Abstract
Sucrose and starch are end products of two segregated gluconeogenic pathways, and their production takes place in the cytosol and chloroplast of green leaves, respectively. According to this view, the plastidial ADP·glucose (ADPG) pyrophosphorylase (AGP) is the sole enzyme catalyzing the synthesis of the starch precursor molecule ADPG. However, a growing body of evidences indicates that starch formation involves the import of cytosolic ADPG to the chloroplast. This evidence is consistent with the idea that synthesis of the ADPG linked to starch biosynthesis takes place in the cytosol by means of sucrose synthase, whereas AGP channels the glucose units derived from the starch breakdown. To test this hypothesis, we first investigated the subcellular localization of ADPG. Toward this end, we constructed transgenic potato plants that expressed the ADPG-cleaving adenosine diphosphate sugar pyrophosphatase (ASPP) from Escherichia coli either in the chloroplast or in the cytosol. Source leaves from plants expressing ASPP in the chloroplast exhibited reduced starch and normal ADPG content as compared with control plants. Most importantly however, leaves from plants expressing ASPP in the cytosol showed a large reduction of the levels of both ADPG and starch, whereas hexose phosphates increased as compared with control plants. No pleiotropic changes in photosynthetic parameters and maximum catalytic activities of enzymes closely linked to starch and sucrose metabolism could be detected in the leaves expressing ASPP in the cytosol. The overall results show that, essentially similar to cereal endosperms, most of the ADPG linked to starch biosynthesis in source leaves occurs in the cytosol.
Starch is the main storage carbohydrate in plants. Its abundance as a naturally occurring compound is surpassed only by cellulose, and it represents the most important carbohydrate in human nutrition. Because of its unique physicochemical characteristics, the use of this polyglucan as a renewable and biodegradable compound is becoming increasingly attractive in practically every industry in existence. Therefore, identification and understanding of all of the factors that are involved in the starch biosynthetic process is critically important for the rational design of experimental traits necessary to improve yields in agriculture, generate starches that might lead to new uses, and produce more elaborated polymers that fit industrial needs.
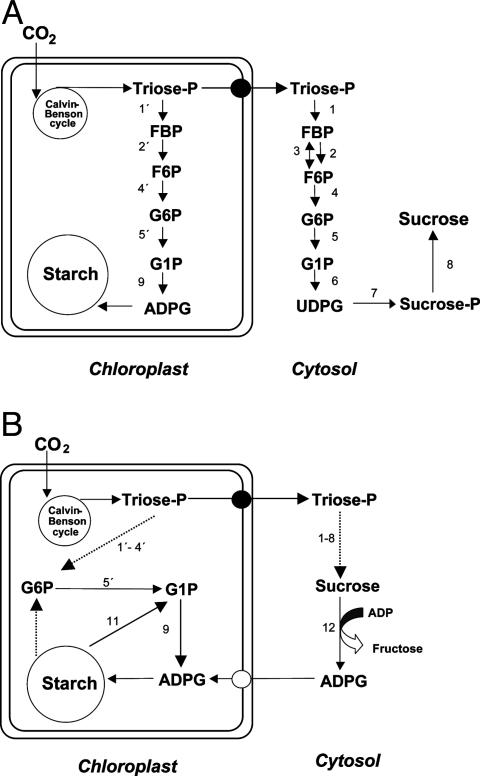
Starch occurring in photosynthetically competent cells is termed “transitory starch,” given the wide diurnal fluctuations of its levels. Since the initial demonstration that ADP·glucose (ADPG) serves as the universal substrate for starch synthase (SS) [International Union of Biochemistry and Microbiology (IUBMB) enzyme database accession no. EC 2.4.1.21] (1, 2), the starch biosynthetic process in green leaves generally has been considered to take place exclusively in the chloroplast and to be segregated from the sucrose biosynthetic process that takes place in the cytosol (Fig. 1A) (3-7). Starch is considered to be the end product of a unidirectional and vectorial pathway in which the chloroplastic ADPG pyrophosphorylase (AGP) (IUBMB accession no. EC 2.7.7.27) is the only enzyme catalyzing the synthesis of ADPG, and it acts as the major limiting step of the gluconeogenic process (8-10). This popular view, consistent with the idea that the chloroplast is a complete photosynthetic unit that can produce starch from the atmospheric CO2 (11, 12), appears to be supported by a wide variety of mutant and transgenic lines in which enzymes and transport machineries have been modulated (13-17). However, there is a growing volume of evidence that points out inconsistencies with such mechanism and previews the likelihood of alternative gluconeogenic pathway(s) in which the ADPG linked to starch biosynthesis is produced in the cytosol (18-20).
Fig. 1.
Suggested pathways of starch synthesis in source leaves. (A) “Classic model” according to which the starch biosynthetic process takes place exclusively in the chloroplast, segregated from the sucrose biosynthetic process taking place in the cytosol. (B) The alternative model in which both sucrose and starch biosynthetic pathways are interconnected by means of the ADPG-synthesizing activity catalyzed by SuSy. In A, starch is shown to be the end product of a unidirectional pathway, whereas B shows starch as an intermediate component of a cyclic gluconeogenic pathway. The enzyme activities that are involved are numbered as follows: 1 and 1′, fructose-1,6-bisphosphate aldolase; 2 and 2′, fructose 1,6-bisphosphatase; 3, PPi:fructose-6-phosphate phosphotransferase; 4 and 4′, phosphoglucoisomerase; 5 and 5′, phosphoglucomutase; 6, UGP; 7, SPS; 8, sucrose phosphate phosphatase; 9, AGP; 10, SS; 11, SP; 12, SuSy.
While investigating the mechanism(s) of adenylate entry into plastids, Pozueta-Romero et al. (20) found that chloroplasts are capable of importing ADPG. Although its physiological relevance was questioned initially (9), this finding carried the inherent supposition that a sizable pool of the ADPG linked to starch biosynthesis in leaves occurs in the cytosol. In this respect, and based on the capacity of cytosolic sucrose synthase (SuSy) (IUBMB accession no. EC 2.4.1.13) to produce ADPG from sucrose and ADP (21-25), an “alternative model” of starch biosynthesis was proposed in which SuSy catalyzes the de novo production of ADPG, which is subsequently imported into the stromal phase of the chloroplast (Fig. 1B) (18, 19). Essentially similar to the case of other gluconeogenic processes occurring in organelles, such as the Golgi cisternae, in which glucose from cytosolic UDP·glucose (UDPG) entering the lumen is transferred rapidly to endogenous acceptors (26), the alternative model of starch biosynthesis assumes that the imported ADPG is coupled rapidly with SS (18).
