Abstract
Vibrio cholerae secretes a Zn-dependent metalloprotease, hemagglutinin/protease (HA/protease), which is encoded by hapA and displays a broad range of potentially pathogenic activities. Production of HA/protease requires transcriptional activation by the quorum-sensing regulator HapR. In this study we demonstrate that transcription of hapA is growth phase dependent and specifically activated in the deceleration and stationary growth phases. Addition of glucose in these phases repressed hapA transcription by inducing V. cholerae to resume exponential growth, which in turn diminished the expression of a rpoS-lacZ transcriptional fusion. Contrary to a previous observation, we demonstrate that transcription of hapA requires the rpoS-encoded σs factor. The cyclic AMP (cAMP) receptor protein (CRP) strongly enhanced hapA transcription in the deceleration phase. Analysis of rpoS and hapR mRNA in isogenic CRP+ and CRP− strains suggested that CRP enhances the transcription of rpoS and hapR. Analysis of strains containing hapR-lacZ and hapA-lacZ fusions confirmed that hapA is transcribed in response to concurrent quorum-sensing and nutrient limitation stimuli. Mutations inactivating the stringent response regulator RelA and the HapR-controlled AphA regulator did not affect HA/protease expression. Electrophoretic mobility shift experiments showed that pure cAMP-CRP and HapR alone do not bind the hapA promoter. This result suggests that HapR activation of hapA differs from its interaction with the aphA promoter and could involve additional factors.
Vibrio cholerae of serogroups O1 and O139, the causative agents of epidemic cholera, adhere to and colonize the small intestine and secrete cholera toxin (CT), which causes the major clinical symptoms of the disease. V. cholerae produces a soluble Zn-dependent metalloprotease (mucinase), hemagglutinin/protease (HA/protease) encoded by hapA (5, 13, 14, 19).
HA/protease can proteolytically activate CT A subunit (6) and the El Tor cytolysin/hemolysin (38) and can hydrolyze several physiologically important proteins such as mucin, fibronectin, and lactoferrin (14). HA/protease perturbs the paracellular barrier of cultured intestinal epithelial cells (31, 50) by acting on tight-junction-associated proteins (51) and promotes the detachment of vibrios from monolayers and mucin (4, 15). Importantly, HA/protease contributes to the reactogenicity of live attenuated cholera vaccine strains (3, 40).
HA/protease belongs to the thermolysin family of bacterial zinc metalloproteases (36). The HA/protease open reading frame (ORF) reveals the presence of a 24-amino-acid signal peptide followed by a 171-amino-acid N-terminal propeptide (19, 36). HA/protease, like V. vulnificus elastase, contains a 10-kDa C-terminal peptide (36). In V. vulnificus the C-terminal peptide mediates hemagglutination and is removed in an autoproteolytic reaction (37). The existence of monoclonal antibodies against HA/protease that neutralize the proteolytic but not the hemagglutinating activity suggests a domain structure similar to that of V. vulnificus elastase (21). HA/protease is secreted via the V. cholerae type II secretion pathway at the cell pole of the single polar flagellum (42, 43).
Expression of hapA requires transcriptional activation by HapR (24), a homologue of V. harveyi LuxR. The regulators LuxO and HapR coordinate cell density-dependent expression of CT, toxin-coregulated pilus (TCP), HA/protease production, motility, and biofilm formation (54). At low cell density, the active form of LuxO (phospho-LuxO) represses hapR, a condition conducive to the expression of aphA (26, 54). At high cell density, LuxO is inactive and HapR is expressed to activate hapA and repress aphA, encoding an activator of tcpPH (26, 47, 54). It appears that V. cholerae harbors three quorum-sensing systems (34). System 1 consists of a CqsA-dependent autoinducer, CAI-1, and its sensor, CqsS (34). System 2 consists of the LuxS-dependent autoinducer CAI-2 and its sensor, LuxPQ, similar to V. harveyi (34). The existence of a system 3 has been inferred genetically (34). The three systems appear to feed the quorum-sensing signal to LuxO/HapR in order to regulate virulence gene expression (34).
Production of HA/protease is influenced by environmental factors other than cell density (2, 45). Secretion of HA/protease is strongly enhanced by nutrient limitation (2). A crp mutant secreted less HA/protease (2). It was also reported that a rpoS insertion mutant secreted less HA/protease (53). However, the protease defect was not fully complemented in trans, and it was not established whether the mutation affected the production or secretion of HA/protease (53). In a later study using a hemagglutination assay for HA/protease, we did not observe the above rpoS dependency (2). These findings suggest that the expression of hapA is more complex and requires the integration of multiple environmental signals through more than one global regulator.
Starvation stress regulators such as relA and the rpoS-encoded stationary-phase σS factor affect intestinal colonization and virulence in V. cholerae (18, 32, 46). In Escherichia coli, the intracellular level of σS is controlled at the level of transcription, translation, and protein stability (20, 48). The relA gene is the genetic determinant of the stringent response. The relA-catalyzed increase in the amount of guanosine tetraphosphate (ppGpp) leads to rapid inhibition of stable RNA synthesis and ultimately to growth arrest (8). A V. cholerae relA mutant showed lower levels of CT and TCP, altered levels of OmpU and OmpT porins, reduced motility, and a 1,000-fold reduction in infant-mouse colonization (18). The effect of relA inactivation on hapA expression was not examined. Quorum-sensing regulators and rpoS cross-regulate their expression in P. aeruginosa (49), but the extent to which such interactions occur in V. cholerae is unknown.
In the present study we show that transcription of hapA is growth phase dependent, requires the rpoS-encoded σS factor, and is enhanced by cyclic AMP (cAMP) receptor protein (CRP). CRP enhanced the transcription of hapR and rpoS, which in turn are required for HA/protease expression. Glucose repressed the hapA gene by inducing cells to resume exponential growth and lowering the level of rpoS transcription. Finally, we used strains containing chromosomal hapA-lacZ and hapR-lacZ transcriptional fusions to demonstrate that hapA is transcribed in response to concurrent cell density and nutrient limitation stimuli.
MATERIALS AND METHODS
Strains and media.
