Abstract
Despite a long history and extensive usage of insoluble aluminum salts (alum) as vaccine adjuvants, the molecular mechanisms underpinning Ag-specific immunity upon vaccination remain unclear. Dendritic cells (DCs) are crucial initiators of immune responses, but little is known about the molecular pathways used by DCs to sense alum and, in turn, activate T and B cells. In this article, we show that alum adjuvanticity requires IL-2 specifically released by DCs, even when T cell secretion of IL-2 is intact. We demonstrate that alum, as well as other sterile particulates, such as uric acid crystals, induces DCs to produce IL-2 following initiation of actin-mediated phagocytosis that leads to Src and Syk kinase activation, Ca2+ mobilization, and calcineurin-dependent activation of NFAT, the master transcription factor regulating IL-2 expression. Using chimeric mice, we show that DC-derived IL-2 is required for maximal Ag-specific proliferation of CD4+ T cells and optimal humoral responses following alum-adjuvanted immunization. These data identify DC-derived IL-2 as a key mediator of alum adjuvanticity in vivo and the Src–Syk pathway as a potential leverage point in the rational design of novel adjuvants.
Introduction
Aluminum salts (alum) have been widely used in vaccines to potentiate humoral- and cell-mediated immune responses; although new adjuvants have been developed and approved for clinical use, alum remains the most widely used vaccine adjuvant (1). Despite its extensive application in vaccines, surprisingly little is known about the mechanisms underpinning alum adjuvanticity. Kool et al. (2) first demonstrated in mice that injection of alum results in the release of uric acid, an endogenous danger signal, which then activates inflammatory dendritic cells (DCs) to prime CD4+ T cells. Shortly after, Flach et al. (3) showed that alum crystals directly interact with membrane lipids on DCs, initiating a signaling cascade that increases cell surface levels of LFA-1 and ICAM-1, which, in turn, enables CD4+ T cells to bind with higher affinity to the DCs. Some studies suggested that the release of dsDNA from damaged and dying host cells, which is induced by alum injection, may contribute to priming of Th2 CD4+ T cells (4, 5) and enhance MHC class II–mediated Ag presentation, prolonging CD4+ T cell interactions with DCs (5). However, a recent report by Noges et al. (6) showed that some of the DNase preparations used in the above studies are contaminated by proteases, casting doubt on the significance of dsDNA in responses to alum.
Despite these recent advances, the immune mediators of alum adjuvanticity remain enigmatic. Significantly, it is still unclear how effector and memory T and B cell responses are initiated and maintained in the context of alum-adjuvanted vaccination. TLR signaling is not required for alum adjuvanticity, because mice deficient in both TLR signaling adaptors MyD88 and TRIF respond normally to alum immunization (7); in addition, mice lacking mast cells, eosinophils, or macrophages do not exhibit defects in endogenous T and B cell responses following alum immunization (8). Although alum adjuvanticity was initially thought to be dependent on the NLRP3 inflammasome (9–11), a number of studies have since demonstrated that NLRP3 and caspase-1 are dispensable at least for the generation of alum-driven Ab responses (4, 8, 12, 13), although their relevance for T cell responses remains unknown.
DCs are professional APCs that are functionally specialized to link the innate and adaptive immune systems by presenting Ags to T cells in conjunction with appropriate costimulatory molecules and secreted cytokines. One such cytokine is IL-2. DCs secrete large amounts of IL-2 following ligation of the dectin-1 receptor by fungal β-glucans (14, 15), but IL-2 production in response to bacterial TLR ligands, such as LPS and CpG (16–18), is lower, which led to controversy about the role of DC-derived IL-2 in immune responses in vivo (19, 20). As a result, few studies focused on DC-derived IL-2 and so we have only a partial understanding of its contributions to immunity (16, 21). For instance, it is unknown whether DC-derived IL-2 can promote Ab responses by sustaining effective CD4+ T cell help or whether this cytokine contributes to responses to nonmicrobial insults, such as the sterile or self-Ags in vaccines and autoimmune diseases.
In this study, we show that the realization of full alum adjuvanticity requires DC-derived IL-2. We demonstrate that alum dose dependently elicits IL-2 secretion from primed DCs, independent of the NLRP3 inflammasome. Mechanistically, alum interacts with the plasma membrane during attempted phagocytosis, leading to activation of the Src and Syk kinases; in turn, these kinases drive calcium mobilization, NFAT translocation, and IL-2 production. Finally, we show that DC-derived IL-2 is required for maximal Ag-specific CD4+ T cell responses and optimal type 2 Ab production in vivo following alum-adjuvanted OVA immunization. These findings demonstrate that the Syk–NFAT–IL-2 axis in DCs is a key pathway underpinning the adjuvanticity of alum and sterile particulates. Furthermore, our work suggests principles to improve vaccine formulation and development, such as the need to activate Syk to elicit significant amounts of adaptive immunity–priming IL-2 from DCs.
Materials and Methods
Animals
C57BL/6J mice were purchased from the Biological Resource Centre (Agency for Science, Technology and Research). B6.SJL-PtprcaPepcb/BoyJ (CD45.1+) and B6.129P2-Il2tm1Hor/J (Il2−/−) mice were purchased from The Jackson Laboratory. Nlrp3tm1Tsc (Nlrp3−/−) mice were provided by J. Tschopp (University of Lausanne, Lausanne, Switzerland), and Pycardtm1Vmd (Asc−/−) mice were obtained from V.M. Dixit (Genentech). B6.129S7-Rag1tm1MomTg(TcraTcrb)425Cbn mice (OT-II-Rag1−/− [OT-II]; Taconic) were mated with CD45.1+ mice, and the F1 progeny (CD45.1+CD45.2+) were used for experiments. CD11c:DTa mice were generated by crossing B6.Cg-Tg(Itgax-cre)1-1Reiz/J (CD11c-Cre) mice with Gt(ROSA)26Sortm1(DTa)Jpmb/J mice (ROSA26-eGFP-DTa) (Jackson Laboratory). Littermates lacking CD11c-Cre (creneg, referred to as DC-Il2+/−) served as negative controls for the offspring inheriting CD11c-Cre (crepos, referred to as DC-Il2−/−). Only crepos animals express the diphtheria toxin in CD11chigh cells, leading to DC depletion and replenishment by Il2−/− precursors. All animals were on a B6 background and were maintained under specific pathogen–free conditions. Experiments were performed under the approval of the Institutional Animal Care and Use Committee in compliance with the Guidelines for Animal Experiments of the Biological Resource Centre (Agency for Science, Technology and Research).
To generate mixed bone marrow chimeras, CD45.1+ mice were irradiated twice (6 Gy) 4 h apart. Il2−/− or Il2+/+ and CD11c:DTa creneg or crepos bone marrow cells were mixed and injected i.v. into irradiated recipients to achieve 1:1 reconstitution. Chimeric mice were allowed to recover for 2 mo after reconstitution before being used for experiments.