Pulse-chase and starch-preloading experiments using isolated chloroplasts (27, 28), intact leaves (29), or cultured photosynthetic cells (30) have provided evidence that chloroplasts can synthesize and mobilize starch simultaneously. This evidence is essentially in agreement with previous reports dealing with gluconeogenic processes in bacteria (31, 32), animals (33, 34), and heterotrophic plant cells (35, 36). Accordingly, the newly proposed mechanism of starch biosynthesis, which is compatible with most, if not all, starch-deficient and excess mutant and transgenic plant described to date, assumes that gluconeogenic enzymes, such as plastid phosphoglucomutase and AGP, play a role in synthesizing ADPG from the glucose units that are derived from the starch breakdown (Fig. 1B) (18, 19). Under circumstances in which plastid phosphoglucomutase and AGP are blocked, the recovery toward starch biosynthesis of the hexose units that are derived from starch breakdown will also be blocked, accompanying a parallel decline of starch accumulation (18, 19).
Transgenic plants expressing microbial enzymes have been used successfully in investigating the mechanisms controlling the partitioning and allocation of carbohydrates in higher plants (7, 37-40). In this respect, we recently found an enzyme, designated as adenosine diphosphate sugar pyrophosphatase (ASPP) (IUBMB accession no. EC 3.6.1.21), that catalyzes the hydrolytic breakdown of ADPG in Escherichia coli to produce AMP and glucose-1-phosphate (G1P) (41). Changes in ADPG hydrolytic activity resulted in altered bacterial glycogen content, strongly indicating that ASPP can be used as a powerful tool for plant metabolic engineering.
To determine the extent to which cytosolic ADPG is involved in transitory starch biosynthesis in leaves, we produced transgenic potato plants expressing ASPP either in the chloroplast or in the cytosol. The rationale behind this approach was that, if a sizable pool of the ADPG linked to starch biosynthesis occurs in the cytosol, cytosolic ADPG hydrolytic activities should lead to a concomitant reduction of both ADPG and starch contents.
The results presented in this work, which show reducing levels both of ADPG and starch in source leaves as a consequence of cytosolic ASPP expression, demonstrate that most of the ADPG linked to starch biosynthesis has a cytosolic localization. The results also demonstrate that fundamentally important mechanisms involved in the starch biosynthetic process continue to be discovered.
Materials and Methods
Plants, Bacterial Strains, and Media. The work was carried out by using WT untransformed potato plants (Solanum tuberosum L. cv Desirée) and plants transformed with either CaMV35S:cauliflower mosaic virus promoter (35S)-β-glucuronidase-intron (GUS-int) (42), 35S-ASPP-nopaline synthase (NOS) or 35S-ChlTPASPP-NOS constructs (see below). Plants were grown individually in pots at ambient CO2 (350 ppm) for 4-6 weeks in growth chambers under an 8 h/16 h light (300 μmol of photons per sec-1/m-2,20°C)/dark (20°C) regime. For biochemical analyses, fully expanded (fifth) source leaves were harvested after 7 h of illumination, immediately quenched in liquid nitrogen, and stored at -80°C for up to 2 months before use.
BL21(DE3) E. coli cells transformed with the pET-ASPP expression vector (41) were grown in LB medium. Agrobacterium tumefaciens cells (strain C58C1:GV2260) (43), transformed with either pCGN35S-ASPP-NOS, pCGN35S-ChlTPASPP-NOS, or 35S-GUS-int were grown in yeast extract broth medium (0.5% beef extract/0.1% yeast extract/0.5% peptone/0.5% sucrose/2 mM MgSO4) with the appropriate selection by using standard techniques (44).
Production of ASPP-Expressing Plants. For the production of plants expressing ASPP in the cytosol, the 640-bp NcoI-XbaI fragment of pASPP (41) was ligated with the corresponding restriction sites of p35S-NOS (5′-CaMV35S-nos-3′) (45) to produce p35S-ASPP-NOS (see Fig. 4, which is published as supporting information on the PNAS web site). This construct was digested successively with the EcoRI, T4 DNA polymerase, and HindIII. The fragment thus released was cloned into the pCGN1548 plant expression vector (46) that had been digested successively with the enzymes XbaI, T4 DNA polymerase, and HindIII to produce pCGN35S-ASPP-NOS.
For the production of plants expressing ASPP in the chloroplast, the nucleotide sequence encoding the chloroplast-transit peptide (ChlTP) of P541 (47) was amplified by PCR using the following primers: 5′-GTCGACAATGGAAACCCTTCTAAAGCCT-3′ (forward) and 5′-GCCATGGGTGCTAAATCAAGAAAGCTAC-3′ (reverse), as well as a bell pepper cDNA library. The resulting PCR product was cloned into pGEM-T Easy (Promega) to produce pGEM-ChlTP (see Fig. 5, which is published as supporting information on the PNAS web site). This construct was digested with SalI and NcoI, and the released fragment was cloned into the corresponding sites of pASPP to produce pChlTP-ASPP. This construct was digested successively with SalI, T4 DNA polymerase, and XbaI. The released fragment was cloned into p35SASPP-NOS that had been digested successively with NcoI, T4 DNA polymerase, and XbaI, giving rise to the p35S-ChlTPASPP-NOS. This construct was digested successively with HindIII, EcoRI, and T4-DNA polymerase, and the released fragment was cloned into pCGN1548 after being digested with HindIII and T4 DNA polymerase to produce pCGN35S-ChlTPASPP-NOS.
Transfer of the chimeric constructs into A. tumefaciens was carried out by electroporation. Subsequent transformation of potato plants was conducted as described by Rocha-Sosa et al. (48). Transgenic plants were selected on kanamycin-containing medium. The presence of the recombinant ASPP-encoding gene aspP was confirmed by Southern blot analyses using radiolabelled aspP as probe. A second screening of the transgenic lines was performed by both ASPP-activity and Western blot analyses using ASPP-specific antisera. Six independent lines per construct were selected and used for all subsequent analyses described in this article.
