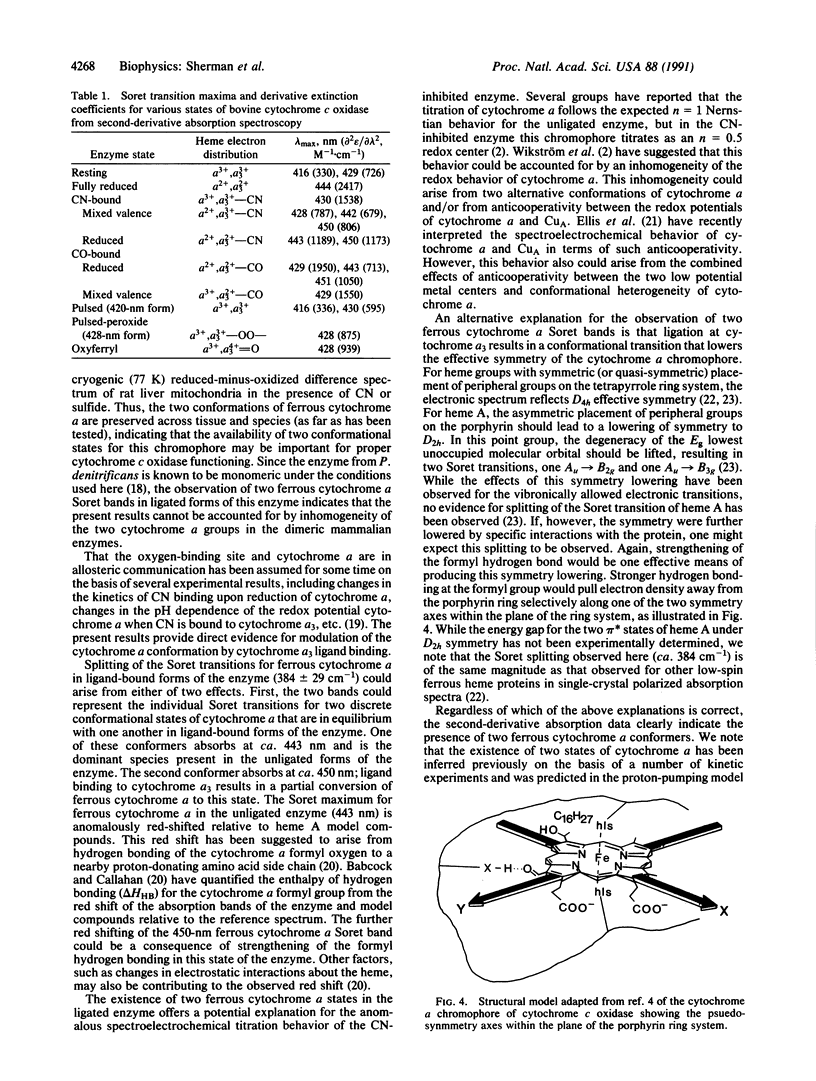
Second-derivative absorption spectra are reported for a variety of oxidation and ligation states of bovine cytochrome c oxidase (ferrocytochrome-c:oxygen oxidoreductase, EC 1.9.3.1). The high resolving power of the second-derivative method allows us to assign the individual electronic transitions of cytochrome alpha and cytochrome alpha 3 in many of these states. In the fully reduced enzyme, one observes a single electronic transition at 444 nm, corresponding to the Soret transition for both ferrous cytochrome alpha and ferrous cytochrome alpha 3. When the cytochrome alpha 3 site is occupied by an exogenous ligand (CN or CO), one observes two absorption bands assignable to the ferrous cytochrome alpha chromophore, one at ca, 443 nm and the other at ca, 450 nm. The appearance of the 450-nm band is dependent only on ligand occupancy at the cytochrome alpha 3 site and not on the oxidation state of the cytochrome alpha 3 iron. These results can be interpreted either in terms of a heterogeneous mixture of two ferrous cytochrome alpha conformers in the cytochrome alpha 3-ligated enzyme or in terms of a reduction in the effective molecular symmetry of the ferrous cytochrome alpha site that results in a lifting of the degeneracy of the lowest unoccupied molecular orbital associated with the Soret pi,pi* transition of cytochrome alpha. In either case, the present data indicate that ferrous cytochrome alpha can adopt two distinct conformations. One possible structural difference between these two states could be related to differences in the strength of hydrogen bonding between the ferrous cytochrome alpha formyl oxygen and a proton donor from an unidentified amino acid side chain of the enzyme. The implications of such modulation of hydrogen-bond strength are discussed in terms of possible mechanisms of proton translocation and electron transfer in the enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock G. T., Callahan P. M. Redox-linked hydrogen bond strength changes in cytochrome a: implications for a cytochrome oxidase proton pump. Biochemistry. 1983 May 10;22(10):2314–2319. doi: 10.1021/bi00279a002. [DOI] [PubMed] [Google Scholar]
- Blair D. F., Bocian D. F., Babcock G. T., Chan S. I. Evidence for modulation of the heme absorptions of cytochrome c oxidase by metal-metal interactions. Biochemistry. 1982 Dec 21;21(26):6928–6935. doi: 10.1021/bi00269a048. [DOI] [PubMed] [Google Scholar]
- Callahan P. M., Babcock G. T. Origin of the cytochrome a absorption red shift: a pH-dependent interaction between its heme a formyl and protein in cytochrome oxidase. Biochemistry. 1983 Jan 18;22(2):452–461. doi: 10.1021/bi00271a031. [DOI] [PubMed] [Google Scholar]
- Ching Y. C., Argade P. V., Rousseau D. L. Resonance raman spectra of CN--bound cytochrome oxidase: spectral isolation of cytochromes a2+, a3(2+), and a3(2+)(CN-). Biochemistry. 1985 Aug 27;24(18):4938–4946. doi: 10.1021/bi00339a032. [DOI] [PubMed] [Google Scholar]