The V. cholerae and E. coli strains, plasmids, and primers used in this work are listed and described briefly in Tables 1 and 2. Strains were grown in Bacto tryptic soy broth (TSB) (Becton, Dickinson & Co.) or Luria-Bertani (LB) broth at 37°C with agitation (250 rpm). When indicated, liquid medium was supplemented with 0.4% d-glucose. The sensitivity to 3-amino-1,2,4-triazole (AT) was determined by using M9 minimal medium supplemented with glucose (0.2%), all amino acids except histidine (4 μg/ml), adenine (1 mM), thiamine (1 mM), and AT (15 mM). Sensitivity to inhibition by serine, methionine, glycine, and leucine (SMGL) was tested in experiments with M9 medium supplemented with glucose (0.2 %), SMGL (100 μg/ml each), adenine (50 μg/ml), thymine (50 μg/ml), and calcium pantothenate (1 μg/ml). Plasmid DNA was introduced into V. cholerae by electroporation (30). Culture media were supplemented with ampicillin (100 μg/ml), tetracycline (1 μg/ml), kanamycin (50 μg/ml), 5-bromo-4-chloro-3-indolyl-β-d-galactopyronoside (X-Gal) (20 μg/ml), or polymyxin B (100 U/ml) as required. Strains AC-V66 (29), KSK394 (46), and DMS-V491 (32) do not produce detectable endogenous β-galactosidase activity and were used as hosts for rpoS, hapR, or hapA-lacZ transcriptional fusions. Growth was monitored by reading the optical density at 600 nm (OD600).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Characteristics |
|---|---|
| V. cholerae strains | |
| C7258 | Wild type, E1 Tor (Peru isolate, 1991) |
| C6709-1 | Wild type, E1 Tor (Peru isolate, 1991) |
| C6706 | Wild type, E1 Tor (Peru isolate, 1991) |
| N16961 | E1 Tor, hapR (naturally occurring frameshift in hapR) |
| AC-V66 | C6709-1, lacZ: res-tet-res (A. Camilli, Tufts University) (29) |
| KSK394 | C6706, crp:: Kmr (K. Skorupski, Dartmouth Medical School) (46) |
| DSM-V491 | C6709-1, ΔrpoS (A. Camilli, Tufts University) (32) |
| AJB2 | AC-V66, hapA::hapA-lacZ, Ampr (this study) |
| AJB3 | KSK394, hapA::hapA-lacZ, Ampr (this study) |
| AJB19 | C7258 relA::pCVDΔrelAK (this study) |
| AJB216 | N16961, ΔaphA (this study) |
| AJB231 | AC-V66, ΔaphA (this study) |
| AJB25 | DSM-V491, hapR::hapR-lacZ (this study) |
| AJB26 | AC-V66, hapR::hapR-lacZ (this study) |
| AJB29 | AC-V66, rpoS::rpoS-lacZ (this study) |
| E. coli strains | |
| DH5α | F− φ80lacZM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− mR+) phoA supE44 λ−thi-1 gyrA96 relA1 |
| SY327λPir | Δ(lac pro) argE(Am) rif nalA recA56 (λpirR6K) (35) |
| SM10λPir | thi thr leu tonA lacY supE recA::RP4-2Tc::Mu (λpirR6K) (35) |
| Plasmids | |
| pBADHisB | Expression vector with araBAD promoter, Ampr (Invitrogen) |
| pALTERex2 | In vitro mutagenesis vector, T7 promoter, Tetr (Promega Corp.) |
| pGP704 | Suicide vector (oriR6K, mobRP4, Ampr) (35) |
| pCVD442 | Suicide vector (oriR6K, mobRP4, sacB, Ampr) (11) |
| pHapLac11 | Plasmid vector containing transcriptional hapA-lacZ fusions (45) |
| pHapRLac2 | Plasmid vector containing transcriptional hapR-lacZ fusions (45) |
| pGPHap10 | KpnI-PstI hapA-lacZ fusion from pHaplac11 in pGP704 (this study) |
| pGPHap13 | KpnI-PstI hapR-lacZ fusion from pHapRlac2 in pGP704 (this study) |
| pCH2 | pACYC184 containing 3.2-kb HindIII hapA insert (19) |
| pBADHapR9 | HapR ORF cloned in pBADHisB (this study) |
| pBADCRP7 | CRP ORF cloned in pBADHisB (this study) |
| pCVDΔrelA | 1,077-bp internal relA fragment cloned in pCVD442 (this study) |
| pCVDΔrelAK | pCVDΔrelA with Kmr in unique NsiI site of relA (this study) |
| pΔAphA | 5′ SphI-BamHI and 3′ BamHI-SacI aphA fragments in pUC19 (this study) |
| pCVDΔAphA | SphI-SacI ΔAphA fragment from pΔAphA cloned in pCVD442 (this study) |
| pRpoS5 | SphI-HindIII rpoS promoter fragment cloned in pUC19 (this study) |
| pRpoSlac5 | SphI-HindIII rpoS DNA replacing hapR promoter in pHapRlac2 (this study) |
| pGPRpoSlacZ | XbaI-ScaI fragment from pRpoSlac5 cloned in pGP704 (this study) |
| pJB43 | 428-bp hapA 5′ fragment in pALTERex2 (this study) |
TABLE 2.
Primers used in this study
| Primer name | Sequence |
|---|---|
| AphA40 | 5′-CGGGAATGCGCAATACTGGTTAACA |
| AphA207 | 5′-CGCTTATCACAGAGGGAACGTAGT |
| AphA1409 | 5′-GCTGGATCCGAACTGGTTGAAGAAT |
| AphA2441 | 5′-GAAGAGCTCGCCAGTTTTTACCATG |
| AphA248 | 5-CCATTAATGAAGGACACCAAGCGC |
| AphA1169 | 5′-GAAGGATCCGATATACCTGCTGATG |
| AphA1742 | 5′-GAGCAGTTTAATGGTCGTTCGCAGA |
| AphA940 | 5′-GGGTGACATAAGCAGCCGAATTTTG |
| Cap123 | 5′-GCAGCCATGACAAAAACGCGTAAC |
| Cap249 | 5′-GCCGGTATGGAGAAACAGTAGAGA |
| Crp892 | 5′-GTTTTACCGTGGGCAGAGAT |
| Crp794 | 5′-CTTGGCGAGTGATCTTGATTTGCA |
| Crp367 | 5′-CATAAGTACCCATCAAAAAGCACGC |
| Crp304 | 5′-GGCGGATCCATGGTTCTAGGTAAAC |
| Crp945 | 5′-GGCAAGCTTACACGAGACGGGTTAT |
| HapA459 | 5′-ACATGCATGCCTGGCTTTCTTATCGA |
| HapA502 | 5′-ACATGCATGCACACGTAGAGTTCACA |
| HapA664 | 5′-GGCCAAGCTTCATTTCTCAATCCTAG |
| HapA456 | 5′-GGCGGATCCTTTCTGGCTTTCTTAT |
| HapA875 | 5′-GCGAATTCCCGTTGTACATCTGCT |
| HapR1100 | 5′-GCCAACTTATACACTGCTTCT |
| HapR1046 | 5′-ATGGAGTAGAAGATGCCGTGGAAC |
| HapR589 | 5′-CGCCTCAAAAACGCAAACTACAACT |
| HapR552 | 5′-AAACTGCAGGACGCATCAATCGAAAA |
| HapR1158 | 5′-CCGGAATTCGCTGCCCAAGAAACTA |
| RelA842 | 5′-GTTCTAGAGGTGATTAAGCTTGCCG |
| RelA1895 | 5′-GTTGCATGCCCCGCTTCGAGATTT |
| RpoS1226 | 5′-CAGCAGTGCCTTATCTCCAT |
| RpoS1003 | 5′-TGTTTGGTTCATCAGCGCACGTTC |
| RpoS576 | 5′-CGATTTTGAAGATGAAGCACTGGAAG |
| RpoS228 | 5′-AAAGCATGCATGTTGAACCTGTCGG |
| RpoS549 | 5′-GGGAAGCTTTTTGGTTACGGTATTG |
| RecA1061 | 5′-CGCTTTACCTTGGCCGATTT |
| RecA578 | 5′-GTGCTGTGGATGTCATCGTTGTTG |
| RecA863 | 5′-CCACCACTTCTTCGCCTTCTTTGA |
Cloning of V. cholerae genes and regulatory sequences.
