Abstract
Introduction
Sickle Cell Disease (SCD) affects 100,000 Americans and more than 14 million people globally, mostly in economically disadvantaged populations, requires early diagnosis after birth and constant monitoring throughout the life-span of the patient.
Areas Covered
Early diagnosis of SCD still remains a challenge in preventing childhood mortality in the developing world due to requirements of skilled personnel and high-cost of currently available modalities. On the other hand, SCD monitoring presents insurmountable challenges due to heterogeneities among patient populations, as well as in the same individual longitudinally. Here, we describe emerging point-of-care micro/nano platform technologies for SCD screening and monitoring, and critically discuss current state-of-the-art, potential challenges associated with these technologies, and future directions.
Expert Commentary
Recently developed microtechnologies offer simple, rapid, and affordable screening of SCD and have the potential to facilitate universal screening in resource-limited settings and developing countries. On the other hand, monitoring of SCD is more complicated compared to diagnosis and requires comprehensive validation of efficacy. Early use of novel microdevices for patient monitoring might come in especially handy in new clinical trial designs of emerging therapies.
Keywords: sickle anemia, sickle cell disease screening, hemoglobinopathies, patient monitoring, point-of-care microtechnologies, electrophoresis, red blood cells, erythrocytes, microfluidics
1. Introduction
Sickle cell disease (SCD) is a genetically inherited debilitating illness, caused by a point mutation in the beta-globin gene, that requires early diagnosis after birth and constant monitoring throughout the life-span of the patient. Sickle cell anemia was first clinically described in the US in 19101, and the mutated heritable sickle hemoglobin molecule was identified in 19492. It is estimated that 100,000 Americans and more than 14 million individuals worldwide3 have SCD, disproportionally in economically disadvantaged populations. SCD is estimated to cost more than $1 billion per year in healthcare costs in the US, while the full economic burden of SCD is likely to be greater considering the additional contributions of productivity loss, uncompensated care, reduced quality of life, and premature mortality4, 5.
The underlying mutation of a single amino acid in the beta chain of sickle hemoglobin (HbS) belies the complex, highly morbid, and sometimes life-threatening clinical phenotype of SCD6, 7. The pathophysiology of SCD is a consequence of abnormal polymerization of HbS and its effects on red cell membrane properties, shape, and density, and subsequent critical changes in inflammatory cell and endothelial cell function. Observed pathophysiologic changes in SCD include alterations in adhesion amongst sickled red blood cells (RBCs) and activated white blood cells (WBCs) and endothelium, and abnormal numbers of circulating endothelial cells and hematopoietic precursor cells. The clinical consequences of SCD are anemia, painful crisis, widespread organ damage, and early mortality4.
Neonatal diagnosis of SCD is critical for the management of the disease, since undiagnosed children are especially in great danger of early mortality due to infections and stroke. Early diagnosis of SCD still remains a critical challenge in preventing childhood mortality in resource limited, developing regions of the world, such as sub-Saharan Africa, due to requirements of skilled personnel and high-cost of instrumentation and testing associated with conventional approaches. SCD diagnosis can be generally achieved through protein or molecular tests in the developed world due to its genetically inherited nature. However, monitoring of SCD patients presents insurmountable challenges due to heterogeneities among patients, as well as in the same individual from time to time, and the multi-system nature of the disease. Furthermore, neither conventional monitoring techniques nor conventional screening tools currently available are feasible for operation at the point of care (POC), impeding easy access to healthcare, as well as exacerbating patients’ quality of life.
Micro/nano platform technologies emerged in the last couple of decades8, 9, through advancements in fabrication techniques and versatile materials, offer unique advantages in overcoming the challenges associated with conventional SCD screening and monitoring tools. This review article describes prominent platform technologies for SCD screening and monitoring and critically discusses current state-of-the-art, potential challenges associated with these technologies, and future directions.
2. Global Scope of SCD
Even though the main birthplace of SCD is Africa, its geographical distribution is now spread worldwide due to migration. Today, SCD is most prevalent in regions of Sub-Saharan Africa, The Americas, Saudi Arabia, India, and Mediterranean countries such as Turkey, Greece, Italy, and South East Asia10, 11. SCD is highly prevalent in malaria endemic regions of the world11. The greatest burden of SCD still lies in Africa, where the number of newborns affected by SCD are estimated to be more than 200,000 annually10, 12, 13. In Africa, SCD is associated with high rate of childhood mortality, 50–90% of African children with SCD die early in their childhood11, 14. In other words, approximately 1,000 babies are born with SCD in Africa every day and more than half die before they are five years old14. The sickle cell carrier frequency across equatorial Africa is between 10% and 40%, which results in an SCD prevalence of at least 2%11. In some parts of Western and Central Africa, the prevalence of sickle cell trait is as high as 25%. SCD also has a high prevalence in central and western regions of India, where approximately 20% of children born with SCD die before the age of two15. Estimated number of people with sickle trait in North America is 2–3 million, whereas the number is 1–2 million in Brazil10. According to the data available, over 6,000 annual births and 100,000–150,000 adults are affected by SCD in Latin America16. According to National Health Service in the UK, the number of people affected by SCD is estimated to be between 12,500 and 15,000, which makes SCD the most common inherited disease in the UK17. 4.2% of the total population in Saudi Arabia is a carrier of sickle cell trait whereas 0.26% is affected by SCD18. In Jamaica, 10% of the total population carries some sort of genetic disorder related to SCD19. In the United States, SCD is the most common inherited blood disorder, and most of the people who suffer from SCD are of African descendent. About 100,000 Americans are affected by SCD, it occurs in about 1 in 365 African-American births, and 1 in every 16,300 Hispanic American births11. Sickle cell trait is estimated to occur in about 1 in 13 African-American births11.
3. SCD Pathophysiology
The pathophysiology of SCD is a consequence of abnormal deoxygenated sickle hemoglobin polymerization and its deleterious effects on RBC membrane, shape, density, deformability, and adhesion. The pathophysiology of SCD mainly consists of anemia, inflammation, hemolysis, vaso-occlusion, and consequent tissue ischemia, pain crisis, and organ damage. Though being the first discovered molecular disease, SCD has been known to be highly complex due to its heterogeneous characteristics in pathophysiology, making it hard to pinpoint the underlining biological mechanisms. Many facets of SCD pathophysiology have been investigated, including hemoglobin polymerization20–22, cellular deformability23–28, adhesion25, 29–34, hemodynamic changes35, 36, and clinical heterogeneity6, 7.
The original powerful observation that sickled red cells show abnormal adhesion to endothelial cells has since been deepened and expanded to describe a complex pathophysiology in which abnormal WBC adhesion also plays an important role. Therefore, along with RBC abnormalities, any approach to understanding SCD pathophysiology must also take into account endothelial, WBC, and platelet activation and adhesion, inflammation, and activation of coagulation37–50. Together, these heterotypic cellular and blood plasma abnormalities, arising ultimately from HbS-polymerization, yield a clinical syndrome that is characterized by acute and chronic pain, cumulative organ damage, and early mortality51, 52.
3.1 Vaso-Occlusion
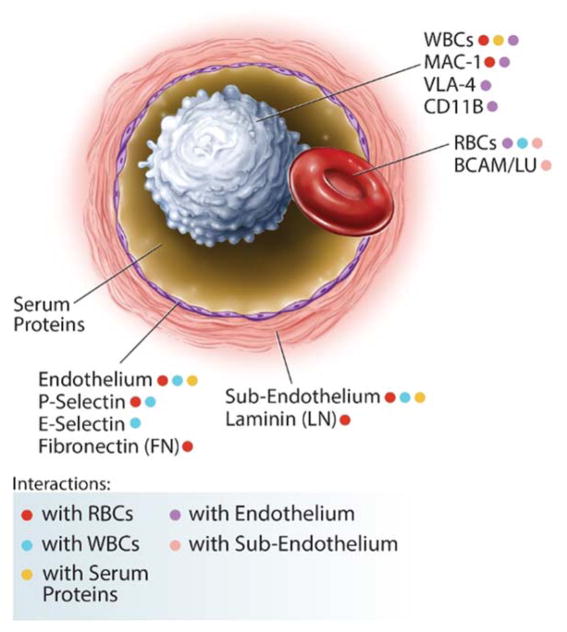
Abnormal adherence to endothelium, by sickle RBCs and WBCs, as a possible root cause of vaso-occlusion and pain, was described in the 1980s, highlighting inflammation and abnormal cellular adhesion as key features of SCD48, 53–55. A myriad of interconnecting abnormal interactions can be envisioned, amongst HbS-containing RBCs, activated WBCs, and activated endothelial cells in SCD (Fig. 1). Key clinical and experimental studies in SCD literature, performed via flow chambers or ex vivo rat mesocecum29, 30, 54, 56, have shown that RBC adhesion and deformability, WBC adhesion and activation57, and endothelial activation contribute to the pathogenesis of vaso-occlusion33, 56, 58, 59 and may correlate with disease severity34, 48, 60, 61. Abnormal RBC adhesion to endothelium has associated with disease activity34, 48 and has diminished with treatment34, 62, with variable but elevated adhesion at clinical baseline. Associations with clinical status have shown using FACS analysis of membrane protein components63–65. However, few longitudinal measurements of adhesion at baseline and with therapy have been performed due to lack of convenient reproducible adhesion assays30, 34.
Figure 1. A subset of interactions between cellular and sub-cellular components in SCD.
Abnormal interactions, amongst HbS-containing RBCs, soluble serum proteins (such as thrombospondin, TSP, and von Willebrand Factor, vWF), cytokine- and WBC- (CD11b+ monocytes) activated endothelial cells (through integrins, integrin receptors, adhesion molecules, and selectins), subendothelial matrix components (including TSP, vWF, fibronectin, and laminin), and activated WBCs (via MAC-1+, LFA-1+, VLA-4+ neutrophils), which themselves also directly adhere to the endothelium.
Abnormal monocyte, neutrophil, platelet, and endothelial cell activation and adhesion are present in SCD, and complementary models of vaso-occlusive crises (VOC) describe initial reticulocyte and neutrophil adhesion to an activated endothelium and/or subendothelial matrix (Laminin, LN; Fibronectin, FN; von Willebrand Factor, vWF), followed by dense (irreversibly sickled) red cell trapping and vaso-occlusion33, 66, 67. Further refinements in the model, based on ex vivo and in vivo experiments, is one in which the endothelium is activated by cytokines and white cells, primarily monocytes, which are themselves activated by sickle RBC-derived factors40, 68–70. These factors combine to increase the adhesiveness of RBCs and white cells, primarily neutrophils and monocytes, to each other and to the endothelium and sub-endothelium, leading to vaso-occlusion. Soluble bridging factors (Thrombospondin, TSP; FN; vWF) are also important, although the interactions are not simply quantified33, 41, 46, 57, 66, 69, 71–75. Further, activated endothelial cells and hematopoietic precursor cells circulate at an unusually high level in SCD40, 48, 76, and correlate with end-organ damage77. Some membrane/cellular interactions have been studied during VOC48, 76, 78, or compellingly demonstrated in animal models57, 79, but broad clinically correlative studies are absent.
3.2 RBC Adhesion and Deformability
A healthy biconcave HbA-containing RBC deforms easily and passes through minuscule vessels and capillaries in the body80–82. Deoxygenated HbS polymerizes inside the red cell83, altering its membrane, shape, and density30, 33, 48, 56, 83–85. These biophysical changes cause reduced deformability, increased stiffness, and abnormal adhesion of the HbS-containing RBC (SCD RBC), and may result in blockage of blood vessels48, 83, 85, 86 and reduced red cell half-life (hemolysis)87, 88.
Sympathetic tone and ‘stress’ signals, such as epinephrine, are modulators of SCD RBC adhesion and of abnormal vascular tone89–93. Importantly, intravascular heme arising from hemolysis impairs endothelial cell function and vascular tone, while triggering WBC activation, inflammation, and activation of coagulation94–98. In SCD, RBC membrane abnormalities include aberrant timing or abnormal persistence during maturation, and abnormal activation, by ‘stress signals’, of surface molecules such as Very Late Antigen-4 (VLA-4), Cluster of Differentiation 36 (CD36), LW glycoprotein, and Basal Cell Adhesion Molecule/Lutheran (BCAM/LU)74, 99–106. Cumulative oxidative damage, resulting in excessive phosphatidylserine (PS) externalization on the SCD RBC membrane, causes abnormal adhesion107, 108. Anti-SCD RBC adhesion therapy has been validated pre-clinically, and, importantly, these targets are beginning to reach clinical trial, including VLA-4 blocking antibodies109, and beta-adrenergic receptor blockade (via an FDA-approved medication, propranalol110) targeting epinephrine-mediated red cell adhesion92, 99, 106, 111, 112. Small molecules (αVβ3 integrin)113 and low molecular weight heparin (P-Selectin)59, 114 were utilized to target RBC adhesion to an activated endothelium specifically, and an oral agent for this purpose is in phase I/II studies in humans (P-selectin)58, 115, 116.
Studies showed that heme and plasma from SCD patients induce neutrophil extracellular traps (NETs) in murine models of SCD97, resulting in capture of RBCs and platelets117, 118. It is not known why hemolysis is more active in some patients87, nor why hemolysis can exacerbate during severe painful crises119–121. SCD RBC deformability associates with hemolysis and adverse clinical outcomes122, without definitive causality123, 124. Adhesion to the endothelium may prolong delay time, and increase polymer formation and fragility as the RBC passes through the vasculature51. Furthermore, an association between hemolysis and increased SCD RBC adhesion to components of the endothelium/sub-endothelial surface has been shown recently34.
3.3 WBC adhesion
Elevated numbers of activated WBCs (monocytes40, 69, 125, 126 and neutrophils42, 127, 128) in SCD patients have long been associated with adverse outcomes in SCD, such as stroke and even early mortality52, 69, 98, 129–132. Moreover, increased rates of endothelial activation and inflammation in SCD induce abnormal leukocyte recruitment to the vessel wall57, 133. The initiation and propagation of vaso-occlusive events subsequently takes place due to interactions between sickle RBCs and adherent leukocytes39. Using an SS mouse model and intra-vital microscopy, Turhan et al. showed that these interactions occurred in post-capillary venules and some of them indeed caused VOC in vivo57. On the other hand, in mice deficient of both E-selectin and P-selectin, vaso-occlusive events did not develop upon TNF-α induction57. Adherent leukocytes and RBC-leukocyte aggregates also distort the local microcirculation that increases the RBC transit time. This phenomenon renders RBCs more susceptible to sickling due to longer exposure to deoxygenation in the microvasculature which could lead to mediated RBC-leukocyte interactions134.