Immunizations
Mice were immunized i.p. with endotoxin-free EndoGrade Ovalbumin protein (10 μg/mouse; Hyglos) or OVA–Alexa Fluor 647 (AF647; Life Technologies) mixed with 1 mg of Imject Alum (Thermo Scientific) in 500 μl of PBS. In adoptive-transfer experiments, CellTrace Violet (Life Technologies)–labeled OT-II CD4+ T cells were injected i.v. via the retro-orbital vein. Immunizations were performed 24 h after adoptive transfer, and proliferation was measured by dye dilution 3 and 7 d later. For OVA-AF647 uptake and DC activation, mediastinal lymph nodes were analyzed 24 h postimmunization. To analyze humoral responses, mice were boosted with OVA/alum 14 d after the priming immunization. Sera were analyzed at days 14, 21, and 28 (0, 7, and 14 d after boosting, respectively).
Cell differentiation and isolation
Bone marrow cells were cultured in GM-CSF–supplemented medium for 7–8 d to generate DCs. Alternatively, 10% L929-conditioned medium was used to derive macrophages. OT-II CD4+ T cells were isolated from spleen and lymph nodes, pushed through a cell strainer, and centrifuged on a Ficoll-Paque (GE Healthcare) density gradient at 600 × g for 20 min at room temperature. Cells at the interface were incubated with anti-CD4 MACS beads (Miltenyi Biotec) and isolated by positive selection. Purified OT-II CD4+ T cells were labeled with CellTrace Violet, according to the manufacturer’s instructions.
Cell stimulation
DCs and macrophages were cultured in 96-well plates at a density of 1 × 105 cells. Cells were primed with ultrapure LPS from Escherichia coli serotype 055:B5 (100 ng/ml; Alexis, Enzo Life Sciences). Primed cells were activated overnight with 250 μg/ml particulates: aluminum hydroxide/magnesium hydroxide Imject Alum (Thermo Scientific), aluminum phosphate Adju-Phos (Brenntag), or SiO2 (Sigma-Aldrich) or monosodium urate (MSU) crystals prepared as described (22). In some experiments, particulates were titrated, as described in the respective figures. Particulates were confirmed to be free from endotoxin contamination using a Limulus amebocyte lysate test. Recombinant IL-1R antagonist (anakinra; Amgen) was used at 750 μg/ml. The following small molecule inhibitors were used: cytochalasin D, PP2 Src inhibitor (Sigma-Aldrich), Syk inhibitor II CAS 227449-73-2 (Calbiochem), cyclosporin A, and FK506 (both from Cell Signaling Technology). EGTA was from Sigma-Aldrich.
Flow cytometry
The following Ab clones from BioLegend and BD Biosciences were used: anti-CD3ε (145-2C11), anti-CD4 (RM4-5), anti-CD8a (53-6.7), anti-CD11c (N418), anti-CD19 (6D5), anti-CD40 (1C10), anti-CD45.1 (A20), anti-CD45.2 (104), anti-CD80 (16-10A1), anti-CD86 (GL-1), anti–I-A/I-E (M5/114.15.2), and anti-TCR Vβ5.1/5.2 (MR9-4). Events were acquired using a BD Fortessa (BD Biosciences). Data were analyzed using FlowJo (TreeStar). In CellTrace Violet–dilution experiments, the Division Index is the average number of cell divisions that a cell in the original population has undergone, including the peak of undivided cells.
ELISA
Cytokine levels in cell-free supernatants were measured using the following kits: OptEIA IL-2 (BD Pharmingen), TNF-α ELISA MAX (BioLegend), and IL-1β DuoSet (R&D Systems). To detect OVA-specific IgM, IgG2b, and IgG1, MaxiSorp plates (Nunc) were coated overnight with 50 μl of 10 μg/ml OVA before mouse sera (diluted 1:100 for IgM and IgG2b, 1:1000 for IgG1) were added and incubated for 2 h at room temperature. After washing, HRP-conjugated goat anti-mouse isotype detection Ab (Southern Biotech) was added and incubated for 1 h at 1:500 dilution, followed by colorimetric development using 3,3′,5,5′-tetramethylbenzidine (Sigma-Aldrich). For OVA-specific IgE, plates were coated with 50 μl of 2 μg/ml anti-mouse IgE capture Ab (LO-ME-3; Invitrogen), and mouse sera diluted at 1:100 were added and incubated for 2 h at room temperature. Biotin-conjugated OVA was added at 3 μg/ml and incubated for 1 h, followed by HRP-conjugated streptavidin before colorimetric development using 3,3′,5,5′-tetramethylbenzidine. All sera stored at −20°C were tested simultaneously to allow comparison of OD. OD values for preimmunization (day 0) were set as background and subtracted from the OD at each time point for each individual mouse.
Statistical analysis
Statistical significance was assessed using unpaired two-tailed t tests. Data were analyzed using Prism 7 (GraphPad).
Results
Alum and other sterile particulates trigger IL-2 release from DCs
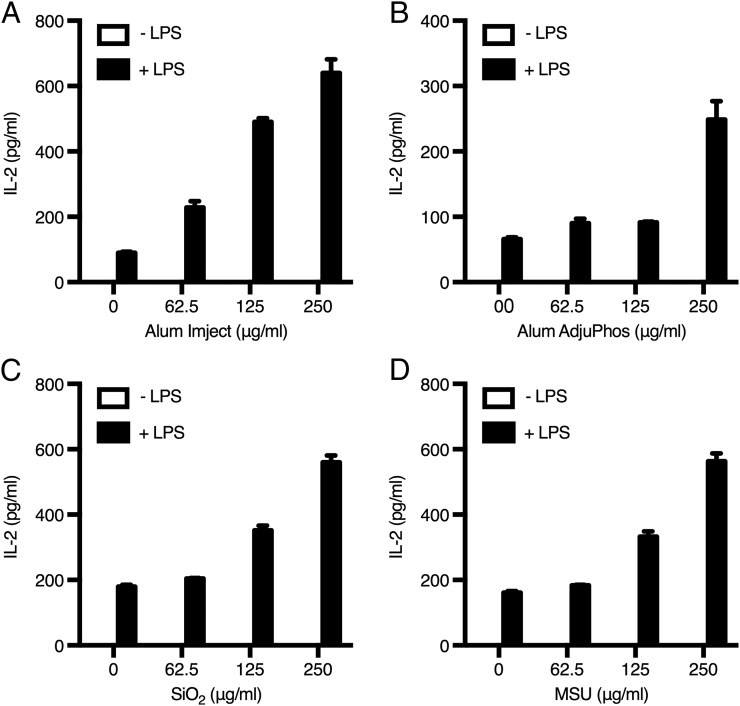
We observed that DCs exposed to either of two commercial forms of alum produced IL-2 in vitro (Fig. 1A, 1B). Priming of DCs with LPS was needed for alum-triggered IL-2 release, in striking similarity to the two-signal requirement for inflammasome activation driven by alum and other particulates (9, 23). LPS alone induced low levels of IL-2 production from DCs, as already reported (16), but alum boosted IL-2 production 3–6-fold (Fig. 1A, 1B). Alum is not the only sterile particulate endowed with biological activity; SiO2 and MSU crystals are able to activate primed APCs by triggering the NLRP3 pathway (22, 24). Therefore, we asked whether these particulates also induced IL-2 production by DCs; similar to alum, SiO2 and MSU dose dependently elicited IL-2 release from primed DCs (Fig. 1C, 1D). The finding that MSU also induces DC-derived IL-2 is of particular relevance, because endogenous uric acid released upon alum injection is known to contribute to its adjuvant effects (2). Moreover, other stimuli, such as CpG, could replace LPS in priming of DCs (Supplemental Fig. 1), suggesting the existence of a common downstream mediator of danger signaling that is involved in priming.