Chromosomal DNA was purified from V. cholerae as described previously (1). Unless otherwise specified, V. cholerae genes and regulatory sequences were amplified from genomic DNA of strain C6709-1 by using the Advantage PCR system (Clontech). All primers were designed based on the DNA sequence of the V. cholerae N16961 genome downloaded from The Institute for Genomic Research database. The ORFs encoding HapR and CRP were amplified with primers HapR552 plus HapR1158 and Crp304 plus Crp945, respectively. The V. cholerae rpoS promoter region was amplified with primers RpoS228 and RpoS549. An internal relA fragment was amplified with primers RelA842 and RelA1895. A 201-bp fully active hapA promoter fragment was amplified with primers HapA459 and HapA664. A second, inactive 158-bp hapA promoter fragment lacking a putative CRP binding site was amplified with primers HapA502 and HapA664. An aphA promoter fragment containing a HapR binding site was amplified with primers AphA40 and AphA207. An E. coli arabinose (araBAD) promoter fragment containing a cAMP-CRP binding site was amplified from pBADHisB with primers Cap123 and Cap249. All amplification products were directionally cloned in pUC19, and both DNA strands were sequenced using the M13 forward and reverse sequencing primers at the Emory University School of Medicine DNA Core facility.
Construction of V. cholerae strains containing chromosomal hapA-lacZ, hapR-lacZ, and rpoS-lacZ transcriptional fusions.
Construction of hapA-lacZ and hapR-lacZ transcriptional fusions was described previously (45). KpnI-PstI fragments containing the hapA-lacZ fusion from pHaplac11 and the hapR-lacZ fusion from pHapRlac2, respectively (Table 1), were subcloned in a suicide vector, pGP704 (35), digested with the above enzymes to generate suicide plasmids pGPHap10 and pGPHap13 (Table 1). To construct V. cholerae strains containing chromosomal rpoS-lacZ fusions, the SphI-HindIII insert in pRpoS5 (Table 1) was subcloned in pHapRlac2, replacing hapR promoter DNA to generate pRpoSlacZ. Next, the rpoS-lacZ fusion was extracted as an XbaI-ScaI fragment and ligated to pGP704 digested with the above enzymes to create the suicide vector pGPRpoSlacZ (Table 1). All suicide vector were constructed in SY327λPir, electroporated to SM10λPir, and transferred by conjugation to receptor V. cholerae strains, and exconjugants were selected in LB agar containing ampicillin and polymyxin B. Suicide vector pGPHap10 was transferred to AC-V66 and KSK293 to generate strains ABJ2 and AJB3, respectively. Suicide vector pGPHap13 was transferred to AC-V66 and DSM-V491 to create strains AJB26 and AJB25, respectively. Finally, pGPRpoSlacZ was transferred to strains AC-V66 to generate AJB29 (Table 1). Integration into the chromosomal hapA or hapR locus was verified by Southern hybridization with a digoxigenin (DIG)-labeled hapA HindIII fragment isolated from plasmid pCH2 (19) or the hapR ORF isolated from pBADHapR9, respectively. Correct integration was confirmed by detection of hapA or hapR in a BglII fragment with a molecular weight higher than in the precursor strains. Correct integration in the rpoS locus in strain AJB29 was verified by Southern hybridization with a DIG-labeled rpoS fragment from pRpoS5. Integration in the rpoS locus of AJB29 was confirmed by the detection of anticipated HindIII junction fragments.
Construction of ΔaphA mutants.
The 5′ and 3′ fragments of the aphA gene were amplified from strain C6709-1 using primer combinations AphA248 plus AphA1169 and AphA1409 plus AphA2441, respectively. The amplified DNAs were sequentially cloned as SphI-BamHI and BamHI-SacI fragments in pUC19, creating an aphA gene with an internal deletion and a frameshift (pΔaphA) (Table 1). The deleted and framshifted aphA gene was transferred to pCVD442 (11) as a SphI-SacI fragment to generate pCVDΔaphA. This plasmid was transferred to E. coli SM10λpir and mobilized by conjugation to V. cholerae N16961 and AC-V66. Exconjugants were selected in LB medium containing ampicillin and polymyxin. Integration into the aphA locus was verified by Southern hybridization using the DIG-labeled 3′ aphA amplification product cloned in pΔaphA. Correct integration was confirmed by the detection of two HindIII junction fragments in exconjugants instead of the single HindIII fragment detected in precursor strains. Exconjugants from N16961 and AC-V66 were allowed to segregate in LB medium and plated in LB agar containing 5% sucrose. Sucrose-resistant clones were tested for ampicillin sensitivity and screened for the deleted aphA allele by PCR using primers AphA940 plus AphA1742. Segregants AJB216 and AJB231 were found to contain the deleted aphA gene.
Disruption of the activity domain of V cholerae relA.
A 1,077-bp V. cholerae relA fragment was amplified as described above and cloned in suicide vector pCVD442 (11) as a SphI-XbaI fragment to generate pCVDΔrelA. A PstI fragment containing the Kmr gene from plasmid pUC4K (Pharmacia) was subsequently inserted in a unique NsiI site located within the highly conserved enzyme activity domain (between essential residues G251 and H354) to create pCVDΔrelAK. This plasmid was transferred by conjugation to V. cholerae C7258 as described above to generate exconjugant AJB19. The organization of the relA locus in exconjugant AJB19 was determined by Southern hybridization analysis of NsiI digests using the DIG-labeled amplified 1,077-bp relA fragment.
RNase protection assays.
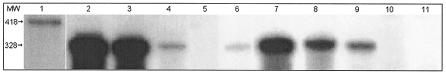
Total RNA was isolated using the RNeasy kit (Qiagen Laboratories). A 5′ fragment of hapA was amplified using primers HapA456 plus HapA875 and cloned in pALTERex2 (Promega Corp.) to generate plasmid pJB43. A 428-bp radiolabeled riboprobe was synthesized by in vitro transcription with T7 RNA polymerase in the presence of 50 μCi of [α-32P]UTP (800 Ci/mmol; Amersham). The riboprobe was purified by polyacrylamide gel electrophoresis, and 2 × 104 to 8 × 104 cpm was annealed with 10 μg of total RNA. Single-stranded RNA was degraded with RNase A and RNase T1 by using the Ambion RPA III kit, and protected fragments were separated in an 8 M urea-5% polyacrylamide gel. After autoradiography, a single 318-bp protected fragment was detected in V. cholerae strains expressing hapA (Fig. 1).
FIG. 1.
RNase protection assay analysis of hapA expression. V. cholerae strains were grown in TSB medium to an OD600 of 2 (deceleration phase). At this point, 10-ml aliquots were withdrawn for total-RNA extraction (lanes 2 to 6) and the remaining culture was further incubated for 16 h (late stationary phase) (lane 7 to 11). Lanes: 1, intact probe (RNase-minus control); 2 and 7, C6706; 3 and 8 C6709-1; 4 and 9, KSK394 (crp); 5 and 10; N16961 (hapR); 6 and 11; DSM-V491 (rpoS). MW, molecular size in base pairs.
RT-PCR assays.