Chloroplast Isolation. Chloroplasts were prepared as described in Haake et al. (16). The final pellet was resuspended in 50 mM Tris, pH 7.8/5 mM MgCl2/1 mM EDTA/2 mM DTT/1% (vol/vol) Triton X-100.
Enzyme Assays. All enzymatic reactions were performed at 37°C. Harvested leaves were immediately freeze-clamped and ground to a fine powder in liquid nitrogen with a pestle and mortar. To assay enzyme activity, 1 g of the frozen powder was resuspended at 4°C in 5 ml of 100 mM Hepes, pH 7.5/2 mM EDTA/5 mM DTT. The suspension was then desalted and assayed for enzymatic activities. We checked that this procedure did not result in loss of enzymatic activity by comparing activity in extracts prepared from the frozen powder and extracts prepared by homogenizing fresh tissue in extraction medium. The following enzymes were assayed according to procedures described in the accompanying references: AGP (ref. 8, except 3 mM 3-phosphoglyceric acid was included in the reaction mixture), SuSy (25), acid invertase (IUBMB accession no. EC 3.2.1.26) (39), ASPP (41), UDPG pyrophosphorylase (UGP) (IUBMB accession no. EC 2.7.7.9) (49), and alkaline pyrophosphatase (APPase) (IUBMB accession no. EC 3.6.1.1) (50). Total SS (IUBMB accession no. EC 2.4.1.21), starch phosphorylase (SP) (IUBMB accession no. EC 2.4.1.1), and amylolytic activities were assayed as described by Sweetlove et al. (35). Measurements of sucrose-phosphate synthase (SPS) (IUBMB accession no. EC 2.3.1.14) were performed in the direction of sucrose-phosphate synthesis. The assay mixture contained 50 mM Hepes (pH 7.5), 5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 10 mM fructose-6-phosphate, and 10 mM UDPG. After 15 min at 37°C, the reactions were stopped by boiling the assay mixture for 30 sec, and sucrose phosphate was determined by HPLC with pulsed amperometric detection on a DX-500 system (Dionex) fitted to a Carbo-Pac PA1 column (250-mm long), as described by Baroja-Fernández et al. (25). We define 1 unit as the amount of enzyme that catalyzes the production of 1 μmol of product per min.
Determination of Soluble Sugars. Soluble sugars were extracted from fully expanded (fifth) leaves of 6-week-old plants essentially as described by Sweetlove et al. (35). Fully expanded leaves were harvested and immediately ground to a fine powder in liquid nitrogen with a pestle and mortar. We resuspended 0.5 g of the frozen powdered tissue in 0.4 ml of 1.4 M HClO4, left it at 4°C for 2 h, and centrifuged it at 10,000 × g for 5 min. The supernatant that we obtained was neutralized with K2CO3 and centrifuged at 10,000 × g. Sucrose, glucose, fructose, G1P, and glucose-6-phosphate (G6P) were determined by HPLC with pulsed amperometric detection on a DX-500 system (Dionex) (25). ADPG and UDPG were measured by HPLC on a system obtained from P. E. Waters and Associates (Kent, England) fitted with a Partisil-10-SAX column as described by Rodríguez-López et al. (51). We checked the effectiveness of the method of nucleotide-sugar extraction by adding known amounts of commercially available ADPG and UDPG to leaf samples (final concentration in the homogenate being 10, 30, and 50 μM). Recoveries for ADPG and UDPG were 98% and 97%, respectively. To further confirm that measurements of these nucleotide sugars were correct, ADPG and UDPG eluted from the Partisil-10-SAX column were enzymatically hydrolyzed with E. coli ASPP (41) and with human UDPG pyrophosphatase (IUBMB accession no. EC 3.6.1.45) (52) respectively.
Analytical Procedures. Protein content was determined by the Bradford method using an XL-100 prepared reagent (Bio-Rad). Starch was measured by using an amyloglucosydase-based test kit (Sigma). Chlorophyll was quantified according to the method of Wintermans and De Mots (53). Photosynthetic parameters of the first fully expanded (fifth) leaves attached to the plants were determined under growth conditions by means of an Lci portable photosynthesis system (ADC BioScientific, Hoddesdon, Herts, United Kingdom) in the same chamber where the leaves were grown.
Results and Discussion
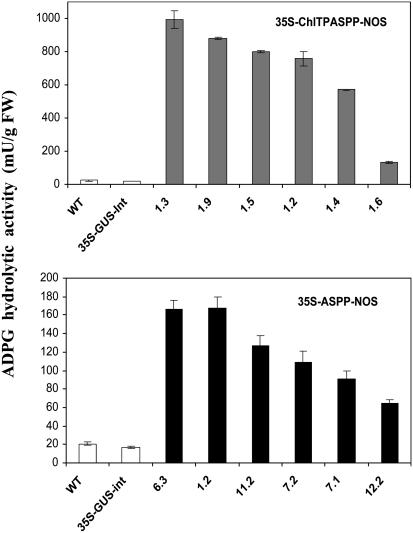
Production of ASPP-Expressing Plants. Potato plants were transformed with either 35S-ASPP-NOS or 35S-ChlTPASPP-NOS (see Materials and Methods) by means of Agrobacterium-mediated gene transfer. We then compared ADPG hydrolytic activities in leaves of ASPP-expressing plants with those of controls (both untransformed and 35S-GUS-int plants). As shown in Fig. 2, ADPG hydrolytic activities in each of the ASPP lines were exceedingly higher than those occurring in the control plants because of the combined action of various nucleotide-sugar hydrolases (54, 55). Western blot analyses using a polyclonal antibody against ASPP displayed a clear immunoreacting band at the expected size (≈26 kDa) in leaves of the ASPP lines, whereas it is not detectable in the control plants (see Fig. 6, which is published as supporting information on the PNAS web site). Thus, overall results show that elevated ADPG hydrolytic activities in both 35S-ASPP-NOS and 35S-ChlTPASPP-NOS plants were due to expression of bacterial ASPP.
Fig. 2.
ADPG hydrolytic activity in source leaves of control (WT and 35S-GUS-int) and ASPP plants (35S-ASPP-NOS and 35S-ChlTPASPP-NOS). Results are given as mean ± SEM of 10 independent plants per line. FW, fresh weight.