FIGURE 1.
Sterile particulates elicit IL-2 release from DCs. DCs were stimulated with Imject alum (A), Adju-Phos alum (B), SiO2 (C), or MSU crystals (D), with or without LPS (100 ng/ml) priming. Cytokine levels were measured in supernatants by ELISA. A representative experiment of four is shown. Error bars represent SD.
Although sterile particulates are able to elicit inflammasome activation in DCs and macrophages (25), stimulation of IL-2 release is a distinctive feature of professional APCs, such as DCs. Indeed, macrophages were unable to produce IL-2 in response to alum or MSU, even upon priming, while retaining the ability to secrete the inflammatory cytokine TNF-α (Supplemental Fig. 2).
The induction of DC-derived IL-2 by particulates is independent of the NLRP3 inflammasome
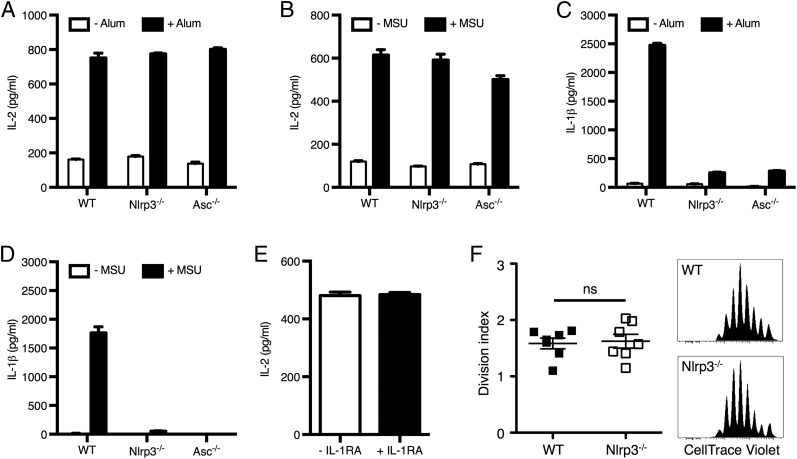
Because all of the sterile particulates tested are well-described activators of the NLRP3 inflammasome, we examined whether the ability of particulates to trigger DC production of IL-2 was dependent on the NLRP3 inflammasome. Interestingly, LPS-primed DCs from wild-type (WT) mice and mice lacking Nlrp3 or Asc (an adaptor protein used in multiple inflammasome subtypes) released comparable amounts of IL-2 in response to alum or MSU (Fig. 2A, 2B). However, as expected, IL-1β release was severely compromised in the culture supernatants of Nlrp3−/− and Asc−/− DCs relative to WT (Fig. 2C, 2D). Furthermore, the neutralization of autocrine IL-1α/β with IL-1RA (anakinra) did not hinder IL-2 production by stimulated WT DCs (Fig. 2E). Finally, Ag-specific CD4+ T cells proliferated normally in alum-immunized Nlrp3−/− recipients (Fig. 2F). Therefore the two pathways elicited by alum and MSU leading to IL-1β and IL-2 release operate independently in DCs.
FIGURE 2.
Particulates trigger DC-derived IL-2 release independent of the NLRP3 inflammasome. WT, Nlrp3−/−, and Asc−/− DCs were primed with LPS (100 ng/ml) and stimulated with alum (A and C) or MSU (B and D). Levels of secreted IL-2 and IL-1β were measured in supernatants. (E) WT DCs were stimulated with alum in the presence or absence of IL-1RA. (F) Proliferation of CellTrace Violet–labeled OT-II CD4+ T cells adoptively transferred into WT or Nlrp3−/− mice immunized with OVA and alum. Representative graphs of CellTrace Violet dilution and consolidated division indexes in mediastinal lymph nodes are shown. (A–E) Graphs representative of three independent experiments are shown. Error bars represent SD. (F) n = 7 mice from three independent experiments. Error bars represent SEM. Statistical significance was assessed using an unpaired two-tailed t test. ns, not significant.
IL-2 release from DCs triggered by sterile particulates is dependent on actin polymerization, Syk and Src kinases, calcium mobilization, and NFAT activation
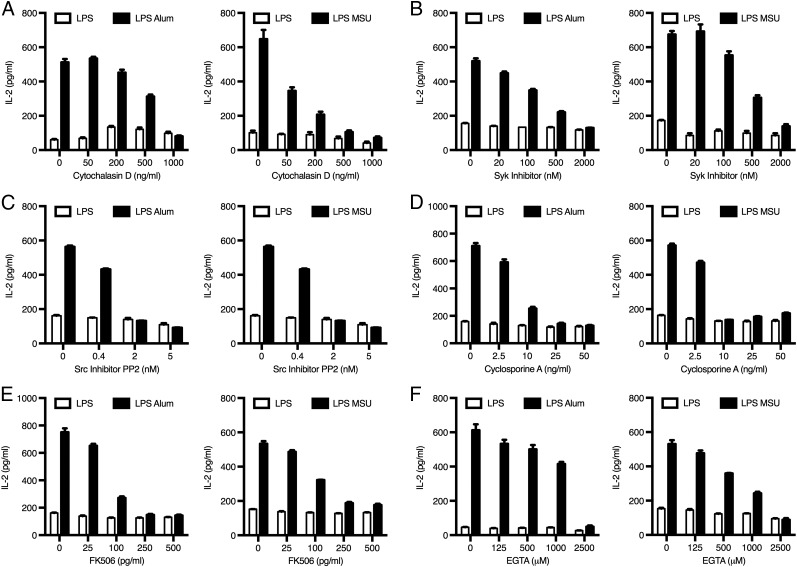
Next, we determined the mechanisms contributing to the ability of particulates to trigger DC production of IL-2. To do this, we first primed DCs with LPS for 30 min, after which inhibitors were added to the culture medium, followed by alum or MSU crystals 10 min later. Such a sequence minimized the likelihood of the inhibitors interfering with DC priming and instead focused on their effects on particulate-induced IL-2 release. This strategy was successful because the levels of IL-2 release induced by LPS alone were unaffected by inhibitor treatment (Fig. 3). We also confirmed that the inhibitors were not causing increased cell death in DC cultures (data not shown).
FIGURE 3.
Particulate-triggered IL-2 release depends on actin polymerization, Syk and Src kinases, and NFAT activation. DCs were primed with LPS (100 ng/ml) for 30 min prior to addition of inhibitors: cytochalasin D (A), Syk inhibitor (B), Src inhibitor PP2 (C), cyclosporin A (D), FK506 (E), and EGTA (F) or vehicle alone. Ten minutes later, cells were stimulated with alum or MSU. IL-2 levels were measured in supernatants by ELISA. All graphs shown are representative of at least three independent experiments. Error bars represent SD.