Reverse transcription PCR (RT-PCR) analysis was performed using the Titanium one-step RT-PCR kit (Clontech). Briefly, 20 ng of total RNA was used as the template for cDNA synthesis. The following primer combinations were used: RpoS1223, RpoS1003, and RpoS576 for rpoS mRNA; HapR1100, HapR1046, and HapR589 for hapR mRNA; Crp892, Crp794, and Crp367 for crp mRNA; and RecA1061, RecA578, and RecA863 for recA mRNA. A control without reverse transcriptase was used for each reaction to exclude chromosomal DNA contamination. For quantitative comparisons, RNA samples were analyzed by real time RT-PCR using the iScript one-step RT-PCR kit with SYBR Green (Bio-Rad Laboratories). Relative expression values (R) were calculated using the equation R = 2−(ΔCt target − ΔCt reference), where Ct is the fractional threshold cycle. The recA mRNA was used as reference.
Expression and purification of His-HapR and His-CRP.
The ORFs encoding HapR and CRP were amplified from C6709-1 as described above and subcloned in pBADHisB (Invitrogen) to generate pBADHapR9 and pBADCRP7, respectively. Both genes were expressed from the arabinose (araBAD) promoter as His6 fusions. His6-HapR and His6-CRP were purified to homogeneity in ProBond columns as specified by the manufacturer (Invitrogen), for induction, cell disruption (sonnication), and affinity purification. Purity was confirmed by the appearance of a single band in a Coomassie blue-stained SDS-PAGE gel that reacted with anti-Xpress antibodies (Invitrogen).
DNA binding assays.
DNA binding assays were conducted using the DIG gel shift procedure (Roche). A 201-bp fully active and HapR-dependent hapA promoter fragment and a 158-bp 5′ deletion fragment lacking promoter activity were amplified as described above. A 162-bp aphA fragment containing a HapR binding site (26) was used as a positive control. Similarly, a 126-bp fragment containing a cAMP-CRP binding site from pBADHisB was used as a positive control for cAMP-CRP binding. Fragments were labeled with DIG and terminal transferase, and binding reactions were conducted as recommended by the kit manufacturer, using 10 ng of labeled DNA. DNAs were separated in a 5% nondenaturing polyacrylamide gel and processed for chemiluminescence detection.
Western blot analysis.
The presence of HA/protease in V. cholerae supernatants was detected with a anti-HA/protease serum and peroxidase-conjugated anti-rabbit immunoglobulin G as described previously (2).
Enzyme assays.
β-Galactosidase activities were measured as described by Miller (33), using the substrate o-nitrophenyl-β-d-galctotopyranoside (ONPG). Specific activities are given in Miller units (1,000 OD420 t−1 v−1 OD600−1), where t is reaction time and v is the volume of enzyme extract per reaction.
RESULTS
Transcription of hapA requires rpoS and is enhanced by CRP.
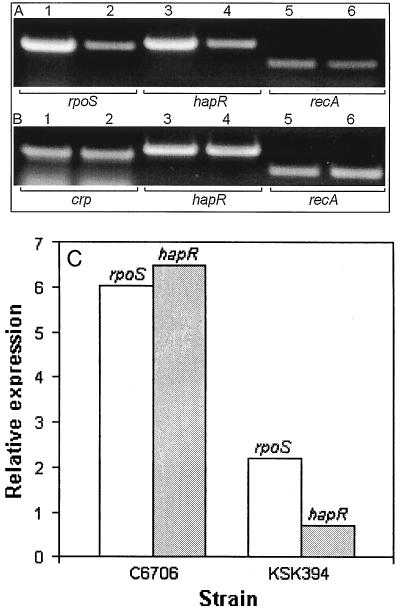
As shown in Fig. 1 (lanes 4 to 6), very little or no hapA mRNA could be detected in crp, hapR, and rpoS mutants in the growth deceleration phase. As cells progressed into the late stationary phase, expression of hapA became less dependent on CRP (Fig. 1, compare lanes 7 and 9) but remained strongly dependent on hapR and rpoS (lane 10 and 11). Interestingly, lower levels of hapA mRNA were detected in cells grown to late stationary phase (Fig. 1, compare lanes 7 and 8 to lanes 2 and 3). We considered the possibility that overexpression of σS in the late stationary phase could diminish the expression of hapR. Strains AJB25 and AJB26 contain a hapR-lacZ fusion integrated in a rpoS and rpoS+ genetic background, respectively (Table 1). When these strains were grown to late stationary phase, the ΔrpoS mutant expressed slightly higher levels of the hapR-lacZ fusion (21.7 ± 2.6 and 13.7 ± 2.1 Miller units for AJB25 and AJB26, respectively).
To study the role of CRP and rpoS in hapA transcription, we first considered the possibility that HapR, CRP, and RpoS could cross-regulate their transcription. We determined the production of rpoS, hapR, and crp mRNA in wild-type strains and isogenic mutants (Fig. 2). Lower levels of rpoS and hapR mRNA were detected in crp mutant KSK394 than in its precursor, C6706 (Fig. 2A). No differences were detected under these conditions (growth deceleration phase) for crp and hapR mRNA production in a ΔrpoS mutant compared to its isogenic precursor (Fig. 2B). Because conventional RT-PCR tends to be biased against samples with more abundant transcripts, total RNA from C6706 and KSK394 was analyzed by real-time RT-PCR for rpoS, hapR, and recA mRNA. As shown in Fig. 2C, strain KSK394 produced substantially lower levels of both rpoS and hapR mRNA.
FIG. 2.
Transcription of rpoS, hapR, and crp in wild-type and isogenic regulatory mutants. V. cholerae strains were grown in TSB to an OD600 of 2, and RT-PCR was conducted as described in Materials and Methods. (A) Lanes: 1, 3, and 5, C6706 (crp+); 2, 4, and 6, KSK394 (crp). (B) Lanes: 1, 3, and 5 C6709-1 (rpoS+); 2, 4, and 6, DMS-V491 (rpoS). The mRNA detected in each lane is written below each panel. (C) Real-time RT-PCR. RNA extracted from three cultures of C6706 and KSK394 was analyzed for the relative expression of rpoS and hapR (targets) with recA mRNA as reference.
Glucose represses hapA by inducing V. cholerae to resume exponential growth.
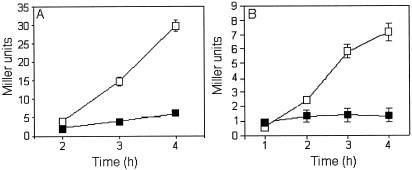
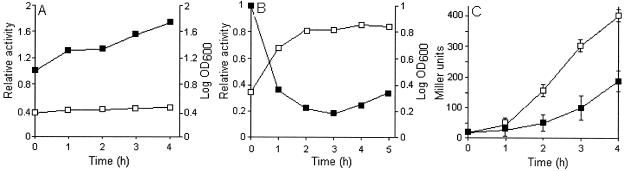
Exconjugant AJB3 (crp mutant) produced five times less β-galactosidase activity than did AJB2 (crp+), confirming that the expression of hapA is enhanced by CRP. However, addition of glucose at the growth deceleration phase repressed the hapA promoter in both strains, demonstrating that glucose repression of hapA is not mediated by CRP (Fig. 3). Repression of hapA by glucose in TSB medium was paralleled by stimulation of growth (Fig. 4A and B). In Fig. 4C we show that addition of glucose reduced the expression of a rpoS-lacZ transcriptional fusion in V. cholerae.
FIG. 3.