Even though both P- and E-selectin are essential for WBC adhesion to the endothelium, E-selectin can further trigger secondary activation signals in the WBC. These signals result in polarized activated αMβ2 integrin (CD11b/CD18 or Mac-1) expression at the leading edge of the crawling neutrophil, and SCD RBC capture135. Surprisingly, inhibition of E-selectin abrogates these effects, whereas inhibition of P-selectin has only a partial effect, which was tested in vivo in VOC using the novel synthetic pan-selectin inhibitor (GMI-1070) with maximal activity against E-selectin136, 137. Many studies in the literature suggest that blocking FcγRIII receptor activity on neutrophils by intravenous immunoglobulin infusions (IVIG) may interrupt Mac-1 activation and RBC capture by neutrophils. NET formation is also inhibited by FcγRIII blockade.
3.4 Endothelial Dysfunction & Inflammation
A growing body of evidence suggests that interplay between vascular dysfunction and high levels of inflammation remarkably contribute to the pathophysiology of SCD40, 42, 138–144. As an endothelial mediator, nitric oxide (NO) has been shown to correlate with the impaired endothelium functioning in sickle cell patients145, 146. Elevated rates of hemolysis in SCD reduce the bioavailability of NO leading to vasoconstriction and further release of pro-inflammatory cytokines into plasma, which activates the endothelium 147. Indeed, it has been shown that circulating endothelial cells are significantly increased in sickle cell patients regardless of their clinical status76. Subsequently, through NO-dependent activation pathways, the adhesion molecules such as VCAM-1, E-selectin, P-selectin, and ICAM-1 are overexpressed on the endothelial layer at significantly higher rates contributing to following vaso-occlusion and painful crises148, 149. Other than NO, endothelium activation is also induced by the adhesion of activated platelets in SCD150.
Furthermore, SCD can be associated with elevated counts of leukocytes, activated platelets, and pro-inflammatory cytokines, all of which are indicators of a marked chronic inflammatory state151–154. Activated monocytes and platelet monocyte aggregates in sickle cell patients trigger endothelial inflammatory response through the nuclear factor κB (NF-κB) pathway155. This interaction is mediated by several cytokines produced by monocytes including tumor necrosis factor α (TNF- α) and interleukin-1β (IL-1β)40. Moreover, invariant natural killer T cells (iNKT) in sickle cell patients overexpress chemokines CXCR3 and IFN-γ that has been shown to mediate pulmonary inflammation156, 157.
4. SCD Screening at the POC
4.1. Ongoing Challenges and Unmet Needs in the Clinic
4.1.1 Developed World
POC screening for SCD in the developed world could allow more cost-effective identification of children at risk. Consistent and economic screening may improve care in these regions with less prevalent hemoglobin gene disorders, and incomplete lab-based support. Even though a well-established universal screening program for SCD is in place in some resource rich-countries, such as the US and UK, uniform newborn screening is not in place in many developed countries due to economic and technical challenges. Recent studies suggest that universal screening could prevent early childhood mortality in SCD, since unscreened patients in low-prevalence regions in developed countries are at greater risk for life-threatening complications during early childhood158–161. Moreover, prevalence of SCD is steadily increasing in European countries due to immigration162–166, requiring additional healthcare support and expanded screening programs, requiring additional healthcare support and expanded screening programs. Screening platforms adapted for mobile phone use in the developing world could increase patient engagement in resource rich settings, by giving patients their own mobile diagnostic. Finally, a cheaper, more-widely available platform could increase access for re-screening, as people reach reproductive age, to allow self-identification in those at risk for transmitting the HbS or HbC genes, i.e., those most at risk for having children with SCD. While this could not fully evaluate genetic risk in all patients, e.g., those with beta thalassemia trait would likely be missed, an accessible, affordable hemoglobin screen, although imperfect, could screen for those at greatest risk for transmitting SCD.
4.1.2 Low Resource Settings
With its origins in sub-Saharan Africa, the Indian subcontinent and the Arabian Peninsula, the sickle β-globin gene has spread throughout the world. It is estimated that more than three quarters of those homozygous for the hemoglobin S gene are born in Africa alone, with half the global burden borne by just three countries: Nigeria, India and Democratic Republic of Congo161. In low-income countries, limited resources for diagnosis and treatment, aggravated by a dearth of government strategies to combat SCD, have led to poor patient outcomes. The World Health Organization (WHO) estimates that more than half of the children born with SCD in sub-Saharan Africa die before the age of 5 years11, 164, 167. This calls for the widespread implementation of affordable and evidence-based interventions that can be integrated into existing health systems to ensure their sustainability. Evidence from high- as well as low-income countries has shown that implementation of a range of interventions, including newborn screening, penicillin prophylaxis, pneumococcal vaccination and parental education significantly reduces morbidity and mortality168–171. However, in low-resource settings, diagnosis of SCD is hampered by the high cost of currently available laboratory methodologies, posing a major barrier to implementing life-saving interventions. Further, limited contact with healthcare delivery systems requires that screening methodologies be timely and generate easily interpretable results to enable initiation of interventions at the POC. The deployment of low-cost, rapid, and accurate POC screening tools will be transformative in helping break the diagnostic barrier. These POC solutions lend themselves to integration into already existing public health programs such as primary immunization, a critical factor in ensuring sustainability in low-resource settings. Generation of easy-to-read results that requires only minimal training for healthcare workers and adaptability to delivery via mobile phone platforms are great assets in employing POC techniques for widespread screening of SCD within public health systems with limited-resources.
4.2. Conventional Techniques for SCD Diagnosis
The diagnosis of homozygous HbSS (sickle cell anemia, SCA) and heterozygous HbSA (sickle cell trait, SCT), HbSC disease, and HbS-β thalassemias are based on the varying percentages and combinations of HbS, HbA, HbF, HbC, and HbA2 present in RBCs. The most basic tests used to identify the presence of sickle hemoglobin are the sickling test and the sickle solubility test172. In sickling test, sodium metabisulfite is used to induce polymerization of sickle hemoglobin and consequent sickling of RBCs by reducing oxygen tension. Then, the diluted blood sample is observed under a microscope to observe the sickled RBCs. Although simple, this test cannot differentiate between HbSS, HbSA, HbSC, or HbS-β thalassemias.
The solubility test works by making HbS insoluble in a concentrated phosphate buffer solution. In this reduced state, HbS precipitates and forms tactoids that refract light creating a turbid solution. The result is compared to positive and negative control blood. An important issue is that these simple screening tests cannot be performed on newborns because of the predominance of HbF at birth. It takes several months after birth for newborns with HbSS (SCA) or HbSA (SCT) to produce significant amounts of HbS, which can be detected with these tests. If used at birth, the tests may produce false-negative results if HbS is less than 10% of the total hemoglobin.
Additional tests are needed to confirm which form of SCD the patient has. There are four tests that are commonly used: hemoglobin electrophoresis, isoelectric focusing (IEF), high performance liquid chromatography (HPLC), and DNA analysis173, 174. The electrophoresis based tests work based on the principle that different Hb types migrate with different velocities when placed in an electric field due to their different net charges. Following is a brief description of each of the aforementioned techniques.
4.2.1 Bench-top Hemoglobin Electrophoresis
Hemoglobin electrophoresis is a laboratory method that can be performed under alkaline or acidic conditions with a variety of sieving materials such as gel or paper165. Under alkaline conditions, hemoglobin types C, A2, S, F, and A have net negative charges and migrate towards the positively charged electrode. Various factors such as charges of the hemoglobin, the pore size of the medium, and the ionic concentration of the buffer solution determine how far each hemoglobin type migrates. The separation of hemoglobin types form visible bands that can be used to identify various hemoglobin disorders. Hb electrophoresis is especially useful for the rapid screening of a small number of samples, and its results can be quantified using densitometry, which may suffer from inaccuracies at very low concentrations. Alkaline Hb electrophoresis displays lower resolution between HbS and HbF, particularly in neonates who have high HbF levels. Finally, electrophoresis carried out in a capillary tube is known as capillary zone electrophoresis (CZE)165. This method allows for the use of higher voltages and shorter run times, which renders it especially advantageous for high-throughput screening.
4.2.2 Isoelectric Focusing
IEF exploits the fact that the net charge of a protein varies with the pH of the surrounding medium. Utilizing this variation, proteins are separated based on their isoelectric points (pI), which can be defined as the point at which a protein possesses zero net charge. The technique uses an applied electrical field across a gel medium with a fixed pH gradient, in which each Hb type becomes immobilized once it reaches its pI. IEF exhibits higher resolution than Hb electrophoresis, thus it is capable of distinguishing between a larger number of Hb variants165, 166. However, due to the larger number of bands that this higher resolution results in, IEF results are harder to interpret164,165. IEF is also more expensive and, as in hemoglobin electrophoresis, quantification is achieved using densitometry which can be inaccurate especially at low hemoglobin concentrations. Despite these challenges, IEF is considered to be the standard for newborn screening, since diagnosis is possible with very small sample volume or even an eluate from a dried blood spot164.
4.2.3 High Performance Liquid Chromatography
HPLC separates a fluid into its components based on molecular size and charge using cation exchange chromatography to identify the various hemoglobin types in a blood sample. HPLC utilizes absorbent materials such as granular silica or other polymers as a sieving medium. A pressure pump drives the fluid through the material, and a computer detects the separation. Unique aspects of this test are full automation and accurate quantification of the hemoglobin levels. These machines are relatively expensive and are not readily available in developing countries. In resource rich countries like the US, HPLC has largely replaced Hb electrophoresis and IEF as a primary screening test. This is because Hb electrophoresis and IEF are labor intensive, time consuming and are not designed to quantify Hb levels. The ability to quantify Hb levels with HPLC makes it useful for monitoring patients who are on hydroxyurea or transfusion therapies175.
4.2.4 DNA Analysis
DNA-based assays can be used to detect the mutations in β globin that produce abnormal Hb176. However, it is generally more expensive than the previously described methods. An earlier popular method for the DNA-based assay utilizes the point mutation on the β-globin gene with restriction enzyme digestion and polymerase chain reaction (PCR). The point mutation, that causes SCD, changes the normal β-globin gene sequence, which removes the restriction site for the restriction enzyme DdeI. During the restriction enzyme digestion of the β-globin gene using DdeI, the gene is split into two fragments if the mutation is not present. However, if the mutation is present, the gene remains as a single large fragment. PCR is then used to amplify the fragments for identification using electrophoresis. Currently, the most robust testing strategy utilizes direct sequencing of β-globin combined with copy number variation analysis of the beta-globin locus.
4.3. Emerging POC Technologies for SCD Screening
The most recent technological trend for SCD screening focusses on adapting available diagnostic tools for feasible operation at the POC, especially in resource-challenged regions. The emerging technologies have veered towards overcoming concerns of cost, fabrication complexity, portability, as well as the need for highly-trained operators associated with conventional techniques. According to their operating principles and detection schemes, the emerging techniques in the past few years can be categorized into four groups: (i) paper-based hemoglobin solubility assays, (ii) lateral flow immunoassays, (iii) density-based separation, and (iv) microengineered electrophoresis. The work reported in each of these categories is reviewed in this section and summarized in Table 1.
Table 1.
Emerging POC technologies for SCD screening
| Technique | Advantages | Disadvantages | Cost per test | Equipment cost | Turnaround time | Sensitivity | Specificity | LOD | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Paper-based hemoglobin solubility | Simple fabrication, ease of use, uses natural color of blood, no batching. | Failure to distinguish between HbSC and HbAS. Blood clotting interferes with the test. Interpretation of results is susceptible to human error. | $0.7 | $300–500 (for automated detection) | 20 mins | 94.2%a | 97.7%a | NA | 177–180 |
|
| |||||||||
| Lateral flow immunoassay (Sickle SCAN) | Ease of use. Rapid results. Absence of auxiliary equipment. | Complex fabrication. Interpretation of results is susceptible to human error. Hb quantification is not supported. | >$5b | NA | 2 mins | 99%c | 99%c | HbA: 40%; HbS: 1%; HbC: 2% | 181 |
|
| |||||||||
| 98.3–100%d | 92.5–100%d | 1–2% | 182 | ||||||
|
| |||||||||
| Lateral flow immunoassay (HemoTypeSC) | Ease of use. Absence of auxiliary equipment. | Complex fabrication. Interpretation of results is susceptible to human error. Hb quantification is not supported. | $0.25e | NA | 20 mins | 100%f | 100%f | HbA: 2.7%; HbS: 3.3%; HbC: 1.3% | 183 |
|
| |||||||||
| Density-based separation | Simple testing procedure. Rapid results. | Need for bulky centrifuge limits applicability to POC setting. Batching required for centrifuge operation. Inability to distinguish between HbAA and HbAS. Inaccuracies due to high HbF levels, health and treatment conditions, and genetic factors affect RBC density. | $0.5 | $150–1,600 | 10 mins | 90–91%g | 88–97%g | 2.8% (dense cells) | 184 |
|
| |||||||||
| Microengineered Electrophoresis (HemeChip) | Low cost. Ease of use. Robustness. Rapid testing. Results comparable to standard electrophoresis tests. Possible integration with mobile devices. Works on the principles of clinical standard electrophoresis test. | High HbF concentration present in newborns less than 4 weeks of age may affect test results. | $0.9 | ~$500h (for automated detection) | <10 mins | 89–100%i | 82–89%i | HbS: 10%; HbF: 10%; HbA:10%; HbC/A2: 3% | 185 |
for the detection of the HbS presence.
based on personal communication.
for the detection of different Hb genotypes.
for the detection of the presence of different Hb types.
estimated material cost only.
for the differentiation between different Hb phenotypes.
for the identification of HbSS and HbSC with two-phase and three-phase AMPS.
initial assessment of automated reader cost.
for differentiating between adjacent bands corresponding to various Hb types.