We observed that inhibition of actin polymerization by cytochalasin D dose dependently reduced particulate-triggered IL-2 release by DCs (Fig. 3A). This finding suggests that phagocytosis or, more likely, binding/retention of particles at the plasma membrane (attempted/frustrated phagocytosis) (3) is necessary for IL-2 release. Because alum and MSU activate Syk in DCs by interacting with cell surface lipid rafts in a receptor-independent manner (3, 26), we next asked whether particulate activation of Syk was necessary for IL-2 release. That was indeed the case, because increasing concentrations of Syk inhibitor markedly reduced IL-2 levels in the culture medium (Fig. 3B). Furthermore, Ab-mediated cross-linking of CD16 or TREM1, which signal through Syk (27), also induced Syk-dependent IL-2 release (Supplemental Fig. 3). Thus, Syk is required for IL-2 secretion in response to fungal β-glucans (14, 15), as well as sterile insults. Src kinases cooperate with Syk to regulate signal transduction at the “phagocytic synapse” (28); accordingly, using the Src inhibitor PP2, we found that Src kinases were also essential for particulate-triggered IL-2 release by DCs (Fig. 3C).
The Syk pathway ultimately leads to calcium mobilization, which, in turn, could trigger calcineurin-dependent activation of NFAT. NFAT was linked to IL-2 release upon microbial challenge (15, 27, 29, 30). Therefore, we asked whether NFAT also regulates IL-2 secretion in response to sterile stimuli, such as alum. Using the calcineurin inhibitors cyclosporin A and FK506 (tacrolimus) to block NFAT translocation to the nucleus, we observed a dose-dependent reduction in IL-2 release (Fig. 3D, 3E). A similar inhibition of particulate-mediated IL-2 secretion was observed in the presence of the calcium-chelating agent EGTA (Fig. 3F), in line with the Ca2+-dependent activation of the calcineurin–NFAT pathway.
Taken together, our findings identify the Syk/Src–Ca2+–NFAT pathway as the master regulator of IL-2 production in DCs upon exposure to sterile particulates.
DC-derived IL-2 is necessary for optimal induction of Ag-specific CD4+ T cell expansion in vivo upon alum immunization
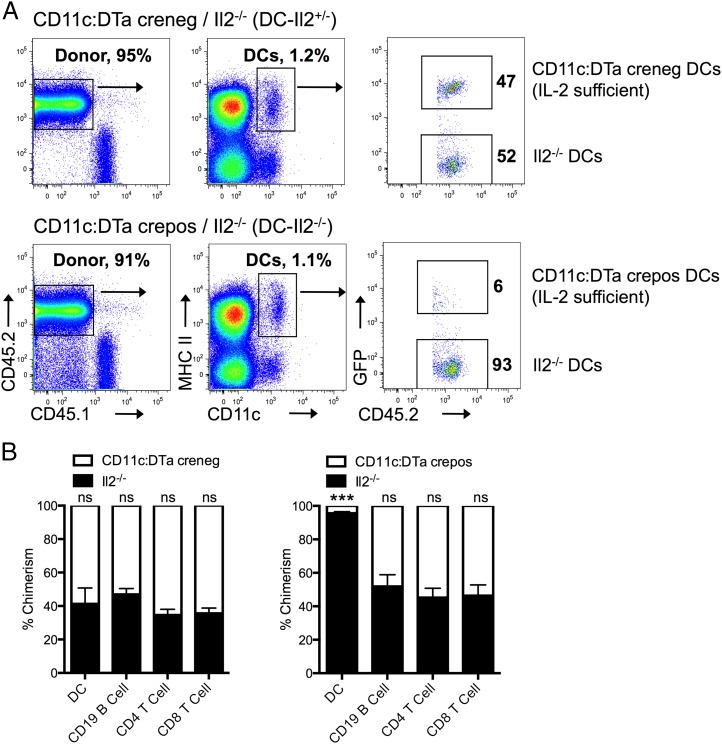
Having demonstrated that particulates induce DC secretion of IL-2 in vitro, we then asked whether in vivo DC production of IL-2 is important for arming adaptive immunity upon alum immunization. We generated a mouse conditionally deficient for IL-2 in DCs by adopting a widely used mixed bone marrow chimera model (31–33). Congenic CD45.1+ mice were lethally irradiated, and their bone marrow was reconstituted with a mixture of CD45.2+GFP+ CD11c:DTa and CD45.2+ Il2−/− bone marrow cells. This setup allows the cell populations (donor versus recipient and IL-2 sufficient versus IL-2 deficient) to be distinguished based on CD45.1, CD45.2, and GFP expression (Fig. 4A). Because DCs generated by CD11c:DTa crepos bone marrow cells are constitutively deleted by diphtheria toxin production prompted by Cre recombinase expression, only Il2−/− DCs populate tissues (Fig. 4B). Hereafter, we refer to CD11c:DTa crepos Il2−/− animals as DC-Il2−/−. As controls, irradiated CD45.1+ mice were reconstituted with donor cells from Il2−/− mice and CD11c:DTa littermates negative for Cre (DC-Il2+/−) or bone marrow cells from Il2+/+ mice and CD11c:DTa littermates positive for Cre (DC-Il2+/+). In these mice, the DC compartment consisted of approximately equal ratios of IL-2–sufficient CD11c:DTa creneg (not expressing diphtheria toxin) and IL-2–deficient cells (Fig. 4B). Moreover, the chimerism of the B and T cell compartments was unaffected in DC-Il2−/− mice compared with DC-Il2+/− mice, illustrating the specificity of DC targeting using this strategy (Fig. 4B).
FIGURE 4.
Generation of CD11c:DTa/Il2−/− mixed bone marrow chimeras to conditionally delete IL-2 in DCs. (A) Representative flow cytometry profile of splenic DCs in chimeric mice. CD45.1 mice were lethally irradiated and reconstituted with a mixture of Il2−/− (GFP−) and CD11c:DTa (GFP+) creneg or crepos bone marrow cells, referred to as DC-Il2+/− and DC-Il2−/−, respectively (see Materials and Methods and Results for details). All donor cells expressed CD45.2. (B) Comparison of chimerism of IL-2–sufficient CD11c:DTa versus Il2−/− cells in the spleens of creneg and crepos chimeras. Data are from at least eight mice per group pooled from three independent experiments. Error bars represent SEM. Statistical significance was assessed using an unpaired two-tailed t test. ***p < 0.001. ns, not significant.
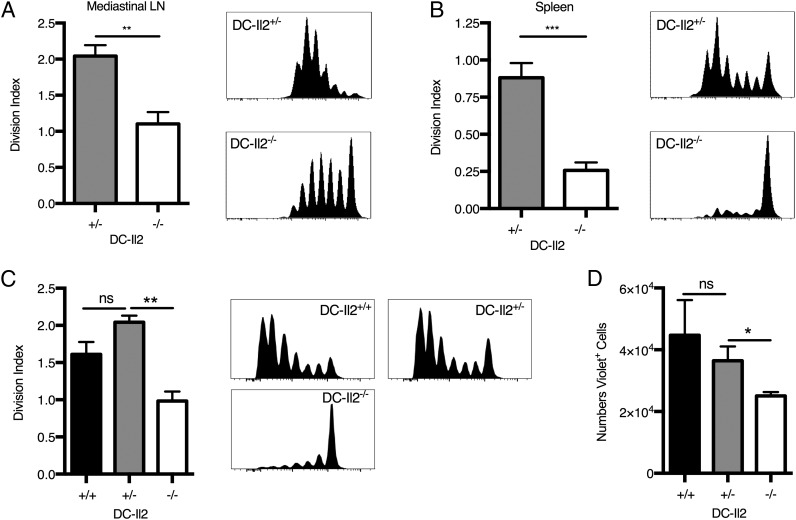
To investigate the effect of DC-derived IL-2 upon responses to alum immunization, we adoptively transferred CellTrace Violet–labeled OT-II CD4+ T cells into DC-Il2+/+, DC-Il2+/−, and DC-Il2−/− mice before immunization with alum-adjuvanted OVA. We observed a significant attenuation of Ag-specific proliferation of OT-II CD4+ T cells in the draining lymph nodes and spleens of DC-Il2−/− animals at 3 d (Fig. 5A, 5B) and 7 d (Fig. 5C) after immunization, whereas T cells expanded normally in the DC-Il2+/+ and DC-Il2+/− control groups (Fig 5A–C). Accordingly, significantly fewer OT-II CD4+ T cells were recovered from DC-Il2−/− spleens compared with DC-Il2+/+ and DC-Il2+/− controls (Fig. 5D). Interestingly, IL-2–mediated alum responses in vivo occurred in the absence of an experimentally applied priming stimulus, such as LPS, reminiscent of particulate-driven NLRP3 activation that requires priming in vitro but not in vivo (22, 34, 35).
