Abstract
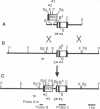
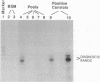
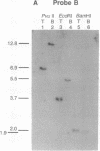
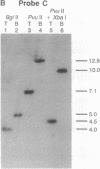
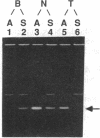
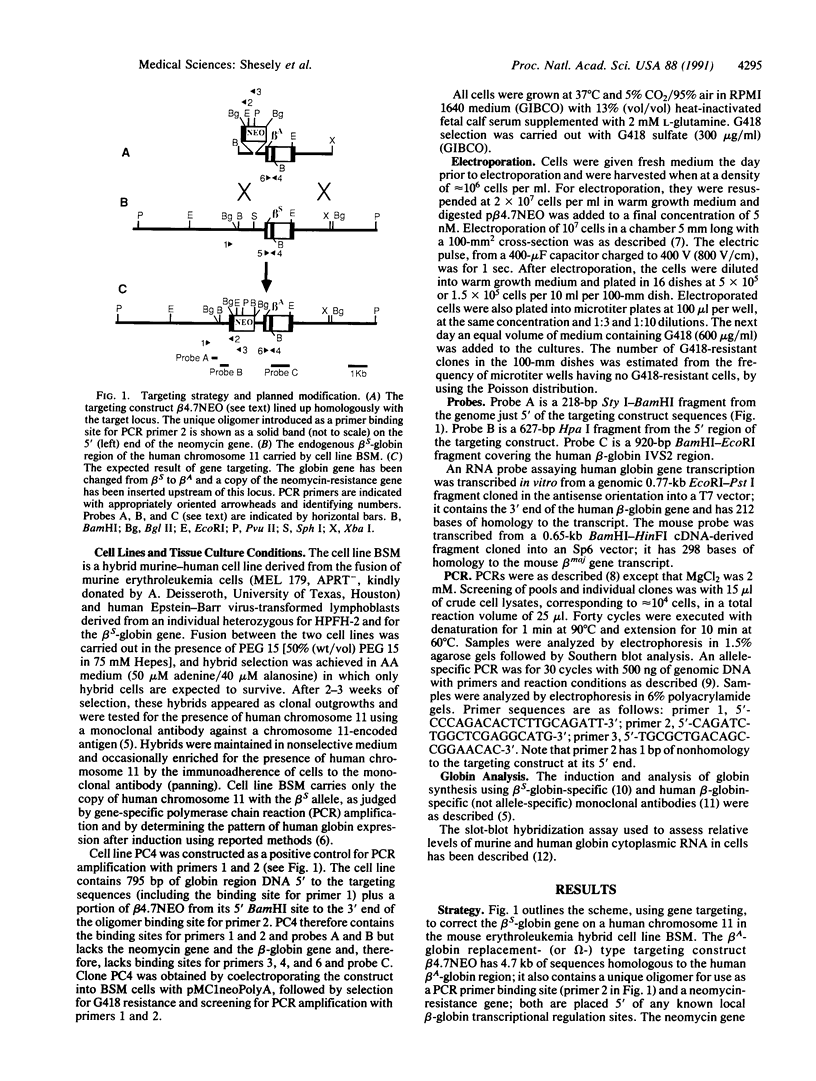
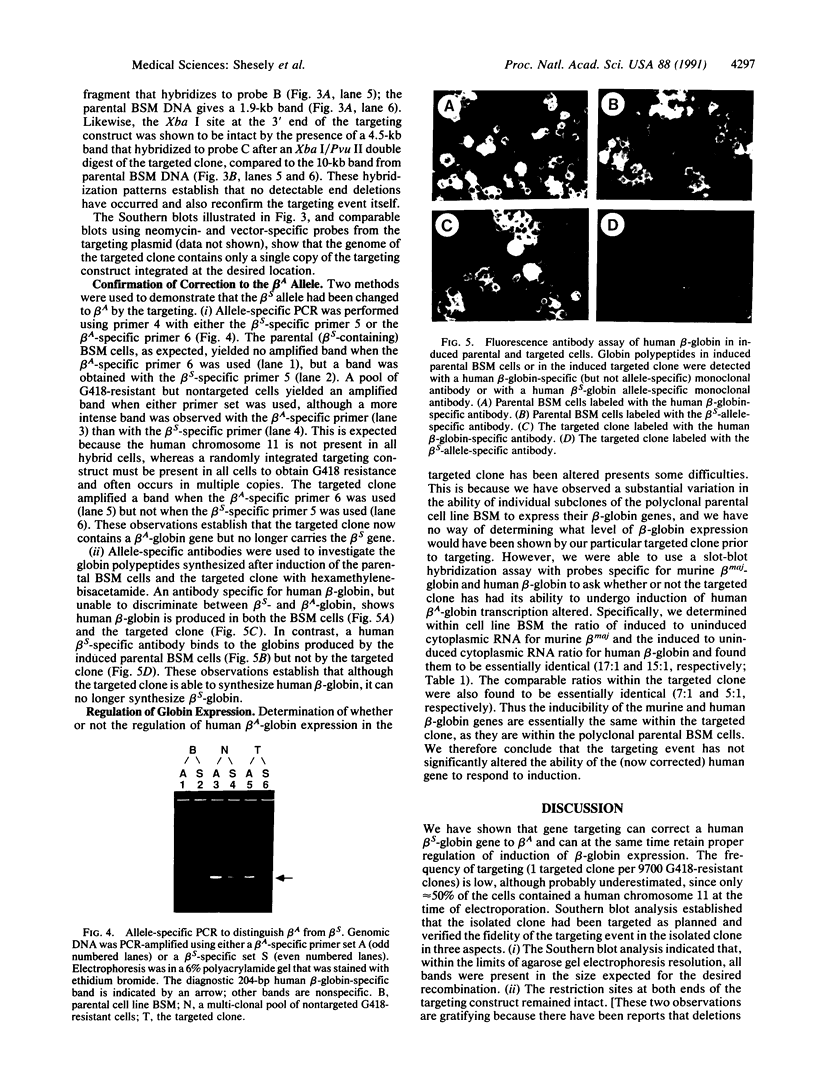
As a step toward using gene targeting for gene therapy, we have corrected a human beta S-globin gene to the normal beta A allele by homologous recombination in the mouse-human hybrid cell line BSM. BSM is derived from a mouse erythroleukemia cell line and carries a single human chromosome 11 with the beta S-globin allele. A beta A-globin targeting construct containing a unique oligomer and a neomycin-resistance gene was electroporated into the BSM cells, which were then placed under G418 selection. Then 126 resulting pools containing a total of approximately 29,000 G418-resistant clones were screened by PCR for the presence of a targeted recombinant: 3 positive pools were identified. A targeted clone was isolated by replating one of the positive pools into smaller pools and rescreening by PCR, followed by dilution cloning. Southern blot analysis demonstrated that the isolated clone had been targeted as planned. The correction of the beta S allele to beta A was confirmed both by allele-specific PCR and by allele-specific antibodies. Expression studies comparing the uninduced and induced RNA levels in unmodified BSM cells and in the targeted clone showed no significant alteration in the ability of the targeted clone to undergo induction, despite the potentially disrupting presence of a transcriptionally active neomycin gene 5' to the human beta A-globin gene. Thus gene targeting can correct a beta S allele to beta A, and the use of a selectable helper gene need not significantly interfere with the induction of the corrected gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boggs S. S., Gregg R. G., Borenstein N., Smithies O. Efficient transformation and frequent single-site, single-copy insertion of DNA can be obtained in mouse erythroleukemia cells transformed by electroporation. Exp Hematol. 1986 Nov;14(10):988–994. [PubMed] [Google Scholar]