Glucose repression does not require CRP. Strains AJB2 (A) and AJB3 (B) containing a hapA-lacZ transcriptional fusion inserted in the hapA locus in a crp+ (AC-V66) and crp (KSK394) background, respectively, were grown in TSB medium to an OD600 of 1. Cultures were divided in half and further incubated for 4-h with (▪) and without (□) glucose. Samples were taken hourly for enzyme assays and OD600 determinations. Each value represents the average of three independent cultures. Error bars indicate the standard deviation of the mean.
FIG. 4.
Correlation between glucose repression and growth stimulation. (A and B) Strain AJB2 was grown to an OD600 of 2, and the culture was divided in half. Glucose was added to one half (B), and the other half was used as a control (A). Samples were taken at 1-h intervals for β-Galactosidase activity (▪) and OD600 readings (□). (C) Strain AJB29 containing a rpoS-lacZ transcriptional fusion was grown in TSB to an OD600 of 1, and the culture was divided in half. Half was supplemented with glucose (▪), and half was used as a control (□). Relative activities in panels A and B refer to the Miller units at the time of glucose supplementation. Final Miller units reported are the mean of three independent cultures. Error bars indicate the standard deviation of the mean.
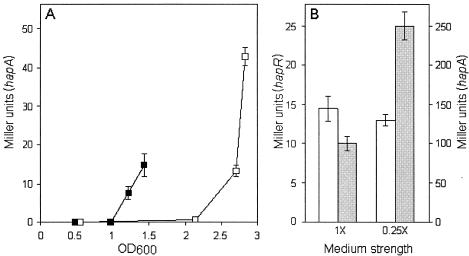
We next considered whether cells induced to enter the stationary phase at lower cell densities could transcribe hapA. To test this possibility, we performed a nutritional downshift experiment. A low level of hapR mRNA was detected by RT-PCR in exponentially growing V. cholerae AJB2 cells at OD600 values as low as of 0.2 (data not shown). V. cholerae AJB2 was grown in rich TSB medium to an OD600 of 0.5, at which hapA expression is not detected, and half of the culture was collected and resuspended in 0.25× TSB. As shown in Fig. 5A, the nutritional downshift had a twofold effect. First, expression of hapA was diminished due to lower levels of HapR at the shift point, indicating that nutrient limitation alone does not fully activate hapA. Second, hapA expression could be detected at a lower optical density. To determine if the expression of hapA at a lower optical density was due to activation of hapR or hapA, strains AJB2 (hapA-lacZ) and AJB26 (hapR-lacZ) were grown to late stationary phase in TSB medium of different strengths. While hapA promoter activity was enhanced by nutrient limitation, the activity of the hapR promoter remained constant (Fig. 5B). We conclude that that expression of hapA requires two concurrent environmental stimuli: high cell density and entry to stationary phase due to nutrient limitation.
FIG. 5.
Effect of a nutritional downshift on hapA promoter activity. (A) V. cholerae AJB2 was grown to an OD600 of 0.5. Half of the culture was centrifuged, and the cells were resuspended in 0.25 × TSB (▪); the remaining cells were kept in 1 × TSB (□). Cultures were analyzed for β-galactosidase activity at different time points. (B) Overnight cultures of strain AJB26 (hapR-lacZ) (open bars) and AJB2 (hapA-lacZ) (shaded bars) were diluted in TSB medium of different strengths and incubated at 37°C until late stationary phase. Cultures were analyzed for hapR- and hapA-driven β-galactosidase expression. The final Miller units reported represent the mean of three independent cultures. Error bars indicate the standard deviation of the mean.
Expression of hapA does not require RelA or AphA.
In E. coli, ppGpp enhances the transcription of rpoS (8, 17, 41) and many rpoS-dependent genes (28). Therefore, we examined the role of relA in the expression of hapA. Southern hybridization showed that strain AJB19 contains two inactive relA alleles: one containing a Kmr insertion in the N-terminal activity domain and a second allele lacking the entire N terminus (data not shown). As expected from the phenotype of E. coli relA mutants (41), strain AJB19 was sensitive to AT and amino acids SMGL in combination (data not shown). Nevertheless, AJB19 produced wild-type levels of HA/protease (Fig. 6, lane 2). We also considered the possibility that HapR could control the transcription of hapA through the regulator AphA, the only known target for HapR (26, 27). Since transcription of aphA is repressed by HapR at high cell density (26), we speculated that aphA could encode a repressor of hapA. This hypothesis predicts that inactivation of aphA should restore HA/protease production in hapR mutant strain N16961 and enhance protease production in hapR+ strain AC-V66. We constructed two strains, each containing a deletion and frameshift mutation in the aphA gene. Strain AJB216, derived from N16961, and strain AJB231, derived from AC-V66, produced significantly reduced levels of CT as a result of the aphA mutation (26) (data not shown). However, the aphA mutation in AJB216 did not restore HA/protease production in N16961 (Fig. 6, lane 4) and AJB231 did not express significantly higher levels of HA/protease than AC-V66 (lane 6).
FIG. 6.
Western blot analysis of HA/protease expression in V. cholerae mutants. Supernatants from V. cholerae strains grown to saturation in TSB medium were concentrated in Centricon-10 centrifugal filters (Amicon Bioseparations), subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% polyacrylamide) and transferred to a polyvinylidene difluoride membrane. Lanes: 1, C7258; 2, AJB19; 3, N16961; 4, AJB216; 5, AC-V66; 6, AJB231; 7, DSM-V491; 8, pure HA/protease.
HapR and CRP alone do not bind hapA.
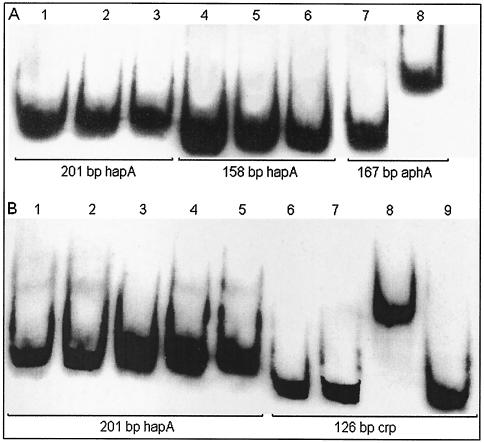
We conducted electrophoretic mobility shift assays using the 201-bp minimal but fully active and HapR-dependent hapA promoter fragment and the a 158-bp inactive fragment which lacks a DNA sequence with dyad symmetry, resembling a cAMP-CRP binding site. Binding experiments revealed that purified His6-HapR and His6-CRP do not bind the active hapA promoter. Both proteins were capable of shifting the positive control fragment containing a HapR binding site from aphA and a CRP site from the arabinose operon (Fig. 7). No electrophoretic mobility shift was detected by adding His6-CRP and His6-HapR together to the binding-reaction mixture.
FIG. 7.
DNA binding assays. (A) His6-HapR. DIG-labeled DNA fragments (10 ng) were incubated with no protein (lane 1), 64 ng of protein (lane 2), 128 ng of protein (lane 3), no protein (lane 4), 64 ng of protein (lane 5), 128 ng of protein (lane 6), no protein (lane 7), and 64 ng of protein (lane 8) (B) His6-CRP. DIG-labeled DNA fragments (10 ng) were incubated with no protein (lane 1), 50 ng of protein and cAMP (lane 2), 50 ng of protein (lane 3), 500 ng of protein and cAMP (lane 4), 500 ng of protein (lane 5), no protein (lane 6), no protein with cAMP (lane 7), 500 ng of protein and cAMP (lane 8), and 500 ng of protein (lane 9). The concentration of cAMP was 500 μM.