FIGURE 5.
DC-derived IL-2 is required for optimal expansion of Ag-specific CD4+ T cells following alum-adjuvanted immunization. Proliferation of CellTrace Violet–labeled OT-II CD4+ T cells adoptively transferred into chimeric mice immunized with OVA (10 μg/mouse) and alum were measured 3 d (A and B) and 7 d (C) postimmunization. Representative graphs of CellTrace Violet dilution and consolidated division indexes in mediastinal lymph nodes (A) and spleens (B and C) are shown. (D) Absolute numbers of CellTrace Violet+ OT-II CD4+ T cells 7 d postimmunization in spleen of Il2+/+, Il2+/−, and Il2−/− mice. (A and B) n = 6 or 7 mice per group from two independent experiments. (C and D) n = 3 mice per group. Error bars represent SEM. *p < 0.05, **p < 0.01, ***p < 0.001, unpaired two-tailed t test. ns, not significant.
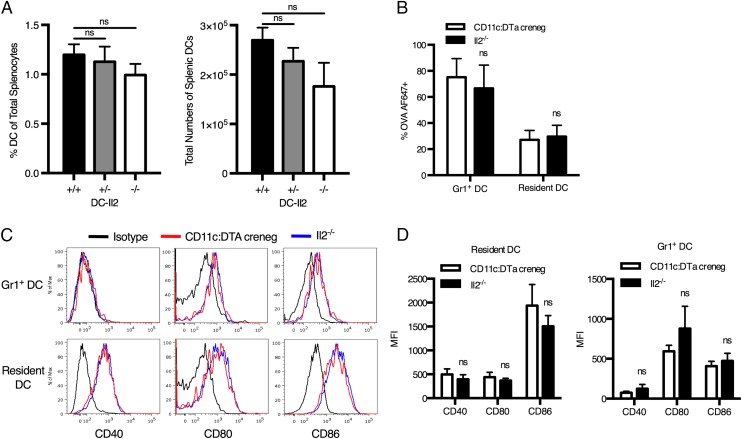
The observed reduction in CD4+ T cell proliferation was decisively due to the complete lack of DC-derived IL-2 in DC-Il2−/− mice: other than IL-2 deficiency, the Il2−/− DC populations were numerically and functionally indistinguishable from IL-2–sufficient (CD11c:DTa creneg) DCs. We did not observe any difference in the percentage or total number of DCs in the spleens of DC-Il2+/+, DC-Il2+/−, and DC-Il2−/− chimeras, indicating that DC deficiency of IL-2 does not impact the ability of Il2−/− bone marrow precursors to differentiate into DCs (Fig. 6A). We also examined the ability of IL-2–deficient DCs to take up the OVA Ag in vivo by injecting DC-Il2+/− mice i.p. with alum mixed with fluorescent OVA-AF647, followed by the measurement of OVA-AF647 uptake and levels of expression of costimulatory molecules of resident and recruited Gr-1+ DCs in the draining lymph nodes. We did not observe any difference in the ability of IL-2–deficient (Il2−/−) or IL-2–sufficient (CD11c:DTa creneg) resident or recruited DCs to acquire OVA-AF647 (Fig. 6B) or to upregulate the expression of CD40, CD80, and CD86 costimulatory molecules (Fig. 6C, 6D).
FIGURE 6.
DC populations from IL-2–sufficient and IL-2–deficient mice are numerically and functionally indistinguishable. (A) Percentages and numbers of DCs in spleens of DC-Il2+/+ (CD11c:DTa crepos/Il2+/+), DC-Il2+/− (CD11c:DTa creneg/Il2−/−), and DC-Il2−/− chimeras. (B) Uptake of OVA-AF647 by IL-2–sufficient CD11c:DTa creneg and IL-2–deficient DCs (Il2−/−) in the mediastinal lymph node of DC-Il2+/− mice 24 h after alum-adjuvanted Ag immunization. DCs were gated as CD11c+MHCII+Gr-1+ (recruited monocyte-derived DCs) and CD11c+MHCIIhiGr-1− (lymph node–resident DCs). (C and D) Median fluorescence intensity (MFI) of costimulatory molecule expression by IL-2–sufficient CD11c:DTa creneg and IL-2–deficient DCs 24 h after injection of alum-adjuvanted OVA-AF647. n = 6 mice from two independent experiments. Error bars represent SEM. Statistical significance was assessed using an unpaired two-tailed t test. ns, not significant.
Alum-driven humoral responses are impaired in the absence of DC-derived IL-2
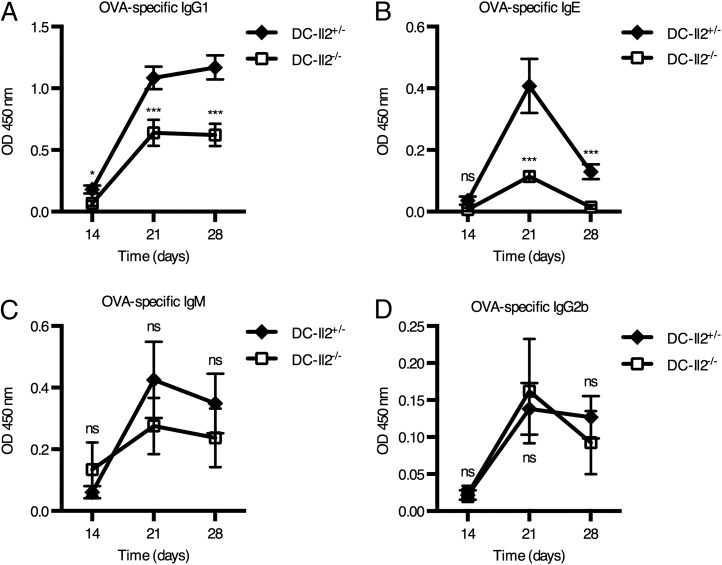
Having characterized the mechanism leading to IL-2 release from DCs stimulated with alum and established its relevance for T cell responses to Ags in vivo, we set out to understand whether humoral responses were also compromised when DC-derived IL-2 is lacking. To this aim, we compared the serum titers of OVA-specific Abs upon alum-adjuvanted prime/boost immunization of DC-Il2−/− and DC-Il2+/− mice. We observed significantly lower levels of serum OVA-specific IgG1 and IgE Abs in DC-Il2−/− mice compared with DC-Il2+/− controls from day 21 postimmunization (Fig. 7A, 7B), whereas the serum IgM and IgG2b levels remained unaffected by IL-2 deficiency (Fig. 7C, 7D). The pattern of Igs affected by the lack of DC-derived IL-2 suggests that this cytokine preferentially contributes to type 2 humoral responses to the OVA model Ag in vivo.
