Abstract
Type I human diabetics and streptozotocin-induced diabetic mice with higher genetically determined levels of angiotensin-converting enzyme have an increased risk of developing nephropathy. However, previous experiments in mice and computer simulations indicate that modest increases in angiotensin-converting enzyme have minimal effects on blood pressure and angiotensin II levels, although bradykinin decreases significantly, inferring that bradykinin is critical for protecting the kidney in diabetics. Here, we confirm this inference by demonstrating that Akita diabetic mice lacking the bradykinin B2 receptor develop overt albuminuria, excreting the equivalent of >550 mg/day albumin in humans, which contrasts with the microalbuminuria (equivalent to <150 mg/day) seen in their simply diabetic littermates. The overt albuminuria is accompanied by a marked increase in glomerular mesangial sclerosis. The importance of bradykinin demonstrated here bears strongly on how current drugs reduce diabetic nephropathy and suggests that B2 receptor-specific agonists merit consideration in this context.
Keywords: maturity onset diabetes of the young, albuminuria, glomerulosclerosis
Differences in expression of the gene coding for angiotensin-converting enzyme (ACE), such as are observed in humans in relation to the insertion/deletion polymorphism in the ACE gene (1) and in mice with different numbers of the gene (2), have minimal effects on angiotensin II (ANGII) levels because the steady-state concentrations of the products of ACE are less sensitive to modest changes in the activity of the enzyme than the concentrations of its substrates. Thus, our recent computer simulations (3, 4) and accompanying experimental data indicate that a 50% increase in ACE causes a <5% increase in steady-state ANGII levels but close to a 20% decrease in bradykinin levels. Hence, our inference that the causative link between genetic differences in ACE expression and diabetic nephropathy (5, 6) is likely to be mediated by the ACE substrate bradykinin. To test this inference, we have combined the following two mutations: a dominant mutation that leads to maturity onset diabetes, Akita (7), resulting from an amino acid change in the insulin 2 gene (Ins2C96Y) (8), and a recessive knockout mutation (Bdkrb2-) (9) in the gene coding for the bradykinin B2 receptor, the receptor predominantly expressed in the normal kidney.
Materials and Methods
Animals. Animals were purchased from The Jackson Laboratory and bred in our mouse facility. The mice having the null allele for the bradykinin B2 receptor (9) were backcrossed to wild-type C57BL/6J at least six times before mating to mice heterozygous for the Akita mutation (Ins2+/C96Y) (7), which already have the C57BL/6J genetic background. All experiments were conducted in accordance with the guidelines of the University of North Carolina Institutional Animal Care and Use Committee. Blood pressures were measured as described (2). Plasma glucose levels were determined with the glucose-oxidase method (Wako, Richmond, VA). Urinary albumin was determined with ELISA (Exocell M, Philadelphia).
Quantitative RT-PCR. Total RNA was extracted from the whole right kidney of mice, and the mRNA expression of the bradykinin B1 and B2 receptors and of megsin and nephrin was determined by quantitative RT-PCR as described (10). Primers for the amplification and probes for detecting and measuring the expression of these genes are provided in Table 3, which is published as supporting information on the PNAS web site. Sense RNAs synthesized from a cDNA fragment cloned into pBluescript II (Stratagene) for each of the genes of interest were used for calibrating quantitation of their messages.
Results
Males with the following four combinations of mutations were generated: wild type at both loci; mutant (homozygous knockout) only at the Bdkrb2 locus; mutant (heterozygous Akita) only at the Ins2 locus; and mutant at both loci. Basic parameters, including body, heart, and kidney weights, as well as blood pressure, plasma glucose, food intake, urine volume, and daily excretion of albumin, were determined for each animal (Table 1). Comparison of the singly mutant B2 receptor knockout males with their wild-type littermates showed that the receptor knockout animals do not differ significantly from wild type in any of these parameters, which is in general agreement with a previous report (11) for the same null mutation. Heterozygous males for the dominant Akita mutation (Akita diabetic) have severe diabetes at 6 months of age with 38.8 ± 1.9 mmol/liter (698 ± 34 mg/dl) plasma glucose. Their body weights are 70% of wild type; they consume more than three times the normal amount of food, and their urine outputs are ≈20 times those of wild type. Doubly mutant males (Akita diabetic and bradykinin B2 receptor null) are indistinguishable from the singly mutant Akita diabetic animals in all these parameters (including plasma glucose, food intake, and urine volume), except for two parameters indicative of nephropathy: kidney weight and albumin excretion.