4.3.1 Paper-Based Hemoglobin Solubility Assay
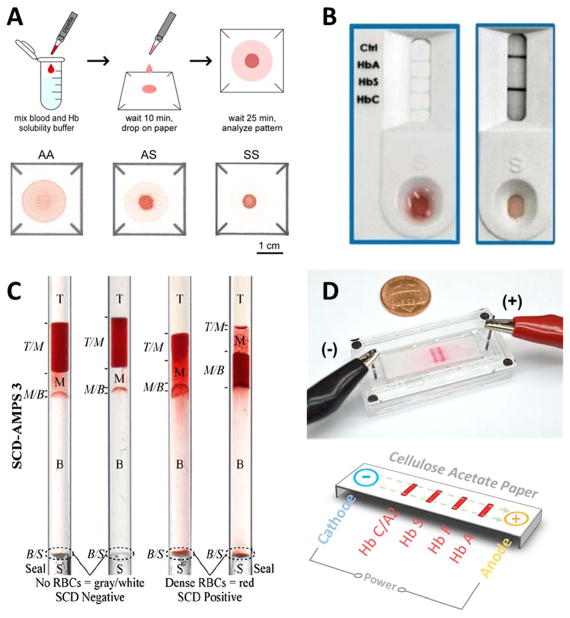
This technique exploits the insolubility of HbS and the filtration properties of the paper substrate used in microfluidic paper-based analytical devices (μPADs) as a means to visually detect the presence of HbS. To perform the test, a drop of blood (20 μL) mixed with a hemoglobin solubility buffer in a 1:10 ratio is applied onto patterned chromatography paper. The difference in capillary action transport of polymerized HbS and other hemoglobin types results in different blood stain patterns (Fig. 2A). These patterns are used to differentiate between HbAA, HbAS and HbSS, and the test process can be completed within 20 minutes177–180. Initial validation results of this test in a resource-limited setting showed that HbS can be visually identified with 94.2 % sensitivity and 97.7 %180. Furthermore, when combined with a custom image analysis algorithm, the relative color intensity of the center spot can be used to quantify Hb concentration in the sample.
Figure 2. Illustrations of the principle of operation of the emerging technologies for SCD diagnosis.
(A) Paper-based Hemoglobin solubility180. A droplet of blood mixed with Hb solubility buffer is dropped on chromatography paper, and a blood stain is allowed to form. The stain on paper is analyzed and the color intensity profiles are used to determine the Hb type in the sample. (B) Sickle Scan™ lateral flow immunoassay181. The test specimen consisting of a drop of blood mixed with Hb solubility buffer is dropped onto the sample loading zone. The solution then diffuses to the test zones where Hb is captured by color-conjugated antibodies. The type of Hb is determined by the appearance of a blue line at the different test zones along the test strip. (C) Density-based separation184. The blood sample is mixed with aqueous polymeric solutions in capillary tubes. Upon centrifugation, the precipitation of a dense RBC layer at the bottom of the tube indicates SCD. (D) Microengineered electrophoresis (HemeChip). After loading the blood sample mixed with DI water into the chip, an applied electric field causes Hb separation. Due to the differences in mobility among Hb types, each type will travel a unique distance across the paper strip.
This paper-based assay offers the advantages of ease of use, low cost ($0.77 per test), simple fabrication, and minimal sample processing as it requires only one step of mixing. Moreover, it utilizes the natural color of blood for detection without resorting to complex color change detection or labels. In addition, tests can be performed individually and no batching is required.
Despite the advantages of this technique, the results may be affected by clotting of the blood samples which would prevent the wicking of the blood through the paper substrate. In addition, the test relies on naked-eye detection which may render it prone to operator error, which can be overcome by pairing the test with an automated image processing algorithm. Moreover, the validation results show that this test cannot accurately distinguish between HbSC and HbAS even when used with automated image processing179, 180. Finally, high levels of HbF, especially present in newborns, prevent the polymerization and precipitation of HbS, and consequently hinder the application of the test to newborn screening171.
4.3.2 Lateral Flow Immunoassays
Kanter et al. reported the testing results of a lateral flow immunosassay, known as Sickle SCAN™, developed to detect the presence of HbA, HbS, and HbC with the unassisted eye181. The assay consists of a test strip, with polyclonal antibodies conjugated with colored nanoparticles immobilized on four different test lines (Fig. 2B). Each line corresponds to one of the three hemoglobin types and the fourth line serves as a control to verify proper operation of the device. Once the test specimen consisting of 5 μL of blood in Hb solubility buffer at a 200:1 ratio is added into the device, the solution diffuses to the test zones where the Hb is captured forming antibody-antigen complexes. Consequently, the appearance of a blue line at any of the test lines signals the presence of the targeted hemoglobin types. A readout can be obtained from the device within 2 minutes. The limit of detection (LOD) varies between different hemoglobin types. The reported LOD values for HbA, HbS, and HbC are 40%, 1%, and 2% respectively, with sensitivity and specificity of 99%.
A recent validation study for this lateral flow assay was reported by McGann et al.182. In this study, sensitivities of 98.3%, 99.5%, 100%, as well as specificity values of 94% 92.5% and 100%, were reported for HbA, HbS, and HbC, respectively, with Hb concentrations as low as 2%. This study also revealed that the presence of high concentrations of HbF did not interfere with the detection of HbS or HbC. Moreover, an evaluation of the shelf-life of the device was performed. The device has been proven to function properly even after storage at 37 °C for 30 days. This technology is proposed as an initial assessment tool since it offers the advantages of ease of use, absence of auxiliary equipment, and short turnaround time. However, the assay relies on human visual interpretation, which may be critical to the test result especially in the case where faint HbA lines in patients with HbAS may be misinterpreted, despite the fact that the band intensities do not correlate with corresponding hemoglobin percentages181. Therefore, a weaker HbA line along with a stronger HbS line might be misinterpreted as HbSβ+, instead of SCD trait. In addition, the assay is not suited for quantitative assessment for the concentrations of the different Hb types. Finally, the immobilization of the antibodies conjugated with nanoparticles increases the fabrication complexity of the assay adding to its cost and limiting its shelf-life especially at high temperature environments without refrigeration or air conditioning.
Recently, a new lateral flow assay has been developed under the name HemoTypeSC™. The assay utilizes monoclonal antibodies specific to HbA, HbS and HbC. The assay consists of: (i) laminated fiberglass sample pads. (ii) nitrocellulose membrane with antibodies deposited on four different locations, corresponding to each of the three hemoglobin types as well as a control, and (iii) a cellulosic wick183. To perform the test, 1μL of blood is diluted in a 1:1000 ratio in distilled water. Next, 15 μL of the diluted blood is applied to the sample pad and the strip is dipped in a sample vial containing red-colored colloidal gold nanoparticles rehydrated in 150 μL of assay buffer. The strip is allowed to wick the liquids for 10 minutes before it is taken out of the vial. Upon visual detection of the test strip, the absence of a red line on one or more of the four specific locations indicates the presence of the corresponding hemoglobin type. A result is obtained from this assay within 20 minutes.
This lateral flow assay was validated by testing 100 patients with the specific Hb types. Identification of HbA, HbS, HbC was reported to be achieved with 100% sensitivity and specificity. The limit of detection was estimated at 2.7% for HbA, 3.3% for HbS, and 1.3% for HbC. The estimated cost of the materials used for the fabrication of this assay was $0.25, while a more realistic cost estimation per test was not provided. As in the previous lateral flow assay, quantification of the results is not possible, and the processes involved in its fabrication are complicated. The assay is able to distinguish between HbAA, HbAS, HbAC, HbSS, HbSC, and HbCC. However, identification of other Hb types such as HbF and HbA2 is not possible. In addition, HbSβ+−thal and HbSβo gave results consistent with HbAS and HbSS, respectively.
4.3.3 Density-Based Separation
Density based separation detects sickled RBCs via cell density measurements using aqueous multiphase systems (AMPS). Kumar et al. developed two- and three-phase AMPS capable of distinguishing dense SCD cells from normal cells with a sensitivity of 90% and a specificity of 97% for the two-phase system. Whereas the detection with the three-phase system had a sensitivity of 91% and a specificity of 88%. The estimated limit of detection was 2.8% for dense cells and the cost per test is around $0.5184.
The test requires 5 μL of blood to be mixed with aqueous polymeric solutions. The mixture is loaded into capillary tubes and centrifuged for 10 minutes. SCD is detected by the precipitation of a dense RBC layer at the bottom of the tubes (Fig. 2C). In addition, the combination of larger centrifugation time and the use of an optical reader enables the distinction between HbSS and HbSC. Further analysis of the sediment layer would also allow for the quantification of the fraction of dense cells. The density-based test is simple and rapid. However, the use of a centrifuge increases the cost of the test and its applicability at the POC. The turnaround time would also be affected since the samples need to be processed in batches. Density-based separation is also incapable of differentiating between HbAA and HbAS. It should also be noted that this test might not be suitable for detection of SCD in newborns since dense RBC cells are not present yet due to high levels of HbF in the first 4–6 months of life. Also, the test might not be accurate for patients with persistent high HbF levels, such as patients with the Arab-Indian haplotype. Furthermore, many health conditions, treatment processes and prescribed medications, as well as genetic factors influence the RBCs density and in turn limit the validity of the test.
4.3.4 Microengineered Electrophoresis
Microengineered electrophoresis (HemeChip) has been recently developed to identify and quantify hemoglobin types including HbC/A2, S, F, and A, among others. As depicted in Fig. 2D, the HemeChip consists of a microfabricated Polymethyl methacrylate (PMMA) chamber housing an electrophoresis cellulose acetate paper strip, which is used to separate hemoglobin types via an applied electric field185. A mobile image processing application has also been developed for automated and objective quantification of HemeChip results at the POC.
The test starts by mixing a blood sample (< 5 μL) with pure or deionized (DI) water to lyse the cells and release Hb, and < 1 μL of the mixture is stamped onto the paper substrate inside the chip. Next, an electric field is applied across integrated electrodes. The electric field causes hemoglobin separation with distinct bands, and due to differences in mobility among the different hemoglobin bands, each type will have a unique travel distance from the application point across the paper strip. Screening is achieved in under 10 minutes, and test results showed 90% sensitivity and 89% specificity in differentiating between HbC/A2 and HbS bands, 89% sensitivity, and 82% specificity in differentiating between HbS and HbF bands, and 100% sensitivity and 86% specificity in differentiating between HbF and HbA bands. Large scale field testing of HemeChip with newborns is pending for assessment of clinical sensitivity and specificity in determining healthy versus diseased states and high risk infants. Since HemeChip works on the principles of current clinical standard electrophoresis method, once the large scale field testing is done, the clinical sensitivity and specificity values of HemeChip are expected to be comparable to those of the standard electrophoresis test (93.1% – 99.9%)172. The assessment of the limit of detection for this technique was carried out using adult blood samples. The limit of detection for adult SCD and SCT were determined to be around 10% for HbS, HbF, and HbA, and 3% for HbC/A2.
HemeChip is low cost ($0.9 per chip), rapid, robust, and accurate. Moreover, hemoglobin detection and quantification results using the HemeChip were shown to be in strong agreement with the standard HPLC and laboratory-scale electrophoresis tests. This technology also offers the advantage of possible integration with mobile devices for more accurate analysis. A potential challenge when using this technology, as with any other newborn screening method, may originate from high percentages of HbF masking other Hb types (e.g., HbS and HbA). Additionally, HemeChip test setup currently utilizes a bench-top power supply. However, due to the low power requirement for the test, this power supply can be replaced by portable rechargeable batteries for real world applications.
5. SCD Monitoring at the POC
5.1. Ongoing Challenges and Unmet Clinical Needs
More than 100,000 Americans and millions worldwide have SCD11. In the US, SCD is estimated to cost >8 million dollars per patient over a 50 year life-span4. Life expectancy of SCD patients has increased significantly, thanks to the introduction of cost-effective interventions such as prophylactic penicillin, widespread vaccination, and hydroxyurea use. Nonetheless, high morbidity, from chronic complications and organ damage, and early mortality are still having a great impact on patients with SCD. To date, correlative studies in SCD have ranged amongst clinical reports, based on tests, interventions, and chart review52, 186–191, and, at the other extreme, SCD population-based genetic analyses of gene polymorphisms192–195. Despite significant advances in the understanding of the fundamental pathophysiology of SCD, we are still without markers that can reliably reflect the clinical course of patients in real time. SCD is unusually susceptible to an examination of cell membrane properties and cellular activation. RBC stiffness, RBC density, RBC & WBC adhesion, WBC repertoire and activation, and whole blood viscosity are excellent candidate biophysical surrogates for disease activity, and some of which (RBC density, RBC and WBC adhesion, and WBC repertoire and activation) are already targets in therapeutic trials.
Many important observations about membrane and cellular abnormalities, and their relationship to clinical complications in SCD, have been made since the 1980’s48, 53–55. However, most pathophysiologic studies have been undertaken in modest numbers of subjects at a single time point and single institution. Further, it has not been feasible for more than one analysis to be performed on a single patient sample, e.g., simultaneous evaluations of membrane properties, inflammatory cell activation, and circulating endothelial cell numbers. Often, promising ‘cutting-edge’ or biologically illuminating correlative tests are too expensive, complex, or difficult to ‘export’ to widespread use outside of a few specialized research centers. Widespread access to longitudinal examination of key pathophysiologic endpoints, such as intercellular adhesion of RBCs, WBCs, and endothelium (and subendothelium), if reproducible and feasible, could provide a critical additional dimension to clinical studies that could improve clinical care, guide clinical trial design, and decrease the physical and financial burden of SCD. Non-biased characterization of cellular biophysical properties should enable more precise targeting of disease modifying interventions in SCD. Most notably, reproducible and clinically feasible serial evaluation of RBC or WBC adhesion and WBC activation could guide intervention if, for instance, MAC-1 activation predominated in some clinical scenarios196, 197, P-selectin adhesion in others115, 137, and iNKT cell expansion in a third156, 157, 198. Finally, the burden of SCD is in resource-limited settings. Therefore, inexpensive and simple POC discriminants (and diagnostics) of disease activity could be extremely valuable tools world-wide161, 177.