Subcellular Localization of ASPP in ASPP-Expressing Plants. Subcellular fractionation studies were performed on leaves of both 35S-ASPP-NOS and 35S-ChlTPASPP-NOS plants to investigate the subcellular localization of ASPP. Employing the centrifugation method of Haake et al. (16), chloroplast preparations were obtained from leaves of both 35S-ASPP-NOS and 35S-ChlTPASPP-NOS plants. As shown in Table 1, comparisons of enzyme activities in fractions obtained at the end of the preparation with those in the initial lysate as well as in the centrifugation step guaranteed no loss of activity during the preparation for any of the enzymes analyzed.
Table 1. Subcellular localization of ASPP in leaves of 35S-ChITPASPP-NOS and 35S-ASPP-NOS plants.
| Centrifugation
|
||||||
|---|---|---|---|---|---|---|
| Lysate
|
Supernatant
|
Chloroplast preparation
|
||||
| Enzyme | Activity, milliunits/mg protein | Activity, milliunits/mg protein | % of lysate | Activity, milliunits/mg protein | % of lysate | Recovery, % |
| 35S-ChITPASPP-NOS | ||||||
| ASPP | 177 ± 18 | 136 ± 11 | 77.0 ± 6.0 | 68 ± 4.7 | 38.5 ± 2.1 | 115 ± 6 |
| APPase | 132 ± 6.8 | 75 ± 5.0 | 56.8 ± 5.0 | 56 ± 4.4 | 42.4 ± 3.3 | 99 ± 4 |
| SPS | 336 ± 35 | 354 ± 16 | 105 ± 7.0 | 23 ± 1.9 | 7.0 ± 1.0 | 112 ± 6 |
| 35S-ASPP-NOS | ||||||
| ASPP | 25 ± 1.6 | 25.5 ± 1.4 | 102 ± 6.2 | 1.6 ± 0.1 | 6.6 ± 0.3 | 108 ± 6.2 |
| APPase | 139 ± 8.1 | 113 ± 7.4 | 82.1 ± 3.3 | 62 ± 2.7 | 45.6 ± 3.7 | 127 ± 3.9 |
| SPS | 497 ± 44 | 467 ± 42 | 94.9 ± 6.7 | 34 ± 3.3 | 6.8 ± 0.3 | 101 ± 6.7 |
Leaf homogenates were prepared and subjected to centrifugation as described by Haake et al. (16). Data are given as mean ± SEM of three independent experiments.
Judging by the activities of the plastid marker APPase, ≈40% of the chloroplasts originally present in the homogenates of 35S-ASPP-NOS and 35S-ChlTPASPP-NOS plants were recovered in the final chloroplast preparations (Table 1). The activities of the contaminating cytosolic marker SPS in the chloroplast preparations were found to be ≈7% of those occurring in the initial homogenates and remained in the supernatant after the centrifugation step.
As shown in Table 1, ≈40% of the ASPP activity originally present in the homogenates of 35S-ChlTPASPP-NOS leaves was found in the chloroplast preparation. With a confidence limit of 95%, there is no significant difference between the ASPP yields in the chloroplast preparations and those of the plastidial marker APPase. Further confirmed by immunocytochemical analyses using ASPP antisera (see Fig. 7, which is published as supporting information on the PNAS web site), these results showed that all ASPP in 35S-ChlTPASPP-NOS plants is associated with chloroplasts.
In clear contrast, and matching the pattern of the cytosolic marker SPS, most of ASPP remained in the supernatant after centrifugation of 35S-ASPP-NOS homogenates, whereas only negligible ASPP activity was found in the chloroplast preparations. These results showed that ASPP locates outside the chloroplast in the 35S-ASPP-NOS plants, presumably in the cytosol.
Extraplastidial ASPP Expression in Potato Leaves Leads to a Large Reduction of both ADPG and Transitory Starch Levels. Having confirmed that ASPP exclusively locates in the chloroplast of 35S-ChlTPASPP-NOS plants and locates outside the chloroplast in the 35S-ASPP-NOS plants, control (both untransformed and 35S-GUS-int plants) and ASPP-expressing plants were characterized for their ADPG and starch contents. Because, in our experience, biochemical analyses are subject to considerable variation, we analyzed 10 plants per line to obtain reliable data.
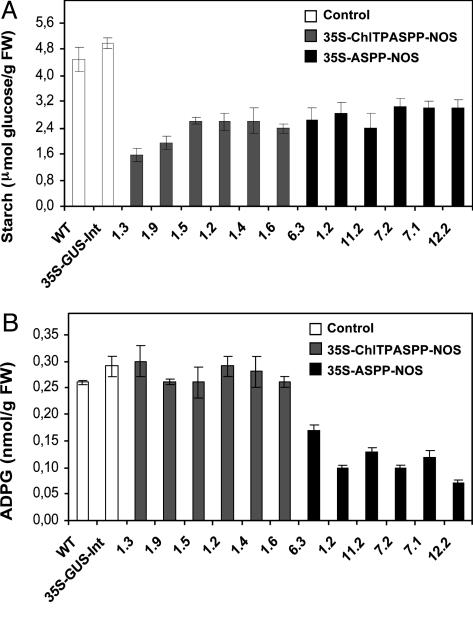
As shown in Fig. 3A, ASPP expression in the chloroplast led to 45-65% reduction of the starch content after 7 h of illumination. Intriguingly however, this reduction of the starch content was not accompanied by any measurable reduction of the intracellular ADPG content (Fig. 3B). Most significantly, 35S-ASPP-NOS plants showed a large reduction (up to 70%) of ADPG content (Fig. 3B), strongly indicating that most of ADPG has an extraplastidial localization. Furthermore, these plants also exhibited a ≈35-50% reduction of the starch content (Fig. 3A), which indicates that extraplastidial ADPG is linked directly to starch biosynthesis in source leaves.
Fig. 3.
ADPG and starch levels in fully expanded source (fifth) leaves from 6-week-old control and ASPP-expressing plants. Leaf samples were taken and quenched in liquid nitrogen at 7 h after the beginning of the light period. Results are given as mean ± SEM of 10 independent plants per line.
Starch levels were studied in both WT and 35S-ASPP-NOS plants throughout the photoperiod. Whereas transitory starch levels in WT leaves rose 5-fold between the start and the end of the light period, this increase was markedly lower in the 35S-ASPP-NOS leaves (see Fig. 8, which is published as supporting information on the PNAS web site). This reduction in the rate of transitory starch accumulation in the 35S-ASPP-NOS leaves was mirrored by a similar reduction in the rate of starch degradation during the night.