DISCUSSION
Very little is known about how environmental factors other than cell density control the transcription of hapA and about the role of CRP and RpoS in this process. Barely detectable hapA mRNA is synthesized in the growth deceleration phase in crp, hapR, and rpoS mutants (Fig. 1). Strong rpoS dependency was also confirmed by the finding that strain DSM-V491 does not support the expression of hapA-lacZ fusions forming white colonies in X-Gal medium. In a previous study, we observed that ΔrpoS mutant DSM-V491 secreted three times less azocasein activity but normal levels of soluble hemagglutinating activity. Since the HA/protease carries both activities, we concluded that HA/protease was not affected in this mutant. In the above study, strains were incubated at 30°C for longer periods (> 24 h). It is possible that rpoS mutants could release other proteases and materials with hemagglutinating activity on prolonged incubation in the stationary phase due to their stress survival defect (53). In Fig. 6 (lane 7) we show that no HA/protease-cross-reacting material could be detected in concentrated supernatants of strain DSM-V491.
Transcription of hapA is strongly enhanced by CRP (Fig. 1). As cells progressed into the late stationary phase, expression of hapA became less dependent on CRP (Fig. 1, lane 9) but remained strongly dependent on hapR and rpoS (lanes 10 and 11). As reported for the regulation of rpoS in E. coli (20), the results shown in Fig. 2A and C suggest that CRP activates hapA in V. cholerae by enhancing the transcription of rpoS. It is noteworthy that a conserved cAMP-CRP binding pentamer is located in the V. cholerae rpoS promoter. As cells progress into the stationary phase, an increase in the level of σS due to enhanced translation or protein stability could partially relieve the CRP dependency of hapA.
Comparison of hapR mRNA in isogenic crp+ and crp strains, using quantitative real-time RT-PCR, suggested that CRP also enhances the transcription of hapR in cells collected at the growth deceleration phase (Fig. 2A and C). The link between CRP and HapR is interesting since it has been reported that V. cholerae quorum-sensing system 3 responds to an intracellular signal that acts through regulators LuxO and HapR (34). Our finding suggests that such internal signal could be cAMP.
We also noticed that cells grown to late stationary phase expressed lower levels of hapA mRNA (Fig. 1, compare lanes 2 and 3 with lanes 7 and 8). A phenomenon described as sigma factor competition has been found in E. coli, by which accumulation of σS lowers the expression of σ70-dependent promoters (39, 48). Because transcription of hapA is subject to a dual regulation requiring both housekeeping and stationary-phase σ factors, we considered the possibility that the lower transcription of hapA in late stationary phase could be due to reduced hapR expression. Strain AJB25 (rpoS) grown to late stationary phase expressed higher levels of a hapR-lacZ fusion than did the isogenic rpoS+ strain, AJB26. However, the increase in expression of hapR in the rpoS mutant was lower than twofold, suggesting that additional factors could be involved.
We have previously reported that secretion of HA/protease is repressed by glucose (2). The presence of a region of dyad symmetry resembling a cAMP-CRP binding site in the hapA promoter suggested a carbon catabolite repression mechanism. However, although the crp exconjugant AJB3 expressed five times less β-galactosidase activity from the hapA promoter, it was still repressed by glucose (Fig. 3). This result demonstrates that repression of hapA transcription by glucose is not mediated by CRP. Accordingly, other sugars that might be under carbon catabolite repression themselves (i.e., galactose, mannose, maltose, sucrose, and glycerol) (16, 25) repressed hapA as efficiently as glucose did (data not shown). Repression of hapA by glucose strongly correlated with growth stimulation (Fig. 4A and B) and diminished the transcription of a rpoS-lacZ chromosomal fusion (Fig. 4C). By analogy to the regulation of σS in E. coli (20), glucose might also act on rpoS translation and σS stability. The above experiment clearly demonstrated that high cell density alone is not enough to activate hapA transcription, since glucose repression was accompanied by an increase in cell density.
When cultures of exponentially growing V. cholerae are induced to enter the stationary phase at a lower cell density, hapA transcription was detected at lower optical densities (Fig. 5A). Two factors could contribute to the expression of hapA at lower cell density: nutrient limitation could increase cAMP levels, leading to a CRP-mediated increase in HapR, and/or nutrient limitation promotes early entry in stationary phase, with accumulation of σS. Although these explanations are not mutually exclusive, the fact that hapR transcription was not significantly enhanced by nutrient limitation (Fig. 5B) suggests that entrance to the stationary phase is the predominant cause of early transcription of hapA. Taken together, our results show that hapA is transcribed in response to concurrent high cell density and nutrient limitation stimuli. It appears advantageous for V. cholerae to prevent hapA expression in populations entering the stationary phase at cell densities not supporting hapR expression, at which the formation of a biofilm is favored (47, 54).
In E. coli, transcription of rpoS and many rpoS-dependent genes is influenced by RelA activity (20). Nevertheless, strain AJB19 produced wild-type levels of HA/protease (Fig. 6). These results strongly suggest that the expression of hapA is not greatly affected by RelA activity. However, since only relA spoT double mutants entirely lack ppGpp (52), it is possible that the relA mutation in AJB19 does not reduce ppGpp levels enough to affect the expression of hapA.
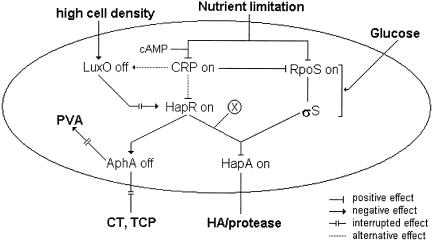
Interestingly, pure His6-CRP or His6-HapR added separately or together did not bind the hapA promoter. This result indicates that HapR activates hapA by a mechanism different from that of its interaction with the aphA promoter. The hapA promoter does not contain the HapR recognition sequence present in aphA (26). The hapA+ phenotypes of mutants AJB216 and AJB231 exclude the possibility of HapR controlling hapA through AphA (Fig. 6). Therefore, HapR could require additional factors to bind hapA or could control the expression of HA/protease through an unidentified regulator. In Fig. 8 we provide a model for the regulation of HA/protease expression in the context of other virulence factors, based on our current findings. The salient features this model are that transcription of hapA requires concurrent expression of hapR and rpoS, CRP enhances hapA transcription through hapR and rpoS, glucose represses hapA by inducing cells to resume exponential growth and lowering rpoS transcription, and HapR requires additional factors to activate hapA.
FIG. 8.
Regulatory interactions involved in hapA transcription regulation. The hapA gene is transcribed in response to two concurrent environmental signals: high cell density and nutrient limitation. Nutrient limitation leads to entry of bacteria into the stationary phase, with enhanced transcription of rpoS and high levels of σS. At high cell density in the stationary phase, HapR is expressed and, in combination with an unidentified factor (X) and σS, activates the transcription of hapA. Glucose represses hapA transcription by inducing cells to resume exponential growth, blocking the pathway to accumulation of σS. The cAMP-CRP complex enhances the transcription of hapA by positively influencing the transcription of rpoS and hapR. CRP could increase HapR levels by acting on luxO or hapR. HapR represses the expression of AphA, required for the production of CT and TCP. In the absence of AphA, pva, encoding penicillin V amidase, is expressed.