Table 1. Characteristics of 6-month-old males having the four combinations of wild-type and mutant Ins2 and Bdkrb2 genes.
| Genotype | Bdkrb2+/+Ins2+/+ | Bdkrb2−/−Ins2+/+ | Bdkrb2+/+Ins2+/C96Y | Bdkrb2−/−Ins2+/C96Y |
|---|---|---|---|---|
| Number | 4 | 4 | 9 | 7 |
| BW, g | 36.8 ± 1.2 | 34.2 ± 3.5 | 24.7 ± 2.0† | 24.6 ± 1.4† |
| HW, mg | 203 ± 16 | 200 ± 31 | 150 ± 11* | 157 ± 12* |
| HW/BW, mg/g | 5.5 ± 0.5 | 5.9 ± 0.6 | 6.3 ± 0.6 | 6.4 ± 0.4 |
| KW, mg | 212 ± 7 | 237 ± 19 | 274 ± 21 | 330 ± 18†‡ |
| KW/BW, mg/g | 5.8 ± 0.3 | 6.9 ± 1.9 | 11.4 ± 0.9† | 13.5 ± 0.5†‡ |
| Blood pressure, mmHg (1 mmHg = 133 Pa) | 118 ± 3 | 124 ± 5 | 123 ± 3 | 126 ± 4 |
| Hematocrit, % | 42.8 ± 2.1 | 44.0 ± 0.5 | 45.9 ± 1.8 | 46.2 ± 1.6 |
| Plasma glucose, mmol/liter | 10.9 ± 1.3 | 8.2 ± 1.4 | 38.8 ± 1.9† | 37.9 ± 1.4† |
| Food intake, g/day | 3.5 ± 0.3 | 3.9 ± 0.8 | 12.2 ± 1.5* | 14.0 ± 2.6* |
| Urine volume, ml/day | 1.3 ± 0.1 | 1.2 ± 0.1 | 26.1 ± 4.6* | 33.0 ± 6.7* |
| Urinary albumin, g/day | 7.6 ± 2.0 | 11.0 ± 3.0 | 54 ± 12* | 209 ± 40†§ |
Data are given as mean ± SEM. BW, body weight; HW, heart weight; KW, kidney weight.
P < 0.05.
P < 0.001 vs. wild type.
P < 0.05.
P < 0.001 vs Bdkrb2+/+ Ins2+/C96Y.
Kidneys are enlarged in human insulin-dependent diabetics, and more so when they have microalbuminuria or overt levels of albumin excretion (12). In the singly mutant Akita diabetic mice, the kidney weight/body weight ratio was 11.4 ± 0.9 versus 5.8 ± 0.3 in wild-type mice (P < 0.05). In the doubly mutant mice, this ratio (13.5 ± 0.5) was significantly greater than in either the singly mutant Akita diabetic mice (P < 0.05) or the wild-type mice (P < 0.001).
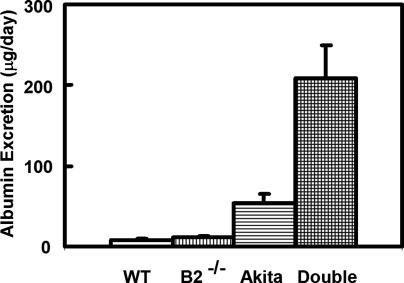
Urinary daily albumin excretion is a more specific indicator of nephropathy than kidney weight. In the double mutants, it reached the level of overt albuminuria (209 ± 40 μg/day, equivalent to >550 mg/day in humans), as shown in Fig. 1 (P < 0.0001 for double mutant versus wild type). This overt albuminuria contrasts with the microalbuminuria (54 ± 12 μg/day, equivalent to <150 mg/day in humans) occurring in the singly mutant Akita diabetic mice (P = 0.005 for double mutant versus Akita).
Fig. 1.