More than half of patients living with SCD are treated with Hydroxyurea (HU) or are receiving regular transfusions to prevent severe and life-threatening complications of SCD. HU is the only FDA- approved drug to treat SCD. Regular blood transfusions are commonly performed in approximately 10% of pediatric and up to 20% of adult patients with SCD. HU increases the overall HbF % and the percentage of red cells containing detectable HbF (F-cells), in children and adults199–201, thereby decreasing the tendency toward intracellular polymerization of HbS199, 202, 203. Importantly, the decision to initiate HU therapy, especially in children, must be thoughtful and therapy must be monitored and adjusted to achieve optimal results. HbF is an important biomarker for efficacy and adherence to treatment202, 204. Greater treatment-related increases in HbF may predict a more robust response to treatment in children205. Patients require frequent (monthly or bi-monthly) blood testing and monitoring, once HU therapy is initiated. These safety labs comprise a CBC and reticulocyte count; the next month’s dose is not dispensed until that day’s blood counts are available. The dose needed for maximal clinical benefit, which may or may not be the maximum tolerated dose (MTD)206, is generally identified within 6 to 8 months of initiating HU therapy, but should be established and assigned only after the patient tolerates the dose for at least 8 weeks52, 58. Because HbF response to hydroxyurea is dose dependent, HbF levels, measured serially, help to establish MTD in individuals202. Clinical trials with escalation to MTD have reported higher percentage of HbF and Hb as well as mean corpuscular volume207. Careful attention to patients’ response to treatment and the resulting individualized therapy has the potential to improve clinical outcomes208. Increases in Hb and HbF associate with a clinical response to HU therapy, and are sustained, especially in children209,199, 210. Close monitoring and follow-up are vital to ensure adherence to treatment and appropriateness of dose.
Healthy red blood cell transfusion (HbA) can be lifesaving and is proven to help prevent complications of SCD. Transfusion can prevent stroke in children at high risk and, post-stroke, may prevent recurrence211. Regular (monthly) blood transfusions are common therapy, utilized in approximately 10% of pediatric and 20% of adult patients with SCD. The primary objective of long-term transfusion programs is to maintain low proportions of HbS in the blood212, often at <30% HbS percentage with a total Hb between 9–12 g/dL213. Ongoing monitoring of HbS and HbA levels during transfusion care informs therapeutic and technical decisions (about the length and frequency of transfusions)214. Accurate administration of blood transfusion limits excessive transfusion and reduces the risks associated with transfusion, such as alloimmunization, hemolytic reactions, and iron overload215. However, current methods to monitor Hb composition require that samples be sent to a lab, resulting in delays in patient feedback, provider decision-making and treatment modification. Patient self-efficacy and provider monitoring and management would benefit from a POC tests that delivered immediate results.
5.2. Currently Available Methods for SCD Monitoring in Clinical Research
Conventional monitoring of SCD patients in the clinic relies on measurement of different blood components quantitatively and qualitatively, including amount of cell types and proteins to physical properties of cells via complete blood count (CBC) test and biochemical assays216–219. Even though other techniques, including flow cytometry, ektacytometry, and flow chambers, have been occasionally utilized in clinical research and in some clinical trials220–223, they are not integrated into routine patient monitoring. Apart from these modalities, HPLC, which was described in detail in Section 4.2., is used for hemoglobin quantification to monitor patients undergoing hydroxyurea therapy or transfusion therapy224, 225.
5.2.1 Complete Blood Count
A typical complete blood count (CBC) test includes WBC count (number of WBCs/μL), RBC count (number of RBCs/μL), hemoglobin, hematocrit (percentage of RBCs in blood), mean corpuscular volume (MCV, average size of a single RBC), mean corpuscular hemoglobin (MCH, average amount of hemoglobin in a single RBC), red cell distribution width (RDW, variation in RBC size), reticulocyte count (amount of immature RBCs), and platelet count. Homozygous SS and heterozygous S/β0 patients typically have lower RBC count, hemoglobin, and hematocrit due to the hemolytic anemia. On the other hand, WBC count, platelet count, and reticulocyte count are elevated, though they can fluctuate. Reticulocyte counts may alter depending on the degree of anemia, due to hemolysis or other causes (including sequestration), and bone marrow responsiveness to anemia. MCV has shown to rise in SCD patients treated with hydroxyurea. Furthermore, RDW is elevated in SCD patients due to increased heterogeneity within RBC subpopulations. Even though CBC is widely used and provides valuable information on blood cell properties, it is insufficient to provide an integrated and complete evaluation of the patient’s status.
5.2.2 Biochemical Assays
Enzymatic and non-enzymatic biochemical markers in blood, measured via activity, reactivity, and immunosorbent assays, can be direct indicators of hepatic dysfunction, kidney damage, endothelial damage, inflammation and intravascular hemolysis. In SCD patients, plasma levels of lactate dehydrogenase (LDH), total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) are used as principal hemolytic markers. ALT, AST, and total bilirubin, along with alkaline phosphatase (ALP) are also indicators of hepatic dysfunction. Serum cystatin c and creatinine levels are used as indicators for kidney function and elevated levels in SCD patients are associated with renal decline. Elevated c-reactive protein levels in blood plasma are associated with acute and chronic inflammation. Moreover, soluble adhesion molecules, including VCAM-1, ICAM-1, E-selectin, and P-selectin, are associated with increased cell adhesion and VOC and can be quantified via enzyme-linked immunosorbent assay (ELISA)226, 227. Even though these soluble adhesion molecules utilized to better understand the SCD pathophysiology and targeted in some clinical trials37, these markers need thorough validation before clinical use. Overall, biochemical assays can be important in measuring several protein and enzymatic markers that are associated with end-organ damage and vasculopathy, however, they are time- and labor-intensive.
5.2.3 Flow Cytometry
Surface characteristics of blood cells are typically measured with conventional techniques, such as fluorescent activated cell sorting (FACS), immunohistochemistry, or microscopic imaging methods. In FACS, cells of interest are isolated, extensively processed, incubated with a fluorescent-labeled antibody raised against a cellular protein (e.g., integrin, receptor, adhesion molecule), and sorted by optical recognition. Measurement by flow cytometry of aberrant surface molecule expression or activation has served as a surrogate for directly measuring abnormal adhesion in humans with SCD63, 196, 228. However, quantitative changes in surface molecule expression (e.g., BCAM/LU) do not always faithfully recapitulate qualitative changes102, 229. Furthermore, FACS requires high-cost instrumentation, skilled personnel, and time- and labor-intensive operation, which limits its application in most clinical and research settings.
5.2.4 Ektacytometry
Ektacytometry has been widely utilized in deformability measurement of RBCs in SCD197,198. Ektacytometry involves a stationary inner cylinder and an outer cup, and between the two a narrow gap where blood fills in230. Varying levels of shear stress can be generated on blood when the outer cup is rotated at different speeds and osmotic gradient is varied from below physiological osmolality to above physiological levels. Elliptical diffraction patterns of sheared cells are obtained using a laser and a lens, and deformability is calculated from the dimensions of the elliptical diffraction pattern230. Despite its frequent use in SCD research studies, ektacytometry lacks physiologically relevant flow conditions that are present in the blood vessels. Further, it is not feasible as a POC analysis tool at the clinic due to high-cost and need for skilled personnel.
5.2.5 Flow Chambers
Flow chambers are composed of a gasket, with inlet, outlet, and vacuum ports, assembled on a glass slide either functionalized with biomolecules or coated with endothelial cells231. Gasket thickness determines the height of the chamber and flow rates pumped into the chamber can be optimized to simulate physiological shear stress levels in the blood vessels. Flow chambers were widely utilized in the early SCD literature to mimic blood cell and vessel endothelium interactions. In particular, they were employed frequently in SCD research to mimic post-capillary venules, where vaso-occlusion occurs, and analyze abnormal adhesion of RBCs to endothelium and endothelium associated proteins29, 54. Even though flow chambers provide high-throughput analysis of cell interactions and ability to mimic physiologically relevant conditions, they require complicated equipment and skilled personnel for operation, which limits their wide-spread application in the clinic and as a research tool.
5.3. Emerging Technologies for SCD Monitoring in Clinical Research
Despite vast knowledge gained in identifying and targeting cellular abnormalities and interactions in SCD over the last 30 years, such expertise has not been translated into clinical care or trial design due to requirements for complicated custom-designed devices, trained technicians, specially collected patient blood samples, and extensive sample processing and manipulation. Groundbreaking studies on cellular adhesion and deformability in SCD relied on complex systems requiring skilled personnel, which include laser diffraction ektacytometry, FACS, and parallel flow chambers. These important proof-of-concept studies used techniques that required extensive preprocessing, separation of cellular populations, and washing of RBCs29, 30, 56. Further, preprocessing wash steps typically removed plasma proteins that contribute to cellular adhesion25, 232. Complex and labor-intensive techniques to measure cellular adhesive interactions have not been used in longitudinal analyses or in large-scale clinical studies, applied at more than one center. These significant technological barriers have hindered the widespread evaluation of abnormal cellular characteristics, clinically and as a research tool.
Recent advances in micro and nano fabrication technologies have yielded microfluidic platforms that can probe single cell behavior and tissue response simultaneously under precisely controlled biological, biophysical, and flow conditions, mimicking physiological systems at baseline and with disease27, 233–244. These technologies have been used to model SCD vasculature83, 240, 244, and are likely to yield important insights about SCD pathophysiology. Furthermore, such advanced modeling of in vivo microvasculature conditions might produce simple, reliable, and rapid platform technologies for personalized medicine applications, from therapeutics to monitoring.
5.3.1 Endothelialized Microchannels
Endothelial cells play an essential role in the pathophysiological manifestations of SCD33, 55, 245. Activation of the endothelial layer mediates cell adhesion and aggregation by further inducing inflammatory response and subsequent complications. In that sense, several researchers have recently directed their focus towards fabricating endothelialized parallel flow chambers and microfluidic devices to better recapitulate the biophysical and hemodynamic properties of the microvasculature environment246, 247. For example, utilizing Human Umbilical Vein Endothelial Cells (HUVEC) coated flow chamber assays, Zennadi et al.111 demonstrated that epinephrine mediated sickle RBC adhesion to the endothelium through LW-αvβ3 interactions. In addition, epinephrine-stimulated RBCs were shown to activate peripheral blood mononuclear cells (PBMC) and promote PBMC adhesion to the endothelium increasing the risk of vaso-occlusive events. Matsui et al.59 reported elevated sickle RBC adhesion to thrombin treated HUVECs in a parallel flow chamber under normoxic conditions. The rolling adhesion of cells showed P-selectin pathway dependency while firm adhesion required additional pathways. Unfractionated heparin was shown to block the P-selectin dependent pathways by diminishing the thrombin-enhanced rolling adhesion of cells59. Stimulation of endothelial cells in vitro through IL-1β was also shown to enhance sickle RBC adhesion to the endothelium in a time-dependent manner68. During the early stages of the activation, RBC adhesion to the HUVEC monolayer exhibited a negligible dependency on integrin α4β1 on the RBC surface. However, nine hours following the IL-β1 stimulation, the adhesion was both α4β1 and VCAM-I mediated. Even though these studies provided valuable insight into cellular adhesive interactions in SCD, they relied on extensive sample pre-processing, removing soluble adhesion molecules and other activation factors in plasma that are critical in vivo.
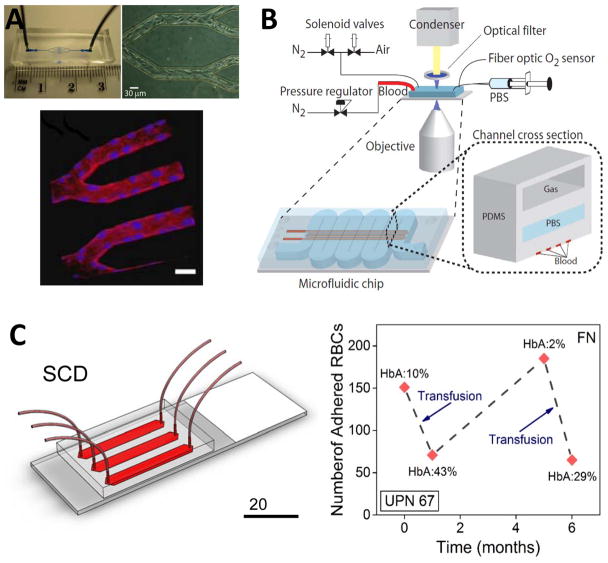
Recently, Tsai et al. developed a microfluidic platform with an inner surface coated by a confluent monolayer of endothelial cells for modeling vascular occlusion and thrombosis in hematologic diseases, such as SCD244 (Fig. 3A). Comparison of the flow behavior of whole blood samples from two patient populations, receiving (HU+) and not-receiving (HU−) hydroxyurea treatment, revealed that HU+ blood achieved higher average velocities within the channels. Moreover, HU− samples caused greater levels of obstruction indicating the efficacy of the drug in SCD. Furthermore, interaction of TNF-α activated Human Lung Microvascular Endothelial Cells (HLMVEC) with TNF-α activated leukocytes resulted in a significant increase in the obstruction of flow as well as a decrease in flow rate. Despite their advantages in high fidelity mimicking of microvasculature, endothelium-based models are not yet widely applicable to large numbers of patients, because of the requirement for labor-intensive and technically challenging tools, and a need for a constant supply of cultured endothelial cells.
Figure 3. Emerging POC technologies for SCD monitoring.