FIGURE 7.
DC-derived IL-2 contributes to efficient Ab production following alum immunization. DC-Il2−/− and DC-Il2+/− chimeric mice were primed/boosted with alum-adjuvanted OVA. Sera were collected just before boosting (day 14) and 1–2 wk postboosting (days 21 and 28). OD of ELISA measurements of OVA-specific IgG1 (A), IgE (B), IgM (C), and IgG2b (D) Abs upon preimmunization background subtraction are shown. n = 11 mice for DC-Il2+/− chimeras, n = 14 for DC-Il2−/− chimeras, from two independent experiments. Error bars represent SEM. *p < 0.05, ***p < 0.001, unpaired two-tailed t test. ns, not significant.
Taken together, our findings on T and B cell functions indicate that DC-derived IL-2 is required for the optimal induction of adaptive immunity in response to alum immunization in vivo.
Discussion
Charles Janeway famously nicknamed adjuvants “the immunologist’s dirty little secret”; although this secret has become less enigmatic in recent years thanks to a string of studies (2, 3, 5, 10, 12), it is still far from being exposed. Yet, it is obvious that the successful design of novel vaccination strategies demands a deeper understanding of the adjuvants delivered to hundreds of millions of people for almost a century, including alum.
In this study, we illuminate a novel mechanism underpinning alum’s function as a vaccine adjuvant. We provide evidence that sterile particulates, alum and uric acid crystals, instruct DCs to release IL-2 and that this IL-2 is necessary for the initiation and maintenance of optimal Ag-specific T and B cell responses following alum-adjuvanted immunization.
Mechanistically, DC-derived IL-2 release requires phagocytosis of alum particles, which activates Src and Syk kinases, ultimately instigating Ca2+ flux and calcineurin-mediated NFAT-dependent IL-2 production. Although the role of the Syk–NFAT–IL-2 axis in DCs is known in antimicrobial (especially antifungal) immunity (21), to our knowledge this is the first report showing its crucial involvement in responses to sterile insults. As such, a natural direction for future investigation will be to ascertain the role of DC-derived IL-2 in autoinflammatory diseases primarily triggered by nonmicrobial danger signals, such as gout, asbestosis, and silicosis.
Because immunization with alum results in the accumulation of uric acid at the site of injection (9), the question that arises is whether alum itself, the released uric acid, or both are responsible for the induction of DC IL-2 production. Data from the Lambrecht group showed that mice receiving the uric acid–degrading enzyme, uricase, together with alum-adjuvanted OVA exhibited attenuated priming of adoptively transferred CD4+ T cells and lower serum titers of OVA-specific Ab subclasses (2, 36). In our DC-Il2−/− mixed chimera mice, which lack DC-derived IL-2, we observed reduced priming of adaptive immunity. Because alum and MSU triggered IL-2 release from DCs in our in vitro experiments, it is likely that DC-derived IL-2 induction mediates direct (alum-mediated) and indirect (MSU-mediated) contributions to alum adjuvanticity.
The role of NLRP3 in mediating alum adjuvanticity has remained a controversial issue. Early studies showed that NLRP3 was important for the alum-stimulated adaptive immune response; however, several studies have since illustrated partial or complete independence from NLRP3 (9, 11, 12, 37). Our data support the paradigm that NLRP3 is dispensable for the adjuvant effect of alum because OT-II CD4+ T cells adoptively transferred into WT or Nlrp3-deficient mice proliferated to a similar extent after alum/OVA immunization. Consistently, DCs from WT, Nlrp3−/−, and Asc−/− mice exhibited comparable levels of IL-2 secretion in response to alum.
The Underhill group recently proposed the concept of the “phagocytic synapse,” based on the observation that only phagocytosis-triggering particulate β-glucans activate dectin-1 signaling, whereas their soluble counterpart does not (28, 38). Given that the dectin-1 pathway is the most potent inducer of IL-2 in DCs (21, 38, 39), it is tempting to speculate that IL-2 might be a hallmark cytokine of phagocytosis in DCs. This notion is in line with our data, given that sterile particulates, such as alum and MSU, are able to strongly enhance IL-2 release, unless actin polymerization is inhibited. Of note, we found that Syk is required for IL-2 production by DCs following alum/MSU stimulation, similar to dectin-1 activation (14, 21), suggesting that Syk is the master regulator of DC-derived IL-2. Consistent with this, we showed that Ab-mediated cross-linking of the Syk-signaling CD16 and Trem-1 receptors caused DCs to secrete abundant amounts of IL-2; moreover, CD16 and Trem-1 are well-known triggers of phagocytosis (40, 41), which supports the paradigm of IL-2 release downstream of phagocytic pathways. Finally, although LPS induces comparatively little IL-2 production by DCs (29), CD14, the LPS coreceptor, also activates Syk upon LPS recognition (42), possibly through plasma membrane lipid sorting driven by its GPI moiety (43–45), which would mirror lipid sorting by alum/MSU (3, 26).
Another unifying theme suggested by our work concerns the recognition of large crystals and particulates, such as alum and MSU, which invariably triggers a convergent pathway involving plasma membrane lipid sorting, Syk activation, and actin-mediated binding. Although downstream events may then diverge, leading to outcomes as varied as upregulation of costimulatory molecules (10, 26), inflammasome activation (46) and, as shown in this article, NFAT-dependent IL-2 production, the upstream mechanism is conserved.
Perhaps the most puzzling issue in the field of particulates is the nature of the priming signal in vivo, which is still unidentified. In vitro, sterile particulates only activate the NLRP3 inflammasome following priming of DCs, usually experimentally elicited with LPS (22). Priming is required to induce pro–IL-1β, as well as to upregulate expression of critical components of the inflammasome complex, thereby making cells responsive to activating stimuli, such as MSU or alum (34). However, patients affected by gout, a disease mediated by MSU-driven NLRP3 activation, do not receive any exogenous priming and yet inhibiting inflammasome-dependent IL-1 activity with the rIL-1R antagonist (anakinra) ameliorates their condition (22, 47, 48). Thus, in vivo priming must come from an endogenous sterile (danger) signal that recapitulates at least those properties of LPS that are needed to induce pro–IL-1β and essential inflammasome components. In our study, we observed a similar pattern for alum-triggered IL-2. In vitro, DCs required prior priming to be responsive to particulates; however, IL-2–dependent in vivo immunizations were performed without the addition of any priming agent. LPS contamination cannot explain our findings, not only because we confirmed that all of our reagents were endotoxin free but, most importantly, because mice doubly deficient in MyD88 and TRIF respond normally to alum vaccination (7). This observation also rules out any potential role for inflammasome-driven IL-1β or necrosis-linked IL-1α (49), both of which signal through MyD88 (50). It is likely that alum injection in vaccination settings or the MSU deposition seen in gout induce a variety of known and unknown danger signals that are able to prime DCs in vivo, enabling IL-2 release upon particulate binding. The danger signals priming DCs for inflammasome activation or IL-2 release in vivo do not necessarily need to be the same. We screened in vitro for potential priming activity of several sterile signals, including HMGB-1, TNF-α, and CD40L, but they were not able to recapitulate the properties of the elusive danger signal.