Daily urinary albumin excretion in 6-month-old male mice. Genotypes are wild type at both the insulin 2 and the bradykinin B2 loci (WT = Ins2+/+, Bdkrb2 +/+); wild type for insulin and homozygous null for the B2 receptor (B2-/- = Ins2+/+, Bdkrb2-/-); heterozygous Akita for insulin and wild type for the B2 receptor (Akita = Ins2+/C96Y, Bdkrb2+/+); and doubly mutant (Double = Ins2+/C96Y, Bdkrb2-/-). P = 0.04, 0.0005, and <0.0001 for wild type versus B2 null, Akita diabetic, and double mutant mice, respectively. P = 0.0003 and <0.0001 for B2 null versus Akita diabetic and double mutant mice, respectively. P = 0.004 for Akita diabetic versus double mutant mice.
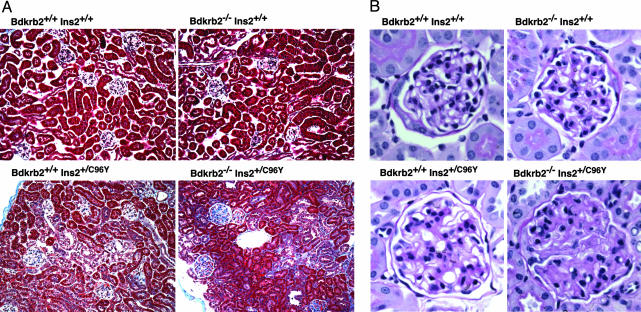
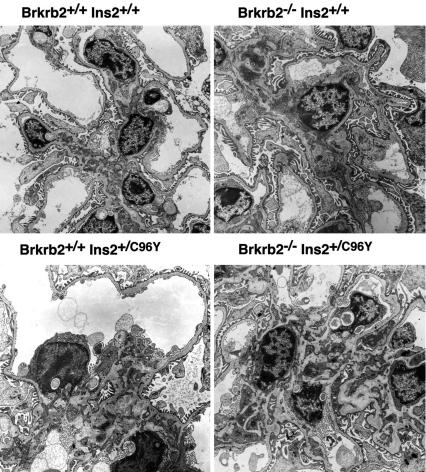
Fig. 2 shows that absence of the bradykinin B2 receptor alone leads to no histological changes in the kidney at 6 months of age. The Akita diabetogenic mutation alone causes some changes, including glycogen deposits in tubular cells and a minor degree of mesangial sclerosis in some glomeruli. In the double mutants, the glycogen accumulation is not obviously different from that in their singly mutant littermates. However, the glomerular mesangial sclerosis is markedly increased and more closely resembles glomerular changes that are seen in human patients with diabetic glomerulosclerosis. Electron micrographs (Fig. 3) confirm the mesangial matrix expansion in the double mutants, but they do not show any obvious changes in the glomerular endothelial cells or podocytes. In general agreement with a previous report (13), immunofluorescence microscopy demonstrates the presence of mesangial Ig in our singly mutant Akita diabetic mice, including IgG, IgA, and IgM, with no obvious difference when the mice also lack the B2 receptor (data not shown).
Fig. 2.
Photomicrographs of the kidneys with the same four genotypes as shown in Fig. 1. (A) Masson's trichrome staining of renal cortex. (Original magnification, ×40.) (B) Periodic acid-Schiff staining of glomeruli counterstained with hematoxylin. (Original magnification, ×400.)
Fig. 3.
Electron micrographs of kidneys with the same four genotypes as shown in Fig. 1. (Original magnification, ×5,000.)
To assess the general status of the four groups of animals and to look for possible compensatory changes, we used quantitative RT-PCR to determine renal mRNA expression of both bradykinin receptors (B1 and B2) and several other informative genes. Table 2 presents the resulting data. Expression of the B2 receptor in the B2 receptor null animals is, as expected (9), indistinguishable from zero. We also confirm a previous report (14) that, in the B2 null animals expression of the bradykinin B1 receptor, expressed only weakly in wild-type kidneys, is markedly enhanced (≈12 times wild type), reaching a level comparable with wild-type expression of the B2 receptor. A similar increase in B1 receptor expression is seen in the singly mutant Akita diabetic animals (to ≈11 times that of wild type), even though expression of the B2 receptor in these animals is also increased (to approximately two times that of wild type). Expression of the B1 receptor in the double mutants is increased further to >25 times that of wild type, becoming greater than wild-type expression of the B2 receptor. However, despite this very marked increase in expression of the B1 receptor in the Akita diabetic B2 receptor null mice, the nephropathy is not prevented.