Large reduction of starch levels in plants expressing ASPP in the cytosol was not limited to leaves. Tubers from 6-month-old plants expressing ASPP in the cytosol were shown to accumulate less starch than tubers of WT plants (F.J.M., E.B.-F., N.A.-C., M.T.M.-Z., and J.P.-R., unpublished data), strongly indicating that most of the ADPG linked to starch biosynthesis in potato tubers has an extraplastidial localization.
Extraplastidial ASPP Expression Leads to a Significant Accumulation of Hexose Phosphates. Leaves from 35S-ChlTPASPP-NOS plants were shown to accumulate normal levels of sucrose, glucose, fructose, G1P, and G6P when compared with control plants (see Table 4, which is published as supporting information on the PNAS web site). In clear contrast, analyses of the 35S-ASPP-NOS leaves revealed contrasting and interesting results.
As shown in Table 2, leaves from both control and 35S-ASPP-NOS plants accumulated nearly identical amounts of sucrose, glucose and fructose. UDPG content in 35S-ASPP-NOS leaves was shown to be normal when compared with control plants. This observation is not surprising because ASPP does not cleave UDPG (41). Interestingly, G1P levels increased up to 2-fold when compared with control plants, which was likely to be a result of the hydrolytic breakdown of cytosolic ADPG catalyzed by ASPP. Furthermore, some transgenic lines were shown to have high levels of G6P. This observation is highly significant because increasing levels of hexose phosphates is characteristic of starch-deficient transgenic plants with altered cytosolic carbon metabolism (38-40).
Table 2. Metabolite levels in the source leaves of control and 35S-ASPP-NOS plants.
| Control
|
35S-ASPP-NOS
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Metabolite | WT | 35S-GUS-Int | 6.3 | 1.2 | 11.2 | 7.2 | 7.1 | 12.2 |
| Glucose | 952 ± 57 | 1,055 ± 64 | 927 ± 56 | 1,079 ± 67 | 996 ± 69 | 757 ± 52 | 908 ± 62 | 1006 ± 63 |
| Fructose | 986 ± 61 | 757 ± 49 | 871 ± 54 | 1,124 ± 68 | 883 ± 60 | 647 ± 46 | 1,041 ± 67 | 706 ± 49 |
| Sucrose | 1,154 ± 66 | 850 ± 59 | 1,141 ± 69 | 858 ± 34 | 1,156 ± 78 | 862 ± 61 | 1,014 ± 62 | 1,430 ± 92 |
| G6P | 245 ± 24 | 318 ± 24 | 397 ± 18* | 263 ± 28 | 367 ± 30* | 306 ± 32 | 400 ± 18* | 356 ± 7* |
| GIP | 23.5 ± 2.3 | 20.5 ± 0.8 | 44.6 ± 4.0* | 48.6 ± 1.8* | 36.9 ± 3.8* | 43.1 ± 1.9* | 35.6 ± 3.9* | 48.4 ± 3.8* |
| UDPG | 52.3 ± 4.5 | 47.7 ± 4.5 | 56.6 ± 7.7 | 53.7 ± 0.5 | 47.1 ± 5.9 | 49.9 ± 1.2 | 49.1 ± 1.0 | 51.9 ± 2.2 |
Leaf samples were taken from 6-week-old plants grown in chambers in ambient CO2 conditions at 20°C and an irradiance of 300 μmol of photons per sec−1/m−2. They were then quenched in liquid nitrogen 7 h after the beginning of the light period. The results are given as mean ± SEM of extracts from 10 independent plants per line. Metabolite levels are given as nmol/g fresh weight.
Values significantly different from the control plants.
Extraplastidial ASPP Expression Is Not Accompanied by Pleiotropic Changes in Enzymes Directly Connected to Starch and Sucrose Metabolism. We measured the maximum catalytic activities of a range of enzymes closely connected to starch and sucrose metabolism. As shown in Table 3, these analyses revealed no significant changes in AGP, APPase, UGP, SuSy, SPS, acid invertase, total SS, SP, and total amylolytic activity. Thus, the overall results indicate that the reduction of both ADPG and starch contents in 35S-ASPP-NOS plants is ascribable to the extraplastidial ADPG breakdown catalyzed by ASPP.
Table 3. Enzyme activities in the leaves of control and 35S-ASPP-NOS potato plants.
| Control
|
35S-ASPP-NOS
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Enzyme | WT | 35S-GUS-int | 6.3 | 1.2 | 11.2 | 7.2 | 7.1 | 12.2 |
| AGP | 130 ± 6 | 131 ± 7 | 120 ± 5 | 139 ± 6 | 137 ± 5 | 140 ± 7 | 119 ± 5 | 120 ± 6 |
| UGP | 102 ± 5 | 123 ± 6 | 100 ± 7 | 110 ± 5 | 106 ± 4 | 120 ± 5 | 120 ± 6 | 114 ± 5 |
| SuSy | 23 ± 2 | 18 ± 1 | 22 ± 3 | 23 ± 3 | 22 ± 3 | 21 ± 1 | 25 ± 4 | 20 ± 1 |
| Acid invertase | 138 ± 10 | 115 ± 9 | 140 ± 10 | 134 ± 9 | 101 ± 8 | 106 ± 6 | 101 ± 7 | 124 ± 9 |
| SPS | 2,820 ± 120 | 3,440 ± 230 | 3,200 ± 190 | 3,600 ± 210 | 4,400 ± 220 | 3,500 ± 310 | 3,380 ± 290 | 4,020 ± 320 |
| APPase | 846 ± 23 | 997 ± 35 | 727 ± 25 | 868 ± 31 | 968 ± 35 | 814 ± 28 | 752 ± 27 | 771 ± 26 |
| Amylolytic activity | 107 ± 5 | 118 ± 4 | 118 ± 8 | 130 ± 7 | 133 ± 10 | 112 ± 2 | 118 ± 3 | 128 ± 9 |
| Total SS | 7.3 ± 1 | 8.6 ± 0.8 | 8.0 ± 0.8 | 8.1 ± 0.8 | 7.2 ± 0.7 | 7.2 ± 0.7 | 9.4 ± 0.9 | 7.5 ± 0.7 |
| SP | 38 ± 7 | 40 ± 7 | 34 ± 7 | 47 ± 10 | 53 ± 14 | 41 ± 10 | 32 ± 8 | 56 ± 17 |
Activities were determined in samples from source leaves of 6-week-old plants grown in chambers in ambient CO2 conditions at 20°C and an irradiance of 300 μmol of photons per sec−1/m−2. Leaf samples were taken and quenched in liquid nitrogen 7 h after the beginning of the light period The results are given as mean ± SEM of extracts from 10 independent plants per line. Enzyme activities are given in milliunits/g fresh weight. Control plants represent both WT and 35S-GUS-int.