It is worth mentioning that human DCs enhance T cell priming by trans-presenting IL-2 via CD25 (51). This finding demonstrates that DC-derived IL-2 is an immune pathway shared by mouse and man, endowing our findings with appeal for clinical translation. Moreover, this example supports the notion that DC-derived IL-2 plays a nonredundant and distinct role in immune responses from T cell–derived IL-2, in line with our data. Therefore, despite the fact that “adaptive” IL-2 has been well characterized, much remains to be learned about “innate” IL-2.
Many novel non-TLR–based adjuvants have been developed as alternatives to alum, including MF59, AS03, and Montanide ISA 720. Therefore, it will be of interest to examine whether these novel adjuvants are likewise dependent on DC-derived IL-2 for efficacy and, if so, whether a common mechanism underlies the many adjuvant systems currently in development.
In conclusion, by showing an important role for DC-derived IL-2 in promoting the adaptive immune response to alum immunization, our results suggest that adjuvants targeting DCs to increase production of IL-2 might enhance vaccine efficacy. Because combinatorial adjuvant systems comprising alum and a TLR ligand, such as monophosphoryl lipid A or CpG, are already in development, we believe that optimizing these combinations to trigger the Syk pathway and, ultimately, IL-2 production might substantially improve vaccine efficacy.
Supplementary Material
Acknowledgments
We thank Lucia Mori, Maria Curotto de Lafaille, and Francesca Zolezzi for critical review and helpful discussions. We are indebted to Steffen Jung for sending CD11c:DTa cells for pilot experiments and to V.M. Dixit for providing Asc−/− mice. We thank Sabrina Nabti for assistance in mice breeding and husbandry, Anis Larbi and team from the Singapore Immunology Network Flow Cytometry core for assistance with cell sorting, and Lucy Robinson of Insight Editing London for manuscript editing.
This research was funded by a Singapore Immunology Network core grant (Agency for Science, Technology and Research, Singapore).
The online version of this article contains supplemental material.
- AF647
- Alexa Fluor 647
- alum
- aluminum salt
- DC
- dendritic cell
- MSU
- monosodium urate
- OT-II
- OT-II–Rag1−/−
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Wen Y., Shi Y. 2016. Alum: an old dog with new tricks. Emerg. Microbes Infect. 5: e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kool M., Soullié T., van Nimwegen M., Willart M. A., Muskens F., Jung S., Hoogsteden H. C., Hammad H., Lambrecht B. N. 2008. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 205: 869–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flach T. L., Ng G., Hari A., Desrosiers M. D., Zhang P., Ward S. M., Seamone M. E., Vilaysane A., Mucsi A. D., Fong Y., et al. 2011. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat. Med. 17: 479–487. [DOI] [PubMed] [Google Scholar]
- 4.Marichal T., Ohata K., Bedoret D., Mesnil C., Sabatel C., Kobiyama K., Lekeux P., Coban C., Akira S., Ishii K. J., et al. 2011. DNA released from dying host cells mediates aluminum adjuvant activity. Nat. Med. 17: 996–1002. [DOI] [PubMed] [Google Scholar]
- 5.McKee A. S., Burchill M. A., Munks M. W., Jin L., Kappler J. W., Friedman R. S., Jacobelli J., Marrack P. 2013. Host DNA released in response to aluminum adjuvant enhances MHC class II-mediated antigen presentation and prolongs CD4 T-cell interactions with dendritic cells. Proc. Natl. Acad. Sci. USA 110: E1122–E1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noges L. E., White J., Cambier J. C., Kappler J. W., Marrack P. 2016. Contamination of DNase preparations confounds analysis of the role of DNA in alum-adjuvanted vaccines. J. Immunol. 197: 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavin A. L., Hoebe K., Duong B., Ota T., Martin C., Beutler B., Nemazee D. 2006. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science 314: 1936–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKee A. S., Munks M. W., MacLeod M. K., Fleenor C. J., Van Rooijen N., Kappler J. W., Marrack P. 2009. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J. Immunol. 183: 4403–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kool M., Pétrilli V., De Smedt T., Rolaz A., Hammad H., van Nimwegen M., Bergen I. M., Castillo R., Lambrecht B. N., Tschopp J. 2008. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J. Immunol. 181: 3755–3759. [DOI] [PubMed] [Google Scholar]
- 10.Li H., Willingham S. B., Ting J. P., Re F. 2008. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J. Immunol. 181: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenbarth S. C., Colegio O. R., O’Connor W., Sutterwala F. S., Flavell R. A. 2008. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453: 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franchi L., Núñez G. 2008. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur. J. Immunol. 38: 2085–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen I. C., Jania C. M., Wilson J. E., Tekeppe E. M., Hua X., Brickey W. J., Kwan M., Koller B. H., Tilley S. L., Ting J. P. 2012. Analysis of NLRP3 in the development of allergic airway disease in mice. J. Immunol. 188: 2884–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers N. C., Slack E. C., Edwards A. D., Nolte M. A., Schulz O., Schweighoffer E., Williams D. L., Gordon S., Tybulewicz V. L., Brown G. D., Reis e Sousa C. 2005. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22: 507–517. [DOI] [PubMed] [Google Scholar]
- 15.Goodridge H. S., Simmons R. M., Underhill D. M. 2007. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J. Immunol. 178: 3107–3115. [DOI] [PubMed] [Google Scholar]
- 16.Granucci F., Vizzardelli C., Pavelka N., Feau S., Persico M., Virzi E., Rescigno M., Moro G., Ricciardi-Castagnoli P. 2001. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat. Immunol. 2: 882–888. [DOI] [PubMed] [Google Scholar]
- 17.Feau S., Facchinetti V., Granucci F., Citterio S., Jarrossay D., Seresini S., Protti M. P., Lanzavecchia A., Ricciardi-Castagnoli P. 2005. Dendritic cell–derived IL-2 production is regulated by IL-15 in humans and in mice. Blood 105: 697–702. [DOI] [PubMed] [Google Scholar]
- 18.Granucci F., Feau S., Angeli V., Trottein F., Ricciardi-Castagnoli P. 2003. Early IL-2 production by mouse dendritic cells is the result of microbial-induced priming. J. Immunol. 170: 5075–5081. [DOI] [PubMed] [Google Scholar]
- 19.Sauma D., Michea P., Lennon-Duménil A. M., Fierro A., Morales J., Rosemblatt M., Bono M. R. 2004. Interleukin-4 selectively inhibits interleukin-2 secretion by lipopolysaccharide-activated dendritic cells. Scand. J. Immunol. 59: 183–189. [DOI] [PubMed] [Google Scholar]
- 20.Schartz N. E., Chaput N., Taieb J., Bonnaventure P., Trébeden-Nègre H., Terme M., Ménard C., Lebbé C., Schimpl A., Ardouin P., Zitvogel L. 2005. IL-2 production by dendritic cells is not critical for the activation of cognate and innate effectors in draining lymph nodes. Eur. J. Immunol. 35: 2840–2850. [DOI] [PubMed] [Google Scholar]