The transcriptional regulation of hapA reveal similarities and important differences compared to related metalloproteases from other members of the Vibrionaceae. A common property appears to be regulation by LuxR homologues such as HapR (V. cholerae), SmcR (V. vulnificus), or VanT (V. anguillarum) (10, 44). HA/protease differs in how its promoter integrates quorum-sensing and nutrient limitation stimuli. V. vulnificus vvpE elastase is transcribed from two promoters: promoter PL, constitutive throughout the logarithmic and stationary phases, and promote PS, dependent on SmcR, RpoS, and CRP (22). In contrast, hapA is not transcribed unless nutrient limitation induces cells to enter the stationary phase (Fig. 8). In V. vulnificus, CRP and SmcR coactivate elastase expression by binding to the PS promoter (23). CRP also binds to V. harveyi lux promoters to regulate luminescence (9). In V. cholerae, CRP activates hapA by increasing the transcription of hapR and rpoS (Fig. 8). CRP also increases the transcription of luxR in V. fischeri (12). These differences may play a role in enhancing the ability of different vibrios to adhere to and colonize their specific niches.
V. cholerae colonizes the distal small intestine, where most luminal nutrients (i.e., simple sugars) have already been absorbed (7). This mucin-rich environment could provide the signal to activate hapA. We have proposed that expression of HA/protease during infection could perturb the protective mucus barrier, promote detachment, and spread the infection along the gastrointestinal tract. (3,4,45). Because the expression of HA/protease also requires high cell density, we would expect HA/protease effects to be more prominent in volunteers ingesting a high inoculum of hapA+ live vaccines parallel with neutralization of stomach acidity.
Acknowledgments
The present study was partially supported by research grant 3506GM008248 from the National Institutes of Health.
We are grateful to Richard A. Finkelstein (University of Missouri School of Medicine) for critically reading the manuscript and for his encouragement.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Short protocols in molecular biology. John Wiley & Sons Inc., New York, N.Y.
- 2.Benitez, J. A., A. J. Silva, and R. A. Finkelstein. 2001. Environmental signals controlling production of hemagglutinin/protease in Vibrio cholerae. Infect. Immun. 69:6549-6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benitez, J. A., L. Garcia, A. J. Silva, H. Garcia, R. Fando, B. Cedre, A. Perez, J. Campos, B. L. Rodriguez, J. L. Perez, T. Valmaseda, O. Perez, A. Perez, M. Ramirez, T. Ledon, M. Diaz, M. Lastre, L. Bravo, and G. Sierra. 1999. Preliminary assessment of the safety and immunogenicity of a new CTXΦ-negative hemagglutinin/protease-defective E1 Tor strain as a cholera vaccine candidate. Infect. Immun. 67:539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benitez, J. A., R. G. Spelbrink, A. J. Silva, T. E. Phillips, C. M. Stanley, M. Boesman-Finkelstein, and R. A. Finkelstein. 1997. Adherence of Vibrio cholerae to cultured differentiated human intestinal cells: an in vitro colonization model. Infect. Immun. 65:3474-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth, B. A., M. Boesman-Finkelstein, and R. A. Finkelstein. 1983. Vibrio cholerae soluble hemagglutinin/protease is a metalloenzyme. Infect. Immun. 42:639-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booth, B. A., M. Boesman-Finkelstein, and R. A. Finkelstein. 1984. Vibrio cholerae hemagglutinin/protease nicks cholera enterotoxin. Infect. Immun. 45:558-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler, S. M., and A. Camilli. 2004. Both chemotaxis and net motility greatly influence the infectivity of Vibrio cholerae. Proc. Natl. Acad. Sci. USA 101:5018-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cashel, M., D. R. Gentry, V. J. Hernandes, and D. Vinella. 1996. The strigent response, p. 1458-1496. In F. C. Neidhardt, R. Curtiss III, J. L.: Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. American Society for Microbiology, Washington, D.C.
- 9.Chatterjee, J., C. M. Miyamoto, A. Zouzoulas, B. Franz Lang, N. Skouris, and E. A. Meighen. 2002. MetR and CRP bind to the Vibrio harveyi lux promoters and regulate luminescence. Mol. Microbiol. 46:101. [DOI] [PubMed] [Google Scholar]
- 10.Croxatto, A., V.J. Chalker, J. Lauritz, J. Jass, A. Hardman, P. Williams, M. Camara, and D.L. Milton. 2002. VanT, a homologue of Vibrio harveyi LuxR, regulates serine, metalloprotease, pigment, and biofilm production in Vibrio anguillarum. J. Bacteriol. 184:1617-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunlap, P. V. 1999. Quorum regulation of luminescence in Vibrio fischeri. J. Mol. Microbiol. Biotechnol. 1:5-12. [PubMed] [Google Scholar]
- 13.Finkelstein, R. A., and L. F. Hanne. 1982. Purification and characterization of the soluble hemagglutinin (cholera lectin) produced by Vibrio cholerae. Infect. Immun. 36:1199-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkelstein, R. A., M. Boesman-Finkelstein, and P. Holt. 1983. Vibrio cholerae hemagglutinin/lectin/protease hydrolyzes fibronectin and ovomucin: F. M. Burnet revisited. Proc. Natl. Acad. Sci. USA 80:1092-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkelstein, R. A., M. Boesman-Finkelstein, Y. Chang, and C. C. Häse. 1992. Vibrio cholerae hemagglutinin/protease, colonial variation, virulence, and detachment. Infect. Immun. 60:472-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganguly, U., and W. B. Greennough III. 1975. Adenosine 3′5′-cyclic monophosphate in Vibrio cholerae. Infect. Immun. 11:343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gropp, M., Y. Strausz, M. Gross, and G. Glaser. 2001. Regulation of Escherichia coli RelA requires oligomerization of the C-terminal domain. J. Bacteriol. 183:570-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haralalka, S., S. Nandi, and R. K. Bhadra. 2003. Mutation in the relA gene of Vibrio cholerae affects in vitro and in vivo expression of virulence factors. J. Bacteriol. 185:4672-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Häse, C. C., and R. A. Finkelstein. 1991. Cloning and nucleotide sequence of the Vibrio cholerae hemagglutinin/protease (HA/protease) gene and construction of an HA/protease- negative strain. J. Bacteriol. 173:3311-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hengge-Aronis, R. 2002. Signal transduction and regulatory mechanisms involved in control of the σS (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 66:373-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honda, T., A. Hata-Naka, K. Lertpocasombat, and T. Miwatani. 1991. Production of monoclonal antibodies against a hemagglutinin/protease of Vibrio cholerae non-O1. FEMS Microbiol. Lett. 62:227-230. [DOI] [PubMed] [Google Scholar]
- 22.Jeong, H. S., K. C. Jeong, H. K. Choi, K.-J. Parks, K.-H. Lee, J. H. Rhee, and S. H. Choi. 2001. Differential expression of Vibrio vulnificus elastase gene in a growth phase-dependent manner by two different types of promoters. J. Biol. Chem. 276:13875-13880. [DOI] [PubMed] [Google Scholar]