Extraplastidial ASPP Expression Does Not Affect the Photosynthetic Capacity of the Plant. Given that both ADPG and starch levels are lower in the 35S-ASPP-NOS plants than in the WT plants, we decided to investigate whether transgenic plants displayed decreased photosynthetic activity. Toward this end, photosynthetic parameters of whole attached leaves in 35S-ASPP-NOS and control plants were compared at light intensities in which the plants were grown (300 μmol of photons per m-2/sec-1) and at ambient CO2 (350 ppm). These analyses revealed no significant changes in the rates of O2 production, transpiration, CO2 stomatal conductance, or substomatal CO2 concentration (see Table 5, which is published as supporting information on the PNAS web site). Therefore, reduction of both ADPG and starch contents in 35S-ASPP-NOS plants cannot be ascribed to reduced photosynthetic capacities but to the extraplastidial hydrolysis of ADPG catalyzed by ASPP.
Extraplastidial Expression of ASPP Does Not Affect the Phenotype and Growth Behavior of the Plant. At no stage during development could we detect any phenotypic difference between the 35S-ASPP-NOS and control plants (both 35S-GUS-int and untransformed plants). No significant differences were observed in protein content, dry weight, plant height, flowering time, and leaf number or size between 35S-ASPP-NOS and control plants (see Table 6, which is published as supporting information on the PNAS web site). Furthermore, there were no significant differences between chlorophyll contents of the ASPP and control plants.
Additional Remarks. The leaf ADPG levels that were reported in this work (≈0.25 nmol/g fresh weight) are marginally low when compared with those of starch-storing organs of dicotyledonous and monocotyledonous plants (2-10 nmol/g fresh weight and 50-250 nmol/g fresh weight, respectively) (56). Concerning the subcellular localization of this nucleotide sugar, results presented in Fig. 3B showing normal levels of ADPG in 35S-ChlTPASPP-NOS plants, and a ≈70% reduction of the normal ADPG content in 35S-ASPP-NOS plants demonstrate that, essentially identical to the case of cereal endosperms (57, 58), most of ADPG has an extraplastidial localization in source leaves. The fact that 35S-ChlTPASPP-NOS leaves have normal ADPG content indicates the possible occurrence of metabolic channels and compartments inside the chloroplast that prevent accessibility of ASPP to some ADPG.
The following observations further strengthen the view that, although ADPG can be synthesized in the chloroplast (12), this nucleotide sugar accumulates in the cytosol. (i) ADPG spontaneously hydrolyzes to AMP and the scarcely metabolizable glucose-1,2-monophosphate under conditions of alkaline pH and high Mg2+ concentration occurring in the illuminated chloroplast (18) (see Fig. 2). Therefore, unless internal compartments that prevent spontaneous hydrolytic breakdown of ADPG occur inside the chloroplast, this molecule cannot accumulate in the chloroplast during active starch biosynthesis. (ii) ADPG transport machinery occurs in chloroplasts (20). In this respect, sequences corresponding to RNAs isolated from leaves are available in databanks that code for chloroplastic membrane proteins that contain a putative KKGGL ADPG-binding motif (18, 59) (see Fig. 9, which is published as supporting information on the PNAS web site). Those proteins share high homology to Brittle-1, a well characterized ADPG translocator occurring in the amyloplasts of maize endosperms (58, 60, 61). (iii) A cytosolic glucan synthase occurs in the cytosol of leaves that utilizes ADPG as the sugar-donor molecule (62). This enzyme has been suggested to be involved in the production of a complex cytosolic heteroglycan (63). (iv) A cytosolic ADPG-cleaving enzyme belonging to the Nudix (Nucleoside diphosphate linked to some other moiety X) hydrolase family of enzymes (64) occurs in plants that [essentially similar to the case of the bacterial counterpart (41)] is likely to be involved in the control of intracellular levels of the ADPG linked to gluconeogenesis (F.J.M., E.B.-F., N.A.-C., M.T.M.-Z., and J.P.-R., unpublished data).
Concerning the source of ADPG accumulating outside the chloroplast, AGP and SuSy are the two known enzymes that can synthesize this nucleotide sugar in plants (19). In contrast to the case of cereal endosperms possessing most of AGP in the extraplastidial compartment (56, 65, 66), AGP locates exclusively in the chloroplast of leaves (67-69). Thus, it is highly conceivable that some ADPG produced in the plastid is exported to the cytosol before it is imported again to the chloroplast.
Although SuSy plays a role in supplying energy for phloem loading in source leaves (70, 71), it is our belief that it is also involved in the production of a sizable pool of the ADPG that is necessary for starch biosynthesis. This view, in which sucrose and starch biosynthetic pathways are connected directly by SuSy (Fig. 1B), is consistent with the occurrence of UGP and SPS antisensed Arabidopsis leaves containing low levels of both sucrose and starch (72, 73).
Taking into account all of the limitations that are inherent in basing conclusions on genetically engineered plants, this article has shown that most of the ADPG linked to starch biosynthesis in leaves occurs outside the chloroplast. Production and characterization of leaves with altered SuSy activity will be critically important to evaluate the importance of this enzyme in the production of cytosolic ADPG.