Table 2. The amount of mRNA in the kidney of the four groups of animals.
| Genotype | Bdkrb2+/+Ins2+/+ | Bdkrb2−/−Ins2+/+ | Bdkrb2+/+Ins2+/C96Y | Bdkrb2−/−Ins2+/C96Y |
|---|---|---|---|---|
| B2 receptor | 32.8 ± 4.3 (100 ± 13) | 0.0 ± 0.0* (0 ± 0) | 67.7 ± 12.7* (207 ± 39) | 0.0 ± 0.0*† (0 ± 0) |
| B1 receptor | 3.1 ± 0.4 (100 ± 13) | 35.2 ± 3.4* (1,132 ± 109) | 37.1 ± 2.1* (1,196 ± 67) | 78.0 ± 8.0*† (2,511 ± 257) |
| Megsin | 0.257 ± 0.034 (100 ± 13) | 0.306 ± 0.040 (119 ± 16) | 0.462 ± 0.049* (180 ± 19) | 0.831 ± 0.105*† (323 ± 41) |
| Nephrin | 1.44 ± 0.19 (100 ± 13) | 1.61 ± 0.16 (112 ± 11) | 3.03 ± 0.39* (210 ± 27) | 4.47 ± 0.28*† (310 ± 19) |
Data are given as pg mRNA of genes of interest in μg total RNA (mean ± SEM). Relative expression levels (% wild type) are shown in parentheses.
P < 0.05 vs. wild type.
P < 0.05 vs. Bdkrb2+/+ Ins2+/C96Y.
Megsin, a member of the serpin family of protease inhibitors, appears to be an inhibitor of matrix protein degradation in the mesangium (15). Its expression is unchanged in the receptor null mice but is increased to approximately two times that of wild type in the singly mutant Akita diabetic mice and to approximately three times that of wild type in the double mutants, which may be related to the extent of mesangial matrix expansion. Expression of nephrin, an essential component of the slit diaphragms of the glomerulus, is increased at early stages in rats that were made diabetic by streptozotocin (16). We found that nephrin expression is approximately two times that of wild type in our Akita diabetic mice and approximately four times that of wild type in the double mutants. This finding is in agreement with the absence of any obvious podocyte losses in the electron micrographs of the glomeruli of these animals.
Discussion
The sequence of events that leads to the increased nephropathy that we observe in diabetic mice lacking the bradykinin B2 receptor is not immediately apparent, although it is clear that the B1 receptor cannot compensate for its absence. Mesangial cells are potential mediators of the glomerular damage that we report in this article because they express the B2 receptor and respond in various ways to bradykinin (17, 18). However, the increase in interstitial fibrosis that we find in the diabetic mice lacking the B2 receptor suggests that the increased nephropathy in the double mutants must involve events additional to those mediated by mesangial cells. Despite remaining questions, our experiments bear directly on the interpretation of the well known merits of ACE inhibitors in preventing diabetic nephropathy. Thus, current thinking usually ascribes this protection to decreases in ANGII levels. Our data suggest that increases in bradykinin levels are also important and that B2 receptor-specific agonists are likely to be protective without the side effects induced by stimulating B1 receptors.
In summary, we conclude that the bradykinin system plays a critical role in protecting the kidney from damage caused by diabetes mellitus, and we emphasize the importance of enhancing the action of kinins as a means of minimizing this diabetic complication.
Supplementary Material
Acknowledgments
We thank Drs. C. Robert Bagnell, Romulo E. Colindres, and HyungSuk Kim (University of North Carolina, Chapel Hill), as well as Thomas M. Coffman (Duke University, Durham, NC) for their help. This work was supported by National Institutes of Health Grants HL49277, HL70523, and HL71266, American Heart Association Grant 0435230N, Burroughs Wellcome Fund Innovation Award in Functional Genomics 1001254, and a fellowship from the Sankyo Foundation of Life Science.
Abbreviations: ACE, angiotensin-converting enzyme; ANGII, angiotensin II.