- Copeland R. A., Chan S. I. Proton translocation in proteins. Annu Rev Phys Chem. 1989;40:671–698. doi: 10.1146/annurev.pc.40.100189.003323. [DOI] [PubMed] [Google Scholar]
- Ellis W. R., Jr, Wang H., Blair D. F., Gray H. B., Chan S. I. Spectroelectrochemical study of the cytochrome a site in carbon monoxide inhibited cytochrome c oxidase. Biochemistry. 1986 Jan 14;25(1):161–167. doi: 10.1021/bi00349a023. [DOI] [PubMed] [Google Scholar]
- Gelles J., Blair D. F., Chan S. I. The proton-pumping site of cytochrome c oxidase: a model of its structure and mechanism. Biochim Biophys Acta. 1986;853(3-4):205–236. doi: 10.1016/0304-4173(87)90002-4. [DOI] [PubMed] [Google Scholar]
- Hartzell C. R., Beinert H. Components of cytochrome c oxidase detectable by EPR spectroscopy. Biochim Biophys Acta. 1974 Dec 19;368(3):318–338. doi: 10.1016/0005-2728(74)90178-9. [DOI] [PubMed] [Google Scholar]
- Kumar C., Naqui A., Chance B. Peroxide interaction with pulsed cytochrome oxidase. Optical and EPR studies. J Biol Chem. 1984 Oct 10;259(19):11668–11671. [PubMed] [Google Scholar]
- Ludwig B., Grabo M., Gregor I., Lustig A., Regenass M., Rosenbusch J. P. Solubilized cytochrome c oxidase from Paracoccus denitrificans is a monomer. J Biol Chem. 1982 May 25;257(10):5576–5578. [PubMed] [Google Scholar]
- Ludwig B., Schatz G. A two-subunit cytochrome c oxidase (cytochrome aa3) from Paracoccus dentrificans. Proc Natl Acad Sci U S A. 1980 Jan;77(1):196–200. doi: 10.1073/pnas.77.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmström B. G. Cytochrome oxidase: some unsolved problems and controversial issues. Arch Biochem Biophys. 1990 Aug 1;280(2):233–241. doi: 10.1016/0003-9861(90)90325-s. [DOI] [PubMed] [Google Scholar]
- Morgan J. E., Blair D. F., Chan S. I. The reactivity of pulsed cytochrome c oxidase toward carbon monoxide. J Inorg Biochem. 1985 Mar-Apr;23(3-4):295–302. doi: 10.1016/0162-0134(85)85038-8. [DOI] [PubMed] [Google Scholar]
- Ray G. B., Copeland R. A., Lee C. P., Spiro T. G. Resonance Raman evidence for low-spin Fe2+ heme a3 in energized cytochrome c oxidase: implications for the inhibition of O2 reduction. Biochemistry. 1990 Apr 3;29(13):3208–3213. doi: 10.1021/bi00465a009. [DOI] [PubMed] [Google Scholar]
- Vanneste W. H. The stoichiometry and absorption spectra of components a and a-3 in cytochrome c oxidase. Biochemistry. 1966 Mar;5(3):838–848. doi: 10.1021/bi00867a005. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Gilmour M. V. The low-temperature spectral properties of mammalian cytochrome oxidase. I. The enzyme in intact rat-liver mitochondria. Biochim Biophys Acta. 1967 Jul 5;143(1):52–61. doi: 10.1016/0005-2728(67)90108-9. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Lindsay J. G., Brocklehurst E. S. Heme-heme interaction in cytochrome oxidase. Biochim Biophys Acta. 1972 Feb 28;256(2):277–286. doi: 10.1016/0005-2728(72)90058-8. [DOI] [PubMed] [Google Scholar]
- Witt S. N., Chan S. I. Evidence for a ferryl Fea3 in oxygenated cytochrome c oxidase. J Biol Chem. 1987 Feb 5;262(4):1446–1448. [PubMed] [Google Scholar]