Supplementary Material
Acknowledgments
We thank Vanesa Rubio, Maria José Villafranca, and Sorospen Barón for expert technical support; Dr. Luis Cañas and María Dolores Gómez for fantastic technical assistance in the immunocytochemical analyses of ASPP-expressing plants; Drs. Jose María Romero and Angel Mérida (Instituto de Bioquímica Vegetal y Fotosíntesis, Seville, Spain) for critical readings of the manuscript; and Dr. Tansy Chia (John Innes Centre, Norwich, United Kingdom) for helpful discussions. This work was supported by Comisión Interministerial de Ciencia y Tecnología and Fondo Europeo de Desarrollo Regional Grant BIO2001-1080 and the government of Nafarroa. A.M.V. was supported by the Spanish Ministry of Culture and Education for financial support. M.T.M.-Z. was supported by a predoctoral fellowship from the Spanish Ministry of Culture and Education.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ADPG, ADP·glucose; AGP, ADPG pyrophosphorylase; ASPP, adenosine diphosphate sugar pyrophosphatase; APPase, alkaline pyrophosphatase; ChlTP, chloroplast-transit peptide; GUS-int, β-glucuronidase-intron; G1P, glucose-1-phosphate; G6P, glucose-6-phosphate; NOS, nopaline synthase; SP, starch phosphorylase; SPS, sucrose-phosphate synthase; SS, starch synthase; SuSy, sucrose synthase; UDPG, UDP·glucose; UGP, UDPG pyrophosphorylase; 35S, CaMV:cauliflower mosaic virus promoter.
References
- 1.Recondo, E. & Leloir, L. F. (1963) Biochem. Biophys. Res. Commun. 6, 85-88. [DOI] [PubMed] [Google Scholar]
- 2.Murata, T., Minamikawa, T. & Akazawa, T. (1963) Biochem. Biophys. Res. Commun. 6, 439-443. [Google Scholar]
- 3.Huber, S. C. & Bickett, D. M. (1984) Plant Physiol. 74, 445-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stitt, M., Kürzel, B. & Heldt, H. W. (1984) Plant Physiol. 75, 554-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stitt, M. & Quick, W. P. (1989) Physiol. Plantarum 77, 633-641. [Google Scholar]
- 6.Neuhaus, H. E. & Stitt, M. (1990) Planta 182, 445-454. [DOI] [PubMed] [Google Scholar]
- 7.Sonnewald, U. (1992) Plant J. 2, 571-581. [PubMed] [Google Scholar]
- 8.Müller-Röber, B., Sonnewald, U. & Willmitzer, L. (1992) EMBO J. 11, 1229-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okita, T. W. (1992) Plant Physiol. 100, 560-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stark, D. M., Timmerman, K. P., Barry, G. F., Preiss, J. & Kishore, G. M. (1992) Science 258, 287-292. [DOI] [PubMed] [Google Scholar]
- 11.Arnon, D. I. (1955) Science 122, 9-16. [DOI] [PubMed] [Google Scholar]
- 12.Heldt, H. W., Chon, C. J., Maronde, D., Herold, A., Stankovic, Z. S., Walker, D. A., Kraminer, A., Kirk, M. R. & Heber, U. (1977) Plant Physiol. 59, 1146-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caspar, T., Huber, S. C. & Somerville, C. (1985) Plant Physiol. 79, 11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riesmeier, J. W., Flügge, U. -I., Schulz, B., Heineke, D., Heldt, H. -W., Willmitzer, L. & Frommer, W. B. (1993) Proc. Natl. Acad. Sci. USA 90, 6160-6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kossmann, J., Sonnewald, U. & Willmitzer, L. (1994) Plant J. 6, 637-650. [Google Scholar]
- 16.Haake, V., Zrenner, R., Sonnewald, U. & Stitt, M. (1998) Plant J. 14, 147-157. [DOI] [PubMed] [Google Scholar]
- 17.Lytovchenko, A., Bieberich, K., Willmitzer, L. & Fernie, A. R. (2002) Planta 215, 802-811. [DOI] [PubMed] [Google Scholar]
- 18.Baroja-Fernández, E., Muñoz, F. J., Akazawa, T. & Pozueta-Romero, J. (2001) Plant Cell Physiol. 42, 1311-1320. [DOI] [PubMed] [Google Scholar]
- 19.Pozueta-Romero, J., Muñoz, F. J., Rodríguez-López, M., Baroja-Fernández, E. & Akazawa, T. (2003) JSPP Lett., 24-31.
- 20.Pozueta-Romero, J., Ardila, F. & Akazawa, T. (1991) Plant Physiol. 97, 1565-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delmer, D. P. (1972) J. Biol. Chem. 247, 3822-3828. [PubMed] [Google Scholar]
- 22.Silvius, J. E. & Snyder, F. W. (1979) Plant Physiol. 64, 1070-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakai, T., Konishi, T., Zhang, X.-Q., Chollet, R., Tonouchi, N., Tsuchida, T., Yoshinaga, F., Mori, H., Sakai, F. & Hayashi, T. (1998) Plant Cell Physiol. 39, 1337-1341. [DOI] [PubMed] [Google Scholar]
- 24.Porchia, A. C., Curatti, L. & Salerno, G. L. (1999) Planta 210, 34-40. [DOI] [PubMed] [Google Scholar]
- 25.Baroja-Fernández, E., Muñoz, F. J., Saikusa, T., Rodríguez-López, M., Akazawa, T. & Pozueta-Romero, J. (2003) Plant Cell Physiol. 44, 500-509. [DOI] [PubMed] [Google Scholar]
- 26.Neckelmann, G. & Orellana, A. (1998) Plant Physiol. 117, 1007-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stitt, M. & Heldt, H. W. (1981) Biochim. Biophys. Acta 638, 1-11. [Google Scholar]
- 28.Fox, T. C. & Geiger, D. R. (1984) Plant Physiol. 76, 763-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott, P. & Kruger, N. J. (1995) Plant Physiol. 108, 1569-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lozovaya, V. V., Zabotina, O. A. & Widholm, J. M. (1996) Plant Physiol. 111, 921-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belanger, A. E. & Hatfull, G. (1999) J. Bacteriol. 181, 6670-6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guedon, E., Desvaux, M. & Petitdemange, H. (2000) J. Bacteriol. 182, 2010-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.David, M., Petit, W. A., Laughlin, M. R., Shulman, R. G., King, J. E. & Barrett, E. J. (1990) J. Clin. Invest. 86, 612-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massillon, D., Bollen, M., de Wulf, H., Overloop, K., Vanstapel, F., Van Hecke, P. & Stalmans, W. (1995) J. Biol. Chem. 270, 19351-19356. [DOI] [PubMed] [Google Scholar]