(A) An endothelialized microfluidic platform to model microvascular occlusion in vitro247. Brightfield microscopy shows the immobilized endothelial cells 48 hours after the cells are seeded in the microfluidic device. The cultured cells round up the rectangular cross-section mimicking in vivo blood vessel geometry and size scale. Immobilized endothelial cells round up the rectangular cross-section mimicking in vivo blood vessel geometry and size scale. The endothelialized microfluidic platform can recapitulate altered blood flow and occlusive events in physiologically relevant flow conditions. The influence of hydroxyurea treatment of sickle cell patients on cell adhesion and subsequent microvascular occlusion is also observed using fluorescent microscopy. Reproduced with permission from [247] (B) A microfluidic platform to probe blood rheology of SCD patient blood samples in physiologically relevant flow and oxygen tension conditions243. Blood flow conductance can be measured in microfluidic channels at the bottom layer, whereas the channel at the top layer are filled with N2 to deoxygenate the blood through gas diffusion across the separating PDMS membrane. Reproduced with permission from [243] (C) SCD Biochip as a functional RBC adhesion assay to monitor SCD34. Adhesion of RBCs to endothelium and sub-endothelium associated proteins in physiologically relevant size scale and flow conditions are analyzed and associated with clinical course of SCD patients. SCD Biochip provides rapid, fully enclosed, standardized, and pre-processing-free analysis of RBC adhesion in whole SCD patient blood samples, enabling longitudinal studies.
5.3.2 Sickle Blood Rheology in Microfluidic Channels
The rheology of blood is dominated by RBC deformability, which is affected by changes in local flow conditions, such as high shear rate induced by vessel size248. In SCD, hemoglobin polymerization triggered by deoxygenation and subsequent decrease in cell deformability dramatically affects blood rheology (e.g., increase in viscosity), most specifically during microcirculation when a single RBC occupies the vessel. Moreover, low deformability dramatically decreases the shear thinning capability of blood, which is imperative to prevent any occlusion in post-capillary venules. The flowing suspension of soft cells (RBCs), that change their morphology and rheology relatively quickly upon deoxygenation is a key source for the vasoocclusion heterogeneity in SCD. Two fundamental time scales in vasoocclusion are the polymerization time (τp) and kinetic time (τk). The polymerization time scale is a function of HbS concentration whereas the kinetic time scale is a function of the pressure gradient, vessel diameter and the effective viscosity of blood. The ratio of these two time scales governs the vasoocclusion phenomenon242.
Microfluidic systems provide several advantages in studying rheological properties of blood in SCD, including physiologically relevant size scale and flow conditions. In the SCD literature, microfluidic channels have been utilized to probe both individual sickle RBC rheology (occurs at a length scale of 10 μm)240, and whole blood rheology along with vaso-occlusion dynamics (occurs at a length scale of 100 μm)243. Du et al.240, developed a microfluidic system incorporating micropillar arrays forcing single RBCs to squeeze and deform through microgaps. Moreover, microfluidic channels were deoxygenated by gas diffusion through a PDMS membrane separating another layer of microchannels injected with N2, allowing analysis of RBC deformability at hypoxic conditions.
To study the effect of deoxygenation on kinematic rheology and vaso-occlusion process, Higgins et al.242 and Wood et al.243 utilized a polydimethylsiloxane (PDMS) microfluidic channel, fabricated via photolithography and soft lithography. Blood in the microfluidic channels was deoxygenated by gas diffusion through a gas membrane separating a second layer of microchannels242, 243 (Fig. 3B). Decreased rate of conductance upon deoxygenation was analyzed in SCD patient blood samples and associated with clinical course of the corresponding patients243. Blood samples from benign SCD patients showed no drop of conductance upon deoxygenation, whereas blood samples from severe SCD patients showed large and rapid change in conductance following deoxygenation. Furthermore, the effect of HbF fraction on the rate of conductance was analyzed and it was shown that conductance rate of hydroxyurea treated patients, with increased HbF levels, were comparable to untreated severe samples.
5.3.3 RBC Adhesion in Microphysiological Flow
Despite the remarkable insights about abnormal RBC adhesion in SCD that have been made, there remain gaps in knowledge about these complex adhesive interactions. Because of their technically challenging and labor-intensive nature, currently available techniques do not allow rapid measurement of diverse cellular adhesive events in the clinic simultaneously, e.g., abnormal RBC adhesion to fibronectin, laminin, and P-selectin. Further, there is no established ‘atlas’ of abnormal adhesive events, examined longitudinally and in a standardized manner in a large heterogeneous population of SCD patients (HbSS and HbSC, children and adults) under a range of clinical circumstances and with and without treatment. Neither the topography of adhesive events for an individual patient, nor for the SCD population as a whole is known. Better knowledge of the nature and scope of abnormal adhesive events is critical to the goals of establishing associations with clinical outcomes and successfully identifying therapeutic targets in clinical trials.
Recently, a versatile microfluidic platform (SCD Biochip, Fig. 3C) mimicking the dimensions of the post-capillary venule was developed for evaluation of RBC adhesion and deformability. Whole blood samples were flowed through microchannels functionalized with endothelium associated proteins (FN and LN) at physiological shear stress levels (1–5 dyne/cm2). The SCD Biochip allows rapid, standardized, and pre-processing-free analysis of whole blood samples, incorporating effects of both cellular and plasma components27. No work prior to SCD Biochip has analyzed cellular adhesion directly using whole blood (taken from patients being seen in clinic) at the micro-vasculature scale.
In SCD Biochip, among blood samples with different hemoglobin phenotypes, the number of adhered RBCs was significantly higher in HbSS blood samples compared to HbSC/Sβ+-thalassemia blood samples or HbAA blood samples, both for FN and LN34, 249. It was shown that HbS-containing RBCs, were heterogeneous in deformability, adhesion strength, and in the number of adhesion sites27, 28. Based on morphological and biophysical characteristics (i.e., deformation in flow), adhered HbS-containing RBCs were categorized as deformable or non-deformable. While deformable RBCs formed a single attachment site, non-deformable RBCs displayed multiple attachment points.
Moreover, RBC adhesion to FN- and LN-coated microchannels in SCD patient blood samples was analyzed, focusing on markers of hemolysis, including an elevated reticulocyte count and an elevated serum lactate dehydrogenase (LDH95, 250, 251) level, since hemolysis may play a critical role in VOC and organ damage in SCD252. Non-deformable HbS-containing RBCs adhered more strongly to FN under flow conditions, which may be the result of their multiple attachment sites. Deformable and non-deformable HbS-containing RBCs at a physiologically relevant shear stress (1 dyne/cm2)27 were quantified, and shown to have a significant association between adhered non-deformable RBCs (%) and serum LDH levels in adult SCD subjects. It is plausible that adherent non-deformable cells are causally associated with hemolysis, due to prolonged delay time in the vasculature51, 253. These findings from the SCD Biochip support an association between deformability of adhered RBCs and hemolysis. In addition, RBC adhesion to FN or LN was significantly higher in subjects with low HbF (<8%) compared with those from subjects with high HbF (≥8%). This is in agreement with the known beneficial effect of fetal hemoglobin in SCD254–256.
Using SCD Biochip, samples from over 100 SCD patients, obtained primarily during outpatient clinic visits, were examined. Furthermore, a small number of these individuals were examined longitudinally using SCD Biochip, performed ≥1 month apart34. For example, in UPN 67 (Unique Patient Identifier 67), monitored over 6 months, RBC adhesion to FN decreased after two episodes of transfusion (Fig. 3C). Overall, these results obtained via SCD Biochip support the idea that changes in RBC adhesion can reflect clinical state and treatment response in SCD. However, these results need to be validated and expanded by increasing the number of subjects, expanding the longitudinal scope, and broadening the range of adhesive interactions that are studied.
6. Expert Commentary
WHO has declared SCD as a public health priority164, 167, 257. More recently, American Society of Hematology, in partnership with other organizations, launched the ‘Sickle Cell Disease Coalition’ and called for action to improve the patient care in SCD11. Estimated 50–80% of the babies born with SCD in Africa die before the age of 5 due to lack of diagnosis followed by basic treatment and care14, 167. Current laboratory-based screening platforms, although widely available in developed countries, are not feasible for operation at the POC in the resource-limited settings and in the developing world due to high infrastructure and operational costs, as well as the need for skilled operators. Moreover, typical turnaround time for screening test results in low-resource environment is too long (2–6 weeks). In such settings, it is critical to screen the baby, obtain the results, and inform the parents before they leave the testing center, otherwise, tested newborns may be lost to follow-up before the test results are available. Therefore, there is a need for simple, rapid, and mobile analyses of hemoglobin types in newborn blood with which to screen for hemoglobinopathies while the baby is still on-site. WHO estimates that more than 70% of SCD related deaths are preventable with simple, cost-efficient interventions, such as early POC newborn screening followed by treatment and care258. Recently developed novel microtechnologies offer simple, rapid, and affordable screening of SCD and have the potential to facilitate universal screening in developing countries. Smart utilization and wide-spread application of these technologies may revolutionize SCD screening in resource-limited settings and dramatically decrease early mortality due to SCD.
Efficacy in both HU and transfusions is reflected in changes in Hb composition. Close monitoring of HbF can reflect adherence to treatment and appropriateness of HU dose. Current monitoring relies on laboratory tests causing significant delays in patient feedback and treatment optimization. A rapid POC test that quantifies HbS, HbF and HbA, performed during a scheduled HU monitoring visit, would allow for personalized and precise dose adjustment. Importantly, communication of results immediately and directly to the patient (without the wait for lab results) may improve adherence to, and clinical efficacy of, HU therapy. Furthermore, it is known that real-time assessment of HbS and HbA levels prior to and during transfusions can improve the accuracy of treatment, there is no rapid real-time POC monitoring test for HbS and HbA levels in blood. A rapid POC test that quantifies HbS and HbA would help clinical management, monitor therapeutic response, improve clinical efficacy and safety, and may ultimately reduce the cost of care while improving patient satisfaction.
In SCD monitoring, current analyses performed at the clinic to evaluate the course of the disease only provide single-faceted information and largely lack complex cellular/tissue level response, which have restricted our ability to integrate complex biophysical phenomena into our understanding of SCD. Further, the relative contribution of red cell vs. white cell interactions as well as endothelium response and rheological changes in individual patients with SCD, at baseline, with clinical disease activity, and following therapeutic interventions has not been studied in a sizable SCD population, using reproducible, unbiased, and standardized evaluations. Development of novel microtechnologies for measuring the impact of interventions on cell/tissue biophysical properties, such as RBC adhesion and deformability, may accelerate the development or adoption of new pharmaceutical and treatment approaches. Clinically feasible measurements are especially important as abnormal adhesive interactions emerge as therapeutic targets116, 136, 137, 259, 260. Indeed, we do not yet have the tools to tell us whether there is a difference amongst patients in degree or type of aberrant cellular adhesion; that is, do some patients show exaggerated RBC adhesion when compared with other patients, who show exaggerated WBC adhesion. Absent a clinical adhesion assay, current and future studies of anti-adhesion strategies in SCD, antibody- or drug-based, may underestimate the response of patients who have predominant abnormalities in one targeted pathway more than another, e.g., differential sensitivity to agents that target MAC-1 activation, BCAM/LU phosphorylation, or selectin binding. Alternatively, overall cellular adhesion of all cell types, and therefore sensitivity to a range of targeted agents, may increase in a patient who has striking abnormalities in a single cell type.
7. Five-year View and Future Perspectives
POC technologies for disease screening and patient monitoring are attracting increasing attention with each passing year due to improvements in their cost-effectiveness, speed, and user-friendly operation. Such technologies not only enable a better access to healthcare by millions of underserved people but also improve patients’ quality of life by allowing quick result turn around at home or at the clinical site.
Diagnosis
Some of the screening platforms for SCD described in this review are already in field trials in Sub-Saharan Africa, whereas others have been validated in local hospitals in the US. Therefore, it is plausible for these technologies to be in market in the next couple of years, especially in developing regions of the world including Africa, India, and Southeast Asia, where the need is more urgent and the demand is the greatest. High HbF levels have shown to be problematic for all screening platforms, decreasing sensitivity and specificity of the test. However, HbF levels continually decrease in babies over the course of the first six months after birth, and at six weeks, HbF level decreases to be around 60%. This timing is especially opportune for POC screening as this time frame aligns well with typical immunization schedules for children in Africa. When these devices are proved to be effective, reliable, and cost-efficient in the developing world, there is a great potential for their use in developed countries: (1) where the infrastructure for universal screening is not in place, as in most European Countries, and (2) where these cost-efficient diagnostic alternatives can replace bulky, complicated, and costly traditional techniques, as in the US.
Monitoring
On the monitoring front, which is much more complicated in comparison to diagnosis and requires thorough validation of efficacy, early use of the novel microdevices might come in especially handy in the design of clinical trials for emerging therapies, most of which are performed in developed countries. Since all these advanced microtechnologies are based on mimicking human microvasculature features and biophysical properties, measurements and evaluations performed in these devices reflect cellular/tissue level responses, such as cell adhesion and vaso-occlusion. Therefore, such platforms can be utilized in therapeutic clinical trials targeting biophysical interactions in human vasculature. These platforms may be used for selective patient enrollment in trials and evaluating impact of therapeutics in enrolled patient blood samples. However, the long-term goal for all of these microtechnologies are patient monitoring at the POC, whether it can be home or clinic, either as a complimentary to other blood tests or a stand-alone monitoring modality. Successful development of such novel monitoring technologies can also provide a glimpse of hope for SCD patients in the developing world, where insufficient number of healthcare providers and infrastructure impedes proper patient tracking and follow-ups.
8. Key Issues.
SCD affects 100,000 Americans and more than 14 million people globally, disproportionally in economically disadvantaged populations.
SCD requires early diagnosis after birth and constant monitoring throughout the life-span of the patient.
Currently available screening and monitoring tools are not feasible for operation at the point of care (POC), which increase the economic burden with hospital visits and costly tests, as well as exacerbate patients’ quality of life.
The emerging POC technologies offer cost-efficient, rapid, and reliable screening of SCD, which is essential to facilitate universal screening programs in developing countries.
The emerging microfluidic platforms mimicking and modeling biophysical conditions of SCD microvasculature can reflect cellular/tissue level responses, providing unique capabilities for efficient, reliable, and convenient monitoring of SCD patients at clinical baseline, with disease, and under treatment.