- 21.Zelante T., Wong A. Y., Ping T. J., Chen J., Sumatoh H. R., Vigano E., Hong Bing Y., Lee B., Zolezzi F., Fric J., et al. 2015. CD103 dendritic cells control Th17 cell function in the lung. Cell Rep. 12: 1789–1801. [DOI] [PubMed] [Google Scholar]
- 22.Martinon F., Pétrilli V., Mayor A., Tardivel A., Tschopp J. 2006. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241. [DOI] [PubMed] [Google Scholar]
- 23.Davis B. K., Wen H., Ting J. P. 2011. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 29: 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yazdi A. S., Guarda G., Riteau N., Drexler S. K., Tardivel A., Couillin I., Tschopp J. 2010. Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1α and IL-1β. Proc. Natl. Acad. Sci. USA 107: 19449–19454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guarda G., Braun M., Staehli F., Tardivel A., Mattmann C., Förster I., Farlik M., Decker T., Du Pasquier R. A., Romero P., Tschopp J. 2011. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34: 213–223. [DOI] [PubMed] [Google Scholar]
- 26.Ng G., Sharma K., Ward S. M., Desrosiers M. D., Stephens L. A., Schoel W. M., Li T., Lowell C. A., Ling C. C., Amrein M. W., Shi Y. 2008. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity 29: 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colonna M. 2007. All roads lead to CARD9. Nat. Immunol. 8: 554–555. [DOI] [PubMed] [Google Scholar]
- 28.Goodridge H. S., Reyes C. N., Becker C. A., Katsumoto T. R., Ma J., Wolf A. J., Bose N., Chan A. S., Magee A. S., Danielson M. E., et al. 2011. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature 472: 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanoni I., Ostuni R., Capuano G., Collini M., Caccia M., Ronchi A. E., Rocchetti M., Mingozzi F., Foti M., Chirico G., et al. 2009. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature 460: 264–268. [DOI] [PubMed] [Google Scholar]
- 30.Tassi I., Cella M., Castro I., Gilfillan S., Khan W. N., Colonna M. 2009. Requirement of phospholipase C-gamma2 (PLCgamma2) for Dectin-1-induced antigen presentation and induction of TH1/TH17 polarization. Eur. J. Immunol. 39: 1369–1378. [DOI] [PubMed] [Google Scholar]
- 31.Zanoni I., Spreafico R., Bodio C., Di Gioia M., Cigni C., Broggi A., Gorletta T., Caccia M., Chirico G., Sironi L., et al. 2013. IL-15 cis presentation is required for optimal NK cell activation in lipopolysaccharide-mediated inflammatory conditions. Cell Reports 4: 1235–1249. [DOI] [PubMed] [Google Scholar]
- 32.Hochweller K., Striegler J., Hämmerling G. J., Garbi N. 2008. A novel CD11c.DTR transgenic mouse for depletion of dendritic cells reveals their requirement for homeostatic proliferation of natural killer cells. Eur. J. Immunol. 38: 2776–2783. [DOI] [PubMed] [Google Scholar]
- 33.Mortier E., Woo T., Advincula R., Gozalo S., Ma A. 2008. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J. Exp. Med. 205: 1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauernfeind F. G., Horvath G., Stutz A., Alnemri E. S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B. G., Fitzgerald K. A., et al. 2009. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183: 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H., Ambade A., Re F. 2009. Cutting edge: necrosis activates the NLRP3 inflammasome. J. Immunol. 183: 1528–1532. [DOI] [PubMed] [Google Scholar]
- 36.Kool M., Willart M. A., van Nimwegen M., Bergen I., Pouliot P., Virchow J. C., Rogers N., Osorio F., Reis e Sousa C., Hammad H., Lambrecht B. N. 2011. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity 34: 527–540. [DOI] [PubMed] [Google Scholar]
- 37.Spreafico R., Ricciardi-Castagnoli P., Mortellaro A. 2010. The controversial relationship between NLRP3, alum, danger signals and the next-generation adjuvants. Eur. J. Immunol. 40: 638–642. [DOI] [PubMed] [Google Scholar]
- 38.Hernanz-Falcón P., Joffre O., Williams D. L., Reis e Sousa C. 2009. Internalization of Dectin-1 terminates induction of inflammatory responses. Eur. J. Immunol. 39: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LeibundGut-Landmann S., Gross O., Robinson M. J., Osorio F., Slack E. C., Tsoni S. V., Schweighoffer E., Tybulewicz V., Brown G. D., Ruland J., Reis e Sousa C. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8: 630–638. [DOI] [PubMed] [Google Scholar]
- 40.Radsak M. P., Salih H. R., Rammensee H. G., Schild H. 2004. Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses: differential regulation of activation and survival. J. Immunol. 172: 4956–4963. [DOI] [PubMed] [Google Scholar]
- 41.Fossati G., Moots R. J., Bucknall R. C., Edwards S. W. 2002. Differential role of neutrophil Fcgamma receptor IIIB (CD16) in phagocytosis, bacterial killing, and responses to immune complexes. Arthritis Rheum. 46: 1351–1361. [DOI] [PubMed] [Google Scholar]
- 42.Zanoni I., Ostuni R., Marek L. R., Barresi S., Barbalat R., Barton G. M., Granucci F., Kagan J. C. 2011. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147: 868–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pugin J., Kravchenko V. V., Lee J. D., Kline L., Ulevitch R. J., Tobias P. S. 1998. Cell activation mediated by glycosylphosphatidylinositol-anchored or transmembrane forms of CD14. Infect. Immun. 66: 1174–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki K. G., Fujiwara T. K., Edidin M., Kusumi A. 2007. Dynamic recruitment of phospholipase C gamma at transiently immobilized GPI-anchored receptor clusters induces IP3-Ca2+ signaling: single-molecule tracking study 2. J. Cell Biol. 177: 731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki K. G., Fujiwara T. K., Sanematsu F., Iino R., Edidin M., Kusumi A. 2007. GPI-anchored receptor clusters transiently recruit Lyn and G alpha for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1. J. Cell Biol. 177: 717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hara H., Tsuchiya K., Kawamura I., Fang R., Hernandez-Cuellar E., Shen Y., Mizuguchi J., Schweighoffer E., Tybulewicz V., Mitsuyama M. 2013. Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat. Immunol. 14: 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.So A., De Smedt T., Revaz S., Tschopp J. 2007. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res. Ther. 9: R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kono H., Chen C. J., Ontiveros F., Rock K. L. 2010. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J. Clin. Invest. 120: 1939–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C. J., Kono H., Golenbock D., Reed G., Akira S., Rock K. L. 2007. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 13: 851–856. [DOI] [PubMed] [Google Scholar]
- 50.Dinarello C. A. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27: 519–550. [DOI] [PubMed] [Google Scholar]
- 51.Wuest S. C., Edwan J. H., Martin J. F., Han S., Perry J. S., Cartagena C. M., Matsuura E., Maric D., Waldmann T. A., Bielekova B. 2011. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat. Med. 17: 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.