- Constantoulakis P., Knitter G., Stamatoyannopoulos G. On the induction of fetal hemoglobin by butyrates: in vivo and in vitro studies with sodium butyrate and comparison of combination treatments with 5-AzaC and AraC. Blood. 1989 Nov 1;74(6):1963–1971. [PubMed] [Google Scholar]
- Deisseroth A., Hendrick D. Activation of phenotypic expression of human globin genes from nonerythroid cells by chromosome-dependent transfer to tetraploid mouse erythroleukemia cells. Proc Natl Acad Sci U S A. 1979 May;76(5):2185–2189. doi: 10.1073/pnas.76.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetschman T., Maeda N., Smithies O. Targeted mutation of the Hprt gene in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8583–8587. doi: 10.1073/pnas.85.22.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin M., Elledge S. J., Davis R. W., Berg P. Gene targeting at the human CD4 locus by epitope addition. Genes Dev. 1990 Feb;4(2):157–166. doi: 10.1101/gad.4.2.157. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Smithies O. Recombinant fragment assay for gene targetting based on the polymerase chain reaction. Nucleic Acids Res. 1988 Sep 26;16(18):8887–8903. doi: 10.1093/nar/16.18.8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990 Sep 21;62(6):1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Nandi A. K., Roginski R. S., Gregg R. G., Smithies O., Skoultchi A. I. Regulated expression of genes inserted at the human chromosomal beta-globin locus by homologous recombination. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3845–3849. doi: 10.1073/pnas.85.11.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T. H., Mcguire T. C., Lim G., Garzel E., Nute P. E., Stamatoyannopoulos G. Identification of Haemoglobin S in red cells and normoblasts, using fluorescent anti-Hb S antibodies. Br J Haematol. 1976 Sep;34(1):25–31. doi: 10.1111/j.1365-2141.1976.tb00170.x. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Stamatoyannopoulos G. Analysis of human hemoglobin switching in MEL x human fetal erythroid cell hybrids. Cell. 1986 Aug 1;46(3):469–476. doi: 10.1016/0092-8674(86)90667-7. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Enver T., Takegawa S., Anagnou N. P., Stamatoyannopoulos G. Activation of developmentally mutated human globin genes by cell fusion. Science. 1988 Nov 18;242(4881):1056–1058. doi: 10.1126/science.2461587. [DOI] [PubMed] [Google Scholar]
- Pyati J., Kucherlapati R. S., Skoultchi A. I. Activation of human beta-globin genes from nonerythroid cells by fusion with murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3435–3439. doi: 10.1073/pnas.77.6.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid L. H., Shesely E. G., Kim H. S., Smithies O. Cotransformation and gene targeting in mouse embryonic stem cells. Mol Cell Biol. 1991 May;11(5):2769–2777. doi: 10.1128/mcb.11.5.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M. J., Nissen L., Collins C. Homologous recombination in hybridoma cells: dependence on time and fragment length. Mol Cell Biol. 1990 Sep;10(9):4466–4472. doi: 10.1128/mcb.10.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Farquhar M., Lindsley D., Brice M., Papayannopoulou T., Nute P. E. Monoclonal antibodies specific for globin chains. Blood. 1983 Mar;61(3):530–539. [PubMed] [Google Scholar]
- Stanton B. R., Reid S. W., Parada L. F. Germ line transmission of an inactive N-myc allele generated by homologous recombination in mouse embryonic stem cells. Mol Cell Biol. 1990 Dec;10(12):6755–6758. doi: 10.1128/mcb.10.12.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Willing M. C., Nienhuis A. W., Anderson W. F. Selective activation of human beta-but not gamma-globin gene in human fibroblast x mouse erythroleukaemia cell hybrids. Nature. 1979 Feb 15;277(5697):534–538. doi: 10.1038/277534a0. [DOI] [PubMed] [Google Scholar]
- Wu D. Y., Ugozzoli L., Pal B. K., Wallace R. B. Allele-specific enzymatic amplification of beta-globin genomic DNA for diagnosis of sickle cell anemia. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2757–2760. doi: 10.1073/pnas.86.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]