Acknowledgments
Authors acknowledge Cleveland Institute of Art Student, Grace Gongaware for crafting the scientific illustration. U. A. Gurkan would like to thank the Case Western Reserve University, University Center for Innovation in Teaching and Education (UCITE) for the Glennan Fellowship, which supports the scientific art program and the art student internship at Case Biomanufacturing and Microfabrication Laboratory. This work was supported by the Case-Coulter Translational Research Partnership (CCTRP) and the Clinical and Translational Science Collaborative (CTSC) of Cleveland [grant# UL1TR000439] from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health (NIH) and NIH roadmap for Medical Research, the Doris Duke Charitable Foundation [grant #2013126], National Heart Lung and Blood Institute [grant #R01HL133574], NIH Center for Accelerated Innovation at Cleveland Clinic (NCAI-CC), and National Science Foundation CAREER Award [grant #1552782]. Authors acknowledge the Center for Integration of Medicine & Innovative Technology (CIMIT) Student Technology Prize for Primary Healthcare for supporting this study. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Declaration of Interest
Y. Alapan, U. Gurkan, J. Little and R. Ung have a financial interest in Hemex Health (licensor of the HemeChip Technology) including licensed intellectual property, stock ownership, and consulting. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been annotated as:
* Of interest
** Of considerable interest
- 1.Herrick J. Peculiar elongated and sickle-shaped red blood cell corpuscles in a case of severe anemia. Archives of Internal Medicine. 1910 [PMC free article] [PubMed] [Google Scholar]
- 2.Pauling L, Itano HA, et al. Sickle cell anemia, a molecular disease. Science. 1949;109(2835):443. [PubMed] [Google Scholar]
- 3.Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86(6):480–487. doi: 10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballas SK. The cost of health care for patients with sickle cell disease. Am J Hematol. 2009;84(6):320–322. doi: 10.1002/ajh.21443. [DOI] [PubMed] [Google Scholar]
- 5.Kauf TL, Coates TD, Huazhi L, Mody-Patel N, Hartzema AG. The cost of health care for children and adults with sickle cell disease. Am J Hematol. 2009;84(6):323–327. doi: 10.1002/ajh.21408. [DOI] [PubMed] [Google Scholar]
- 6.Ballas SK, Mohandas N. Sickle red cell microrheology and sickle blood rheology. Microcirculation. 2004;11(2):209–225. doi: 10.1080/10739680490279410. [DOI] [PubMed] [Google Scholar]
- 7.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337(11):762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 8.Alapan Y, Icoz K, Gurkan UA. Micro- and nanodevices integrated with biomolecular probes. Biotechnol Adv. 2015;33(8):1727–1743. doi: 10.1016/j.biotechadv.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unal M, Alapan Y, Jia H, Varga AG, Angelino, Keith, Aslan M, Sayin I, Han C, Yanxia J, Zhang Z, Gurkan UA. Micro and Nano-scale Technologies for Cell Mechanics. Nanobiomedicine. 2014;1(5):1–29. doi: 10.5772/59379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, Temperley WH, Williams TN, Weatherall DJ, Hay SI. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381(9861):142–151. doi: 10.1016/S0140-6736(12)61229-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The State of Sickle Cell Disease: 2016 Report. American Society of Hematology; 2016. [Google Scholar]
- 12.Diallo D, Tchernia G. Sickle cell disease in Africa. Curr Opin Hematol. 2002;9(2):111–116. doi: 10.1097/00062752-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Diallo DA. Sickle cell disease in Africa: current situation and strategies for improving the quality and duration of survival. Bull Acad Natl Med. 2008;192(7):1361–1372. discussion 1372–1363. [PubMed] [Google Scholar]
- 14.Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in Africa: a neglected cause of early childhood mortality. Am J Prev Med. 2011;41(6 Suppl 4):S398–405. doi: 10.1016/j.amepre.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tewari S, Rees D. Morbidity pattern of sickle cell disease in India: a single centre perspective. Indian J Med Res. 2013;138(3):288–290. [PMC free article] [PubMed] [Google Scholar]
- 16.Huttle A, Maestre GE, Lantigua R, Green NS. Sickle cell in Latin America and the United States [corrected] Pediatric Blood & Cancer. 2015;62(7):1131–1136. doi: 10.1002/pbc.25450. [DOI] [PubMed] [Google Scholar]
- 17.Inheriting sickle cell anaemia - Live Well - NHS Choices. National Health Service; (UK): 2016. [Google Scholar]
- 18.Jastaniah W. Epidemiology of sickle cell disease in Saudi Arabia. Ann Saudi Med. 2011;31(3):289–293. doi: 10.4103/0256-4947.81540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asnani MR, McCaw-Binns AM, Reid ME. Excess risk of maternal death from sickle cell disease in Jamaica: 1998–2007. Plos One. 2011;6(10):e26281. doi: 10.1371/journal.pone.0026281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hebbel RP. Beyond hemoglobin polymerization: the red blood cell membrane and sickle disease pathophysiology. Blood. 1991;77(2):214–237. [PubMed] [Google Scholar]
- 21.Ferrone FA. Polymerization and sickle cell disease: a molecular view. Microcirculation. 2004;11(2):115–128. doi: 10.1080/10739680490278312. [DOI] [PubMed] [Google Scholar]
- 22.Noguchi CT, Schechter AN. Sickle hemoglobin polymerization in solution and in cells. Annu Rev Biophys Biophys Chem. 1985;14:239–263. doi: 10.1146/annurev.bb.14.060185.001323. [DOI] [PubMed] [Google Scholar]
- 23.Nash GB, Johnson CS, Meiselman HJ. Mechanical properties of oxygenated red blood cells in sickle cell (HbSS) disease. Blood. 1984;63(1):73–82. [PubMed] [Google Scholar]
- 24.Brandao MM, Fontes A, Barjas-Castro ML, Barbosa LC, Costa FF, Cesar CL, Saad ST. Optical tweezers for measuring red blood cell elasticity: application to the study of drug response in sickle cell disease. Eur J Haematol. 2003;70(4):207–211. doi: 10.1034/j.1600-0609.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 25.Mohandas N, Evans E. Sickle erythrocyte adherence to vascular endothelium. Morphologic correlates and the requirement for divalent cations and collagen-binding plasma proteins. Journal of Clinical Investigation. 1985;76(4):1605–1612. doi: 10.1172/JCI112144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byun H, Hillman TR, Higgins JM, Diez-Silva M, Peng ZL, Dao M, Dasari RR, Suresh S, Park Y. Optical measurement of biomechanical properties of individual erythrocytes from a sickle cell patient. Acta Biomaterialia. 2012;8(11):4130–4138. doi: 10.1016/j.actbio.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alapan Y, Little JA, Gurkan UA. Heterogeneous red blood cell adhesion and deformability in sickle cell disease. Sci Rep. 2014;4:7173. doi: 10.1038/srep07173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alapan Y, Matsuyama Y, Little JA, Gurkan UA. Dynamic deformability of sickle red blood cells in microphysiological flow. TECHNOLOGY. 2016;0(0):1–9. doi: 10.1142/S2339547816400045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montes RA, Eckman JR, Hsu LL, Wick TM. Sickle erythrocyte adherence to endothelium at low shear: role of shear stress in propagation of vaso-occlusion. Am J Hematol. 2002;70(3):216–227. doi: 10.1002/ajh.10145. [DOI] [PubMed] [Google Scholar]
- 30.Hillery CA, Du MC, Montgomery RR, Scott JP. Increased adhesion of erythrocytes to components of the extracellular matrix: isolation and characterization of a red blood cell lipid that binds thrombospondin and laminin. Blood. 1996;87(11):4879–4886. [PubMed] [Google Scholar]
- 31.Kasschau MR, Barabino GA, Bridges KR, Golan DE. Adhesion of sickle neutrophils and erythrocytes to fibronectin. Blood. 1996;87(2):771–780. [PubMed] [Google Scholar]
- 32.Bartolucci P, Brugnara C, Teixeira-Pinto A, Pissard S, Moradkhani K, Jouault H, Galacteros F. Erythrocyte density in sickle cell syndromes is associated with specific clinical manifestations and hemolysis. Blood. 2012;120(15):3136–3141. doi: 10.1182/blood-2012-04-424184. [DOI] [PubMed] [Google Scholar]
- 33.Kaul DK, Finnegan EM, Barabino GA. Sickle Red Cell-Endothelium Interactions. Microcirculation. 2009;16(1):97–111. doi: 10.1080/10739680802279394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34**.Alapan Y, Kim C, Adhikari A, Gray KE, Gurkan-Cavusoglu E, Little JA, Gurkan UA. Sickle cell disease biochip: a functional red blood cell adhesion assay for monitoring sickle cell disease. Transl Res. :2016. doi: 10.1016/j.trsl.2016.03.008. Describes a POC microfluidic technology for monitoring RBC adhesion and its clinical associations in SCD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaul DK, Fabry ME, Windisch P, Baez S, Nagel RL. Erythrocytes in sickle cell anemia are heterogeneous in their rheological and hemodynamic characteristics. J Clin Invest. 1983;72(1):22–31. doi: 10.1172/JCI110960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei H, Karniadakis GE. Probing vasoocclusion phenomena in sickle cell anemia via mesoscopic simulations. Proc Natl Acad Sci U S A. 2013;110(28):11326–11330. doi: 10.1073/pnas.1221297110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manwani D, Frenette PS. Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Hematology Am Soc Hematol Educ Program. 2013;2013:362–369. doi: 10.1182/asheducation-2013.1.362. [DOI] [PubMed] [Google Scholar]
- 38.Ataga KI, Orringer EP. Hypercoagulability in sickle cell disease: a curious paradox. Am J Med. 2003;115(9):721–728. doi: 10.1016/j.amjmed.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Frenette PS. Sickle cell vaso-occlusion: multistep and multicellular paradigm. Curr Opin Hematol. 2002;9(2):101–106. doi: 10.1097/00062752-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion. Blood. 2000;96(7):2451–2459. [PubMed] [Google Scholar]
- 41.Barabino GA, Wise RJ, Woodbury VA, Zhang B, Bridges KA, Hebbel RP, Lawler J, Ewenstein BM. Inhibition of sickle erythrocyte adhesion to immobilized thrombospondin by von Willebrand factor under dynamic flow conditions. Blood. 1997;89(7):2560–2567. [PubMed] [Google Scholar]
- 42.Hofstra TC, Kalra VK, Meiselman HJ, Coates TD. Sickle erythrocytes adhere to polymorphonuclear neutrophils and activate the neutrophil respiratory burst. Blood. 1996;87(10):4440–4447. [PubMed] [Google Scholar]
- 43.Wick TM, Moake JL, Udden MM, McIntire LV. Unusually large von Willebrand factor multimers preferentially promote young sickle and nonsickle erythrocyte adhesion to endothelial cells. Am J Hematol. 1993;42(3):284–292. doi: 10.1002/ajh.2830420308. [DOI] [PubMed] [Google Scholar]
- 44.Kaul DK, Nagel RL. Sickle cell vasoocclusion: many issues and some answers. Experientia. 1993;49(1):5–15. doi: 10.1007/BF01928783. [DOI] [PubMed] [Google Scholar]
- 45.Mohandas N, Evans E. Rheological and adherence properties of sickle cells. Potential contribution to hematologic manifestations of the disease. Ann N Y Acad Sci. 1989;565:327–337. doi: 10.1111/j.1749-6632.1989.tb24180.x. [DOI] [PubMed] [Google Scholar]
- 46.Wick TM, Moake JL, Udden MM, Eskin SG, Sears DA, McIntire LV. Unusually large von Willebrand factor multimers increase adhesion of sickle erythrocytes to human endothelial cells under controlled flow. J Clin Invest. 1987;80(3):905–910. doi: 10.1172/JCI113151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hebbel RP, Visser MR, Goodman JL, Jacob HS, Vercellotti GM. Potentiated adherence of sickle erythrocytes to endothelium infected by virus. J Clin Invest. 1987;80:1503–1506. doi: 10.1172/JCI113233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med. 1980;302(18):992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- 49.Whelihan MF, Mooberry MJ, Zachary V, Bradford RL, Ataga KI, Mann KG, Key NS. The contribution of red blood cells to thrombin generation in sickle cell disease: meizothrombin generation on sickled red blood cells. J Thromb Haemost. 2013;11(12):2187–2189. doi: 10.1111/jth.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim MY, Ataga KI, Key NS. Hemostatic abnormalities in sickle cell disease. Curr Opin Hematol. 2013;20(5):472–477. doi: 10.1097/MOH.0b013e328363442f. [DOI] [PubMed] [Google Scholar]
- 51.Hofrichter J, Ross PD, Eaton WA. Kinetics and mechanism of deoxyhemoglobin S gelation: a new approach to understanding sickle cell disease. Proc Natl Acad Sci U S A. 1974;71(12):4864–4868. doi: 10.1073/pnas.71.12.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease. Life expectancy and risk factors for early death. New England Journal of Medicine. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 53.Hebbel RP, Eaton JW, Steinberg MH, White JG. Erythrocyte/endothelial interactions and the vasocclusive severity of sickle cell disease. Prog Clin Biol Res. 1981;55:145–162. [PubMed] [Google Scholar]
- 54.Barabino GA, McIntire LV, Eskin SG, Sears DA, Udden M. Endothelial cell interactions with sickle cell, sickle trait, mechanically injured, and normal erythrocytes under controlled flow. Blood. 1987;70(1):152–157. [PubMed] [Google Scholar]