- 23.Jeong, H. S., M. H. Lee, K.-H. Lee, S.-J. Park, and S. H. Choi. 2003. SmcR and cyclic AMO receptor protein coactivate Vibrio vulnificus vvpE encoding elastase through the RpoS-dependent promoter in synergistic manner. J. Biol. Chem. 278:45072-45081. [DOI] [PubMed] [Google Scholar]
- 24.Jobling, M. G., and R. K. Holmes. 1997. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol. Microbiol. 26:1023-1034. [DOI] [PubMed] [Google Scholar]
- 25.Kolb, A., S. Busby, H. Buc, S. Garges, and S. Adhya. 1993. Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 62:749-795. [DOI] [PubMed] [Google Scholar]
- 26.Kovacikova, G., and K. Skorupski. 2002. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol. Microbiol. 46:1135-1147. [DOI] [PubMed] [Google Scholar]
- 27.Kovacikova, G., E. Lin, and K. Skorupski. 2003. The virulence factor activator AphA links quorum sensing to pathohgenesis and physiology in Vibrio cholerae by repressing the expression of a penicillin amidase gene on the small chromosome. J. Bacteriol. 185:4825-4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kvint, K., A. Farewell, and T. Nystrom. 2000. RpoS-dependent promoters require guanosine tetraphosphate for induction even in the presence of high levels of σS. J. Biol. Chem. 275:14795-14798. [DOI] [PubMed] [Google Scholar]
- 29.Lee, S. H., D. L. Hava, M. K. Waldor, and A. Camilli. 1999. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell 99:625-634. [DOI] [PubMed] [Google Scholar]
- 30.Marcus H., J. M. Ketley, J. B. Kaper, and R. K. Holmes. 1990. Effect of DNAse production, plasmid size and restriction barrier on transformation of Vibrio cholerae by electroporation and osmotic shock. FEMS Microbiol. Lett. 68:149-154. [DOI] [PubMed] [Google Scholar]
- 31.Mel, S. F., K. J. Fullner, S. Wimer-Mackin, W. I. Lencer, and J. J. Mekalanos. 2000. Association of protease activity in Vibrio cholerae vaccine strains with decrease in transcellular epithelial resistance of polarized T84 intestinal cells. Infect. Immun. 68:6487-6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merrell, D. S., A. D. Tischler, S. H. Lee, and A. Camilli. 2000. Vibrio cholerae requires rpoS for efficient intestinal colonization. Infect. Immun. 68:6691-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, J. H. 1971. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 34.Miller, M. B., K. Skorupski, D. H. Lenz, R. K. Taylor, and B. L. Bassler. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303-314. [DOI] [PubMed] [Google Scholar]
- 35.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane protein and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyoshi, S., and S. Shinoda. 2002. Microbial metalloproteases and pathogenesis. Microbes Infect. 2:91-98. [DOI] [PubMed] [Google Scholar]
- 37.Miyoshi, S.-H., H. Wakae, K.-I. Tomochika, and S. Shinoda. 1997. Functional domains of a zinc metalloprotease from Vibrio vulnificus. J. Bacteriol. 179:7606-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagamune, K., K. Yamamoto, A. Naka, J. Matsuyama, T. Miwatani, and T. Honda. 1996. In vitro proteolytic processing and activation of the recombinant precursor of E1 Tor cytolysin/hemolysin (Pro-HlyA) of Vibrio cholerae by soluble hemagglutinin/protease of V. cholerae, trypsin, and other proteases. Infect. Immun. 64:4655-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nogueira, T., and M. Springer. 2000. Post-transcriptional control by global regulators of gene expression in bacteria. Curr. Opin. Microbiol. 3:154-158. [DOI] [PubMed] [Google Scholar]
- 40.Robert, A., A. J. Silva, J. A. Benitez, B. L. Rodriguez, R. Fando, J. Campos, D. K. Sengupta, M. Boesman-Finkelstein, and R. A. Finkelstein. 1996. Tagging a Vibrio cholerae E1 Tor candidate vaccine strain by disruption of its hemagglutinin/protease gene using a novel reporter enzyme: Clostridium thermocellum endoglucanase A. Vaccine 14:1517-1522. [DOI] [PubMed] [Google Scholar]
- 41.Rudd, K. E., B. R. Bochner, M. Cashel, and J. R. Roth. 1985. Mutations in the spoT gene of Salmonella typhimurium: effects on his operon expression. J. Bacteriol. 163:534-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandkvist, M., L. O. Michel, L. P. Hough, V. M. Morales, M. Bagdasarian, M. Koomkey, V. J. DiRita, and M. Bagdasarian. 1997. General secretion pathway (eps) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J. Bacteriol. 179:6994-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott, M. E., Z. Y. Dossami, and M. Sandkvist. 2001. Directed polar secretion of protease from single cells of vibrio cholerae via the type II secretion pathway. Proc. Natl. Acad. Sci. USA 98:13978-13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao, C.-P. and L.-I. Hor. 2001. Regulation of metalloprotease gene expression in Vibrio vulnificus by a Vibrio harveyi LuxR homologue. J. Bacteriol. 183:1369-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva, A. J., K. Pham, and J. A. Benitez. 2003. Hemagglutinin/protease expression and mucin gel penetration in E1 Tor biotype Vibrio cholerae. Microbiology 149:1883-1891. [DOI] [PubMed] [Google Scholar]
- 46.Skorupski, K., and R. K. Taylor. 1997. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin co-regulated pilus in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 94:265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vance, R. E., J. Zhu, and J. J. Mekalanos. 2003. A constitutively active variant of the quorum-sensing regulator LuxO affects protease production and biofilm formation in Vibrio cholerae. Infect. Immun. 71:2571-2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vicente, M., K. F. Chater, and V. De Lorenzo. 1999. Bacterial transcription factors involved in global regulation. Mol. Microbiol. 33:8-17. [DOI] [PubMed] [Google Scholar]
- 49.Whiteley, M., M. R. Parsek, and E. P. Greenberg. 2000. Regulation of quorum sensing by RpoS in Pseudomonas aeruginosa. J. Bacteriol. 182:4356-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu, Z., D. Milton, P. Nybon, A. Sjo, and K. E. Magnusson. 1996. Vibrio cholerae hemagglutinin/protease (HA/protease) causes morphological changes in cultured epithelial cells and perturbs their paracellular barrier function. Microb. Pathog. 21:111-123. [DOI] [PubMed] [Google Scholar]
- 51.Wu, Z., P. Nybom, and K. E. Magnusson. 2000. Distinct effects of Vibrio cholerae hemagglutinin/protease on the structure and localization of the tight junction-associated proteins occludin and ZO-1. Cell. Microbiol. 2:11-17. [DOI] [PubMed] [Google Scholar]
- 52.Xiao, H., M. Kalman, K. Ikehara, S. Zemel, G. Glaser, and M. Cashel. 1991. Residual guanosine 3′5′-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J. Biol. Chem. 266:5980-5990. [PubMed] [Google Scholar]
- 53.Yildiz, F. H., and G. K. Schoolnik. 1998. Role of rpoS in stress survival and virulence of Vibrio cholerae. J. Bacteriol. 180:773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu, J., M. B. Miller, R. E. Vance, M. Dziejman, B. L. Bassler, and J. J. Mekalanos. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 99:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]