- 35.Sweetlove, L. J., Burrell, M. M. & ap Rees, T. (1996) Biochem. J. 320, 493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pozueta-Romero, J. & Akazawa, T. (1993) J. Exp. Bot. 44, Suppl., 297-306. [Google Scholar]
- 37.Sonnewald, U., Hajirezaei, M-R., Kossmann, J., Heyer, A., Trethewey, R. N. & Willmitzer, L. (1997) Nat. Biotech. 15, 794-797. [DOI] [PubMed] [Google Scholar]
- 38.Trethewey, R. N., Fernie, A. R., Bachmann, A., Fleischer-Notter, H., Geigenberger, P. & Willmitzer, L. (2001) Plant Cell Environ. 24, 357-365. [Google Scholar]
- 39.Trethewey, R. N., Geigenberger, P., Riedel, K., Hajirezaei, M.-R., Sonnewald, U., Stitt, M., Riesmeier, J. W. & Willmitzer, L. (1998) Plant J. 15, 109-118. [DOI] [PubMed] [Google Scholar]
- 40.Urbanczyk-Wochniak, E., Leisse, A., Roessner-Tunali, U., Lytovchenko, A., Reismeier, J., Willmitzer, L. & Fernie, A. R. (2003) Plant Cell Physiol. 44, 1359-1367. [DOI] [PubMed] [Google Scholar]
- 41.Moreno-Bruna, B., Baroja-Fernández, E., Muñoz, F. J., Bastarrica-Berasategui, A., Zandueta-Criado, A., Rodríguez-López, M., Lasa, I., Akazawa, T. & Pozueta-Romero, J. (2001) Proc. Natl. Acad. Sci. USA 98, 8128-8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vancanneyt, G., Schmidt, R., O'Connor-Sanchez, A., Willmitzer, L. & Rocha-Sosa, M. (1990) Mol. Gen. Genet. 220, 245-250. [DOI] [PubMed] [Google Scholar]
- 43.Debleare, R., Bytebier, B., de Greve, H., Debroeck, F., Schell, J., van Montagu, M. & Leemans, J. (1985) Nucleic Acids. Res. 13, 4777-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maniatis, T., Fritsch, E. F. & Sambrook, J. (1982) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 45.Rodríguez-López, M. (2002) Ph.D. dissertation (Public University of Navarra, Navarra, Spain).
- 46.McBride, K. E. & Summerfelt, K. R. (1990) Plant Mol. Biol. 14, 269-276. [DOI] [PubMed] [Google Scholar]
- 47.Houlné, G., Schantz, M.-L., Meyer, B., Pozueta-Romero, J. & Schantz, R. (1994) Curr. Genet. 26, 524-527. [DOI] [PubMed] [Google Scholar]
- 48.Rocha-Sosa, M., Sonnewald, U., Frommer, W. B., Stratmann, M., Schell, J. & Willmitzer, L. (1989) EMBO J. 8, 23-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ciereszko, I., Johansson, H. & Kleczkowski, L. A. (2001) Biochem. J. 354, 67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sweetlove, L. J., Burrell, M. M. & ap Rees, T. (1996) Biochem. J. 320, 487-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodríguez-López, M., Baroja-Fernández, E., Zandueta-Criado, A. & Pozueta-Romero, J. (2000) Proc. Natl. Acad. Sci. USA 97, 8705-8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yagi, T., Baroja-Fernández, E., Yamamoto, R., Muñoz, F. J., Akazawa, T., Hong, K. S. & Pozueta-Romero, J. (2003) Biochem. J. 370, 409-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wintermans, J. F. G. M. & de Mots. A. (1965) Biochim. Biophys. Acta 109, 448-453. [DOI] [PubMed] [Google Scholar]
- 54.Baroja-Fernández, E., Zandueta-Criado, A., Rodríguez-López, M., Akazawa, T. & Pozueta-Romero, J. (2000) FEBS Lett. 480, 277-282. [DOI] [PubMed] [Google Scholar]
- 55.Rodríguez-López, M., Baroja-Fernández, E., Zandueta-Criado, A., Moreno-Bruna, B., Muñoz, F.J., Akazawa, T. & Pozueta-Romero, J. (2001) FEBS Lett. 490, 44-48. [DOI] [PubMed] [Google Scholar]
- 56.Beckles, D. M., Smith, A. M. & ap Rees, T. (2001) Plant Physiol. 125, 818-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu, T-T. Y. & Shannon, J. C. (1981) Plant Physiol. 67, 525-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shannon, J. C., Pien, F.-M. & Liu, K.-C. (1996) Plant Physiol. 110, 835-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furukawa, K., Tagaya, M., Tanizawa, K. & Fukui, T. (1993) J. Biol. Chem. 268, 23837-23842. [PubMed] [Google Scholar]
- 60.Sullivan, T. D., Strelow, L. I., Illingworth, C. A., Phillips, R. L. & Nelson, O. E. (1991) Plant Cell 3, 1337-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shannon, J. C., Pien, F.-M., Cao, H. & Liu, K.-C. (1998) Plant Physiol. 117, 1235-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tacke, M., Yang, Y. & Steup, M. (1991) Planta 185, 220-226. [DOI] [PubMed] [Google Scholar]
- 63.Yang, Y. & Steup, M. (1990) Plant Physiol. 94, 960-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bessman, M. J., Frick, D. N. & O'Handley, S. F. (1996) J. Biol. Chem. 271, 25059-25062. [DOI] [PubMed] [Google Scholar]
- 65.Denyer, K., Dunlap, F., Thorbjornsen, T., Keeling, P. & Smith, A. M. (1996) Plant Physiol. 112, 779-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kleczkowski, L. A. (1996) Trends Plant Sci. 1, 363-364. [Google Scholar]
- 67.Okita, T. W., Greenberg, E., Kuhn, D. N. & Preiss, J. (1979) Plant Physiol. 64, 187-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Echeverria, E. & Boyer, C. (1986) Am. J. Bot. 73, 167-171. [Google Scholar]
- 69.Robinson, N. L. & Preiss, J. (1987) Plant Physiol. 85, 360-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin, T., Frommer, W. B., Salanoubat, M. & Willmitzer, L. (1993) Plant J. 4, 367-377. [DOI] [PubMed] [Google Scholar]
- 71.Fu, H. & Park, W. D. (1995) Plant Cell 7, 1369-1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strand, A., Zrenner, R., Trevanion, S., Stitt, M., Gustafsson, P. & Gardeström, P. (2000) Plant J. 759-770. [DOI] [PubMed]
- 73.Kleczkowski, L. A., Geisler, M., Ciereszko, I. & Johansson, H. (2004) Plant Physiol. 134, 912-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.