- 55.Barabino GA, McIntire LV, Eskin SG, Sears DA, Udden M. Rheological studies of erythrocyte-endothelial cell interactions in sickle cell disease. Prog Clin Biol Res. 1987;240:113–127. [PubMed] [Google Scholar]
- 56.Kaul DK, Fabry ME, Nagel RL. Microvascular sites and characteristics of sickle cell adhesion to vascular endothelium in shear flow conditions: pathophysiological implications. Proc Natl Acad Sci U S A. 1989;86(9):3356–3360. doi: 10.1073/pnas.86.9.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Natl Acad Sci U S A. 2002;99(5):3047–3051. doi: 10.1073/pnas.052522799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsui NM, Borsig L, Rosen SD, Yaghmai M, Varki A, Embury SH. P-selectin mediates the adhesion of sickle erythrocytes to the endothelium. Blood. 2001;98(6):1955–1962. doi: 10.1182/blood.v98.6.1955. [DOI] [PubMed] [Google Scholar]
- 59.Matsui NM, Varki A, Embury SH. Heparin inhibits the flow adhesion of sickle red blood cells to P-selectin. Blood. 2002;100(10):3790–3796. doi: 10.1182/blood-2002-02-0626. [DOI] [PubMed] [Google Scholar]
- 60.Hebbel RP. Adhesive interactions of sickle erythrocytes with endothelium. Journal of Clinical Investigation. 1997;100(11):S83–S86. [PubMed] [Google Scholar]
- 61.Ballas SK, Smith ED. Red blood cell changes during the evolution of the sickle cell painful crisis. Blood. 1992;79(8):2154–2163. [PubMed] [Google Scholar]
- 62.Hillery CA, Du MC, Wang WC, Scott JP. Hydroxyurea therapy decreases the in vitro adhesion of sickle erythrocytes to thrombospondin and laminin. Br J Haematol. 2000;109(2):322–327. doi: 10.1046/j.1365-2141.2000.02040.x. [DOI] [PubMed] [Google Scholar]
- 63.Styles LA, Lubin B, Vichinsky E, Lawrence S, Hua M, Test S, Kuypers F. Decrease of very late activation antigen-4 and CD36 on reticulocytes in sickle cell patients treated with hydroxyurea. Blood. 1997;89(7):2554–2559. [PubMed] [Google Scholar]
- 64.Test ST, Hua M, Styles L. Decreased expression of integrin α4β1 and CD36 on circulating sickle reticulocytes and nucleated red cells following therapy with high dose erythropoietin. Blood. 1994;84(10 Suppl 1):404a. [Google Scholar]
- 65.Setty BN, Kulkarni S, Dampier CD, Stuart MJ. Fetal hemoglobin in sickle cell anemia: relationship to erythrocyte adhesion markers and adhesion. Blood. 2001;97(9):2568–2573. doi: 10.1182/blood.v97.9.2568. [DOI] [PubMed] [Google Scholar]
- 66.Setty BN, Kulkarni S, Stuart MJ. Role of erythrocyte phosphatidylserine in sickle red cell-endothelial adhesion. Blood. 2002;99(5):1564–1571. doi: 10.1182/blood.v99.5.1564. [DOI] [PubMed] [Google Scholar]
- 67.Frenette PS, Atweh GF. Sickle cell disease: old discoveries, new concepts, and future promise. J Clin Invest. 2007;117(4):850–858. doi: 10.1172/JCI30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Natarajan M, Udden MM, McIntire LV. Adhesion of sickle red blood cells and damage to interleukin-1 beta stimulated endothelial cells under flow in vitro. Blood. 1996;87(11):4845–4852. [PubMed] [Google Scholar]
- 69.Perelman N, Selvaraj SK, Batra S, Luck LR, Erdreich-Epstein A, Coates TD, Kalra VK, Malik P. Placenta growth factor activates monocytes and correlates with sickle cell disease severity. Blood. 2003;102(4):1506–1514. doi: 10.1182/blood-2002-11-3422. [DOI] [PubMed] [Google Scholar]
- 70.Zennadi R, Chien A, Xu K, Batchvarova M, Telen MJ. Sickle red cells induce adhesion of lymphocytes and monocytes to endothelium. Blood. 2008;112(8):3474–3483. doi: 10.1182/blood-2008-01-134346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stone PC, Stuart J, Nash GB. Effects of density and of dehydration of sickle cells on their adhesion to cultured endothelial cells. Am J Hematol. 1996;52(3):135–143. doi: 10.1002/(SICI)1096-8652(199607)52:3<135::AID-AJH2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 72.Kaul DK, Nagel RL, Chen D, Tsai HM. Sickle erythrocyte-endothelial interactions in microcirculation: the role of von Willebrand factor and implications for vasoocclusion. Blood. 1993;81(9):2429–2438. [PubMed] [Google Scholar]
- 73.Brittain HA, Eckman JR, Swerlick RA, Howard RJ, Wick TM. Thrombospondin from activated platelets promotes sickle erythrocyte adherence to human microvascular endothelium under physiologic flow: a potential role for platelet activation in sickle cell vaso-occlusion. Blood. 1993;81(8):2137–2143. [PubMed] [Google Scholar]
- 74.Sugihara K, Sugihara T, Mohandas N, Hebbel RP. Thrombospondin mediates adherence of CD36+sickle reticulocytes to endothelial cells. Blood. 1992;80(10):2634–2642. [PubMed] [Google Scholar]
- 75.Finnegan EM, Turhan A, Golan DE, Barabino GA. Adherent leukocytes capture sickle erythrocytes in an in vitro flow model of vaso-occlusion. Am J Hematol. 2007;82(4):266–275. doi: 10.1002/ajh.20819. [DOI] [PubMed] [Google Scholar]
- 76.Solovey A, Lin Y, Browne P, Choong S, Wayner E, Hebbel RP. Circulating activated endothelial cells in sickle cell anemia. N Engl J Med. 1997;337(22):1584–1590. doi: 10.1056/NEJM199711273372203. [DOI] [PubMed] [Google Scholar]
- 77.Strijbos MH, Landburg PP, Nur E, Teerlink T, Leebeek FW, Rijneveld AW, Biemond BJ, Sleijfer S, Gratama JW, Duits AJ, Schnog JJ. Circulating endothelial cells: a potential parameter of organ damage in sickle cell anemia? Blood Cells Mol Dis. 2009;43(1):63–67. doi: 10.1016/j.bcmd.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 78.van Beem RT, Nur E, Zwaginga JJ, Landburg PP, van Beers EJ, Duits AJ, Brandjes DP, Lommerse I, de Boer HC, van der Schoot CE, Schnog JJ, Biemond BJ. Elevated endothelial progenitor cells during painful sickle cell crisis. Exp Hematol. 2009;37(9):1054–1059. doi: 10.1016/j.exphem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Jang JE, Hod EA, Spitalnik SL, Frenette PS. CXCL1 and its receptor, CXCR2, mediate murine sickle cell vaso-occlusion during hemolytic transfusion reactions. J Clin Invest. 121(4):1397–1401. doi: 10.1172/JCI45336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.An X, Mohandas N. Disorders of red cell membrane. Br J Haematol. 2008;141(3):367–375. doi: 10.1111/j.1365-2141.2008.07091.x. [DOI] [PubMed] [Google Scholar]
- 81.Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008;112(10):3939–3948. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lipowsky HH. Microvascular rheology and hemodynamics. Microcirculation. 2005;12(1):5–15. doi: 10.1080/10739680590894966. [DOI] [PubMed] [Google Scholar]
- 83.Barabino GA, Platt MO, Kaul DK. Sickle cell biomechanics. Annu Rev Biomed Eng. 2010;12:345–367. doi: 10.1146/annurev-bioeng-070909-105339. [DOI] [PubMed] [Google Scholar]
- 84.Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364(9442):1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 85.Hoover R, Rubin R, Wise G, Warren R. Adhesion of normal and sickle erythrocytes to endothelial monolayer cultures. Blood. 1979;54(4):872–876. [PubMed] [Google Scholar]
- 86.Ballas SK, Larner J, Smith ED, Surrey S, Schwartz E, Rappaport EF. Rheologic predictors of the severity of the painful sickle cell crisis. Blood. 1988;72(4):1216–1223. [PubMed] [Google Scholar]
- 87.Taylor JGt, Nolan VG, Mendelsohn L, Kato GJ, Gladwin MT, Steinberg MH. Chronic hyper-hemolysis in sickle cell anemia: association of vascular complications and mortality with less frequent vasoocclusive pain. PLoS ONE. 2008;3(5):e2095. doi: 10.1371/journal.pone.0002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bensinger TA, Gillette PN. Hemolysis in sickle cell disease. Arch Intern Med. 1974;133(4):624–631. [PubMed] [Google Scholar]
- 89.Connes P, Coates TD. Autonomic nervous system dysfunction: implication in sickle cell disease. C R Biol. 2013;336(3):142–147. doi: 10.1016/j.crvi.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 90.Sangkatumvong S, Khoo MC, Coates TD. Abnormal cardiac autonomic control in sickle cell disease following transient hypoxia. Conf Proc IEEE Eng Med Biol Soc; 2008; 2008. pp. 1996–1999. [DOI] [PubMed] [Google Scholar]
- 91.Sangkatumvong S, Khoo MC, Kato R, Detterich JA, Bush A, Keens TG, Meiselman HJ, Wood JC, Coates TD. Peripheral vasoconstriction and abnormal parasympathetic response to sighs and transient hypoxia in sickle cell disease. Am J Respir Crit Care Med. 2011;184(4):474–481. doi: 10.1164/rccm.201103-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hines PC, Zen Q, Burney SN, Shea DA, Ataga KI, Orringer EP, Telen MJ, Parise LV. Novel epinephrine and cyclic AMP-mediated activation of BCAM/Lu-dependent sickle (SS) RBC adhesion. Blood. 2003;101(8):3281–3287. doi: 10.1182/blood-2001-12-0289. [DOI] [PubMed] [Google Scholar]
- 93.Maciaszek JL, Andemariam B, Huber G, Lykotrafitis G. Epinephrine modulates BCAM/Lu and ICAM-4 expression on the sickle cell trait red blood cell membrane. Biophys J. 2012;102(5):1137–1143. doi: 10.1016/j.bpj.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 95.Kato GJ, McGowan V, Machado RF, Little JA, Taylor Jt, Morris CR, Nichols JS, Wang X, Poljakovic M, Morris SM, Jr, Gladwin MT. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107(6):2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jison ML, Gladwin MT. Hemolytic anemia-associated pulmonary hypertension of sickle cell disease and the nitric oxide/arginine pathway. Am J Respir Crit Care Med. 2003;168(1):3–4. doi: 10.1164/rccm.2304002. [DOI] [PubMed] [Google Scholar]
- 97.Chen G, Zhang D, Fuchs TA, Manwani D, Wagner DD, Frenette PS. Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood. 2014;123(24):3818–3827. doi: 10.1182/blood-2013-10-529982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, Smith A, Nath KA, Hebbel RP, Vercellotti GM. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123(3):377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Telen MJ. Erythrocyte adhesion receptors: blood group antigens and related molecules. Transfus Med Rev. 2005;19(1):32–44. doi: 10.1016/j.tmrv.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 100.El Nemer W, Wautier MP, Rahuel C, Gane P, Hermand P, Galacteros F, Wautier JL, Cartron JP, Colin Y, Le Van Kim C. Endothelial Lu/BCAM glycoproteins are novel ligands for red blood cell alpha4beta1 integrin: role in adhesion of sickle red blood cells to endothelial cells. Blood. 2007;109(8):3544–3551. doi: 10.1182/blood-2006-07-035139. [DOI] [PubMed] [Google Scholar]
- 101.Brown MD, Wick TM, Eckman JR. Activation of vascular endothelial cell adhesion molecule expression by sickle blood cells. Pediatr Pathol Mol Med. 2001;20(1):47–72. [PubMed] [Google Scholar]
- 102.Johnson C, Telen MJ. Adhesion molecules and hydroxyurea in the pathophysiology of sickle cell disease. Haematologica. 2008;93(4):481–485. doi: 10.3324/haematol.12734. [DOI] [PubMed] [Google Scholar]
- 103.Wick TM, Eckman JR. Molecular basis of sickle cell-endothelial cell interactions. Curr Opin Hematol. 1996;3(2):118–124. doi: 10.1097/00062752-199603020-00003. [DOI] [PubMed] [Google Scholar]
- 104.Swerlick RA, Eckman JR, Kumar A, Jeitler M, Wick TM. Alpha 4 beta 1-integrin expression on sickle reticulocytes: vascular cell adhesion molecule-1-dependent binding to endothelium. Blood. 1993;82(6):1891–1899. [PubMed] [Google Scholar]
- 105.Joneckis CC, Ackley RL, Orringer EP, Wayner EA, Parise LV. Integrin α4β1 and glycoprotein IV (CD36) are expressed on circulating reticulocytes in sickle cell anemia. Blood. 1993;82(12):3548–3555. [PubMed] [Google Scholar]
- 106.Zennadi R, Moeller BJ, Whalen EJ, Batchvarova M, Xu K, Shan S, Delahunty M, Dewhirst MW, Telen MJ. Epinephrine-induced activation of LW-mediated sickle cell adhesion and vaso-occlusion in vivo. Blood. 2007;110(7):2708–2717. doi: 10.1182/blood-2006-11-056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Jong K, Larkin SK, Styles LA, Bookchin RM, Kuypers FA. Characterization of the phosphatidylserine-exposing subpopulation of sickle cells. Blood. 2001;98(3):860–867. doi: 10.1182/blood.v98.3.860. [DOI] [PubMed] [Google Scholar]
- 108.Kuypers FA, Lewis RA, Hua M, Schott MA, Discher D, Ernst JD, Lubin BH. Detection of altered membrane phospholipid asymmetry in subpopulations of human red blood cells using fluorescently labeled annexin V. Blood. 1996;87(3):1179–1187. [PubMed] [Google Scholar]
- 109.Hines PC, Krishnamoorthy S, White J, Gupta D, van der Flier A, Lancelot MM, Peters RT, Jiang H, Hobbs WE, Light DR. Natalizumab Blocks VLA-4 Mediated Red Blood Cell Adhesion and Is a Potential Therapy for Sickle Cell Disease. Blood. 2014;124(21):221–221. [Google Scholar]
- 110.De Castro LM, Zennadi R, Jonassaint JC, Batchvarova M, Telen MJ. Effect of propranolol as antiadhesive therapy in sickle cell disease. Clin Transl Sci. 2012;5(6):437–444. doi: 10.1111/cts.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zennadi R, Hines PC, De Castro LM, Cartron JP, Parise LV, Telen MJ. Epinephrine acts through erythroid signaling pathways to activate sickle cell adhesion to endothelium via LW-alphavbeta3 interactions. Blood. 2004;104(12):3774–3781. doi: 10.1182/blood-2004-01-0042. [DOI] [PubMed] [Google Scholar]