References
- 1.Rigat, B., Hubert, C., Alhenc-Gelas, F., Cambien, F., Corvol, P. & Soubrier, F. (1990) J. Clin. Invest. 86, 1343-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krege, J. H., Kim, H. S., Moyer, J. S., Jennette, J. C., Peng, L., Hiller, S. K. & Smithies, O. (1997) Hypertension 29, 150-157. [DOI] [PubMed] [Google Scholar]
- 3.Smithies, O., Kim, H. S., Takahashi, N. & Edgell, M. H. (2000) Kidney Int. 58, 2265-2280. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi, N., Hagaman, J. R., Kim, H. S. & Smithies, O. (2003) Endocrinology 144, 2184-2190. [DOI] [PubMed] [Google Scholar]
- 5.Marre, M., Bernadet, P., Gallois, Y., Savagner, F., Guyene, T. T., Hallab, M., Cambien, F., Passa, P. & Alhenc-Gelas, F. (1994) Diabetes 43, 384-388. [DOI] [PubMed] [Google Scholar]
- 6.Huang, W., Gallois, Y., Bouby, N., Bruneval, P., Heudes, D., Belair, M. F., Krege, J. H., Meneton, P., Marre, M., Smithies, O. & Alhenc-Gelas, F. (2001) Proc. Natl. Acad. Sci. USA 98, 13330-13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshioka, M., Kayo, T., Ikeda, T. & Koizumi, A. (1997) Diabetes 46, 887-894. [DOI] [PubMed] [Google Scholar]
- 8.Wang, J., Takeuchi, T., Tanaka, S., Kubo, S. K., Kayo, T., Lu, D., Takata, K., Koizumi, A. & Izumi, T. (1999) J. Clin. Invest. 103, 27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borkowski, J. A., Ransom, R. W., Seabrook, G. R., Trumbauer, M., Chen, H., Hill, R. G., Strader, C. D. & Hess, J. F. (1995) J. Biol. Chem. 270, 13706-13710. [DOI] [PubMed] [Google Scholar]
- 10.Kim, H. S., Lee, G., John, S. W., Maeda, N. & Smithies, O. (2002) Proc. Natl. Acad. Sci. USA 99, 4602-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milia, A. F., Gross, V., Plehm, R., De Silva, J. A., Jr., Bader, M. & Luft, F. C. (2001) Hypertension 37, 1473-1479. [DOI] [PubMed] [Google Scholar]
- 12.Feldt-Rasmussen, B., Hegedus, L., Mathiesen, E. R. & Deckert, T. (1991) Scand. J. Clin. Lab. Invest. 51, 31-36. [PubMed] [Google Scholar]
- 13.Haseyama, T., Fujita, T., Hirasawa, F., Tsukada, M., Wakui, H., Komatsuda, A., Ohtani, H., Miura, A. B., Imai, H. & Koizumi, A. (2002) Tohoku J. Exp. Med. 198, 233-244. [DOI] [PubMed] [Google Scholar]
- 14.Duka, I., Kintsurashvili, E., Gavras, I., Johns, C., Bresnahan, M. & Gavras, H. (2001) Circ. Res. 88, 275-281. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki, D., Miyata, T., Nangaku, M., Takano, H., Saotome, N., Toyoda, M., Mori, Y., Zhang, S. Y., Inagi, R., Endoh, M., et al. (1999) J. Am. Soc. Nephrol. 10, 2606-2613. [DOI] [PubMed] [Google Scholar]
- 16.Aaltonen, P., Luimula, P., Astrom, E., Palmen, T., Gronholm, T., Palojoki, E., Jaakkola, I., Ahola, H., Tikkanen, I. & Holthofer, H. (2001) Lab. Invest. 81, 1185-1190. [DOI] [PubMed] [Google Scholar]
- 17.Bascands, J. L., Pecher, C., Bompart, G., Rakotoarivony, J., Tack, J. L. & Girolami, J. P. (1994) Am. J. Physiol. 267, F871-F878. [DOI] [PubMed] [Google Scholar]
- 18.Duchene, J., Schanstra, J. P., Pecher, C., Pizard, A., Susini, C., Esteve, J. P., Bascands, J. L. & Girolami, J. P. (2002) J. Biol. Chem. 277, 40375-40383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.