- 112.Delahunty M, Zennadi R, Telen MJ. LW protein: a promiscuous integrin receptor activated by adrenergic signaling. Transfus Clin Biol. 2006;13(1–2):44–49. doi: 10.1016/j.tracli.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 113.Finnegan EM, Barabino GA, Liu XD, Chang HY, Jonczyk A, Kaul DK. Small-molecule cyclic alpha V beta 3 antagonists inhibit sickle red cell adhesion to vascular endothelium and vasoocclusion. Am J Physiol Heart Circ Physiol. 2007;293(2):H1038–1045. doi: 10.1152/ajpheart.01054.2006. [DOI] [PubMed] [Google Scholar]
- 114.Alshaiban A, Muralidharan-Chari V, Nepo A, Mousa SA. Modulation of Sickle Red Blood Cell Adhesion and its Associated Changes in Biomarkers by Sulfated Nonanticoagulant Heparin Derivative. Clin Appl Thromb Hemost. :2015. doi: 10.1177/1076029614565880. [DOI] [PubMed] [Google Scholar]
- 115.Kutlar A, Ataga KI, McMahon L, Howard J, Galacteros F, Hagar W, Vichinsky E, Cheung AT, Matsui N, Embury SH. A potent oral P-selectin blocking agent improves microcirculatory blood flow and a marker of endothelial cell injury in patients with sickle cell disease. Am J Hematol. 2012;87(5):536–539. doi: 10.1002/ajh.23147. [DOI] [PubMed] [Google Scholar]
- 116.Kutlar A, Embury SH. Cellular adhesion and the endothelium: P-selectin. Hematol Oncol Clin North Am. 2014;28(2):323–339. doi: 10.1016/j.hoc.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 117.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107(36):15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10(1):136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vichinsky EP, Styles LA, Colangelo LH, Wright EC, Castro O, Nickerson B. Acute chest syndrome in sickle cell disease: clinical presentation and course. Cooperative Study of Sickle Cell Disease. Blood. 1997;89(5):1787–1792. [PubMed] [Google Scholar]
- 120.Hassell KL, Eckman JR, Lane PA. Acute multiorgan failure syndrome: a potentially catastrophic complication of severe sickle cell pain episodes. Am J Med. 1994;96(2):155–162. doi: 10.1016/0002-9343(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 121.Ataga KI, Reid M, Ballas SK, Yasin Z, Bigelow C, James LS, Smith WR, Galacteros F, Kutlar A, Hull JH, Stocker JW, Investigators ICAS. Improvements in haemolysis and indicators of erythrocyte survival do not correlate with acute vaso-occlusive crises in patients with sickle cell disease: a phase III randomized, placebo-controlled, double-blind study of the Gardos channel blocker senicapoc (ICA-17043) Br J Haematol. 2011;153(1):92–104. doi: 10.1111/j.1365-2141.2010.08520.x. [DOI] [PubMed] [Google Scholar]
- 122.Lamarre Y, Romana M, Lemonne N, Hardy-Dessources MD, Tarer V, Mougenel D, Waltz X, Tressieres B, Lalanne-Mistrih ML, Etienne-Julan M, Connes P. Alpha thalassemia protects sickle cell anemia patients from macro-albuminuria through its effects on red blood cell rheological properties. Clin Hemorheol Microcirc. 2014;57(1):63–72. doi: 10.3233/CH-131772. [DOI] [PubMed] [Google Scholar]
- 123.Billett HH, Kim K, Fabry ME, Nagel RL. The percentage of dense red cells does not predict incidence of sickle cell painful crisis. Blood. 1986;68(1):301–303. [PubMed] [Google Scholar]
- 124.Billett HH, Nagel RL, Fabry ME. Paradoxical increase of painful crises in sickle cell patients with alpha-thalassemia. Blood. 1995;86(11):4382. [PubMed] [Google Scholar]
- 125.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102(7):2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 126.Setty BN, Key NS, Rao AK, Gayen-Betal S, Krishnan S, Dampier CD, Stuart MJ. Tissue factor-positive monocytes in children with sickle cell disease: correlation with biomarkers of haemolysis. Br J Haematol. 2012;157(3):370–380. doi: 10.1111/j.1365-2141.2012.09065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J Clin Invest. 2000;106(3):411–420. doi: 10.1172/JCI9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chang J, Shi PA, Chiang EY, Frenette PS. Intravenous immunoglobulins reverse acute vaso-occlusive crises in sickle cell mice through rapid inhibition of neutrophil adhesion. Blood. 2008;111(2):915–923. doi: 10.1182/blood-2007-04-084061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miller ST, Sleeper LA, Pegelow CH, Enos LE, Wang WC, Weiner SJ, Wethers DL, Smith J, Kinney TR. Prediction of adverse outcomes in children with sickle cell disease. N Engl J Med. 2000;342(2):83–89. doi: 10.1056/NEJM200001133420203. [DOI] [PubMed] [Google Scholar]
- 130.Wongtong N, Jones S, Deng Y, Cai J, Ataga KI. Monocytosis is associated with hemolysis in sickle cell disease. Hematology. :2015. doi: 10.1179/1607845415Y.0000000011. [DOI] [PubMed] [Google Scholar]
- 131.Elmariah H, Garrett ME, De Castro LM, Jonassaint JC, Ataga KI, Eckman JR, Ashley-Koch AE, Telen MJ. Factors associated with survival in a contemporary adult sickle cell disease cohort. Am J Hematol. 2014;89(5):530–535. doi: 10.1002/ajh.23683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Anyaegbu CC, Okpala IE, Akren’Ova YA, Salimonu LS. Peripheral blood neutrophil count and candidacidal activity correlate with the clinical severity of sickle cell anaemia (SCA) Eur J Haematol. 1998;60(4):267–268. doi: 10.1111/j.1600-0609.1998.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 133.Okpala I. Leukocyte adhesion and the pathophysiology of sickle cell disease. Curr Opin Hematol. 2006;13(1):40–44. doi: 10.1097/01.moh.0000190108.62414.06. [DOI] [PubMed] [Google Scholar]
- 134.Eaton WA, Hofrichter J. The biophysics of sickle cell hydroxyurea therapy. Science. 1995;268(5214):1142–1143. doi: 10.1126/science.7539154. [DOI] [PubMed] [Google Scholar]
- 135.Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15(4):384–391. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Telen MJ, Wun T, McCavit TL, De Castro LM, Krishnamurti L, Lanzkron S, Hsu LL, Smith WR, Rhee S, Magnani JL, Thackray H. Randomized phase 2 study of GMI-1070 in SCD: reduction in time to resolution of vaso-occlusive events and decreased opioid use. Blood. 2015;125(17):2656–2664. doi: 10.1182/blood-2014-06-583351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chang J, Patton JT, Sarkar A, Ernst B, Magnani JL, Frenette PS. GMI-1070, a novel pan-selectin antagonist, reverses acute vascular occlusions in sickle cell mice. Blood. 2010;116(10):1779–1786. doi: 10.1182/blood-2009-12-260513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu H, Wandersee NJ, Guo YH, Jones DW, Holzhauer SL, Hanson MS, Hogg N, Densmore JC, Kaul S, Hillery CA, Pritchard KA., Jr HMGB1 Release and TLR4-Mediated Inflammation In Sickle Cell Disease At Baseline and During Acute Vaso-Occlusive Crisis. Blood. 2013;122(21):181–181. [Google Scholar]
- 139.Keleku-Lukwete N, Suzuki M, Otsuki A, Tsuchida K, Katayama S, Hayashi M, Naganuma E, Moriguchi T, Tanabe O, Engel JD, Imaizumi M, Yamamoto M. Amelioration of inflammation and tissue damage in sickle cell model mice by Nrf2 activation. Proc Natl Acad Sci U S A. 2015;112(39):12169–12174. doi: 10.1073/pnas.1509158112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Inwald DP, Kirkham FJ, Peters MJ, Lane R, Wade A, Evans JP, Klein NJ. Platelet and leucocyte activation in childhood sickle cell disease: association with nocturnal hypoxaemia. Br J Haematol. 2000;111(2):474–481. doi: 10.1046/j.1365-2141.2000.02353.x. [DOI] [PubMed] [Google Scholar]
- 141.Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation. 2004;11(2):129–151. [PubMed] [Google Scholar]
- 142.Fadlon E, Vordermeier S, Pearson TC, Mire-Sluis AR, Dumonde DC, Phillips J, Fishlock K, Brown KA. Blood polymorphonuclear leukocytes from the majority of sickle cell patients in the crisis phase of the disease show enhanced adhesion to vascular endothelium and increased expression of CD64. Blood. 1998;91(1):266–274. [PubMed] [Google Scholar]
- 143.Bowers AS, Reid HL, Greenidge A, Landis C, Reid M. Blood viscosity and the expression of inflammatory and adhesion markers in homozygous sickle cell disease subjects with chronic leg ulcers. PLoS One. 2013;8(7):e68929. doi: 10.1371/journal.pone.0068929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Benkerrou M, Delarche C, Brahimi L, Fay M, Vilmer E, Elion J, AG-PM, Elbim C. Hydroxyurea corrects the dysregulated L-selectin expression and increased H(2)O(2) production of polymorphonuclear neutrophils from patients with sickle cell anemia. Blood. 2002;99(7):2297–2303. doi: 10.1182/blood.v99.7.2297. [DOI] [PubMed] [Google Scholar]
- 145.Moncada S, Radomski MW, Palmer RM. Endothelium-derived relaxing factor: Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochemical Pharmacology. 1998;37(13):2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- 146.Belhassen L, Pelle G, Sediame S, Bachir D, Carville C, Bucherer C, Lacombe C, Galacteros F, Adnot S. Endothelial dysfunction in patients with sickle cell disease is related to selective impairment of shear stress-mediated vasodilation. Blood. 2001;97(6):1584–1589. doi: 10.1182/blood.v97.6.1584. [DOI] [PubMed] [Google Scholar]
- 147.Mack AK, Kato GJ. Sickle cell disease and nitric oxide: a paradigm shift? Int J Biochem Cell Biol. 2006;38(8):1237–1243. doi: 10.1016/j.biocel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Graido-Gonzalez E, Doherty JC, Bergreen EW, Organ G, Telfer M, McMillen MA. Plasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisis. Blood. 1998;92(7):2551–2555. [PubMed] [Google Scholar]
- 149.Zhang D, Xu C, Manwani D, Frenette PS. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood. 2016;127(7):801–809. doi: 10.1182/blood-2015-09-618538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Proenca-Ferreira R, Brugnerotto AF, Garrido VT, Dominical VM, Vital DM, de Ribeiro MF, dos Santos ME, Traina F, Olalla-Saad ST, Costa FF, Conran N. Endothelial activation by platelets from sickle cell anemia patients. PLoS One. 2014;9(2):e89012. doi: 10.1371/journal.pone.0089012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bandeira IC, Rocha LB, Barbosa MC, Elias DB, Querioz JA, Freitas MV, Goncalves RP. Chronic inflammatory state in sickle cell anemia patients is associated with HBB(*)S haplotype. Cytokine. 2014;65(2):217–221. doi: 10.1016/j.cyto.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 152.Lanaro C, Franco-Penteado CF, Albuqueque DM, Saad ST, Conran N, Costa FF. Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydroxyurea therapy. J Leukoc Biol. 2009;85(2):235–242. doi: 10.1189/jlb.0708445. [DOI] [PubMed] [Google Scholar]
- 153.Awogu AU. Leucocyte counts in children with sickle cell anaemia usefulness of stable state values during infections. West Afr J Med. 2000;19(1):55–58. [PubMed] [Google Scholar]
- 154.Okpala I. The intriguing contribution of white blood cells to sickle cell disease - a red cell disorder. Blood Rev. 2004;18(1):65–73. doi: 10.1016/s0268-960x(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 155.Wun T, Cordoba M, Rangaswami A, Cheung AW, Paglieroni T. Activated monocytes and platelet-monocyte aggregates in patients with sickle cell disease. Clin Lab Haematol. 2002;24(2):81–88. doi: 10.1046/j.1365-2257.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 156.Wallace KL, Marshall MA, Ramos SI, Lannigan JA, Field JJ, Strieter RM, Linden J. NKT cells mediate pulmonary inflammation and dysfunction in murine sickle cell disease through production of IFN-gamma and CXCR3 chemokines. Blood. 2009;114(3):667–676. doi: 10.1182/blood-2009-02-205492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Field JJ, Nathan DG, Linden J. Targeting iNKT cells for the treatment of sickle cell disease. Clin Immunol. 2011;140(2):177–183. doi: 10.1016/j.clim.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gulbis B, Cotton F, Ferster A, Ketelslegers O, Dresse MF, Ronge-Collard E, Minon JM, Le PQ, Vertongen F. Neonatal haemoglobinopathy screening in Belgium. J Clin Pathol. 2009;62(1):49–52. doi: 10.1136/jcp.2008.060517. [DOI] [PubMed] [Google Scholar]
- 159.Le PQ, Ferster A, Cotton F, Vertongen F, Vermylen C, Vanderfaeillie A, Dedeken L, Heijmans C, Ketelslegers O, Dresse MF, Gulbis B. Sickle cell disease from Africa to Belgium, from neonatal screening to clinical management. Med Trop (Mars) 2010;70(5–6):467–470. [PubMed] [Google Scholar]
- 160.Gulbis B. Sickle Cell in Focus. 2016. [Google Scholar]
- 161.Odame I. Perspective: We need a global solution. Nature. 2014;515(7526):S10–S10. doi: 10.1038/515S10a. [DOI] [PubMed] [Google Scholar]
- 162.Engert A, Balduini C, Brand A, Coiffier B, Cordonnier C, Dohner H, de Wit TD, Eichinger S, Fibbe W, Green T, de Haas F, Iolascon A, Jaffredo T, Rodeghiero F, Salles G, Schuringa JJ. The European Hematology Association Roadmap for European Hematology Research: a consensus document. Haematologica. 2016;101(2):115–208. doi: 10.3324/haematol.2015.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Aguilar Martinez P, Angastiniotis M, Eleftheriou A, Gulbis B, del Manu Pereira MM, Petrova-Benedict R, Corrons JL. Haemoglobinopathies in Europe: health & migration policy perspectives. Orphanet J Rare Dis. 2014;9:97. doi: 10.1186/1750-1172-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sickle-Cell Disease in the African Region: Current Situation and the Way Forward. AFR/RC56/17. World Health Organization Regional Committee for Africa; 2006. [Google Scholar]
- 165*.Bain BJ. Haemoglobinopathy Diagnosis. Malden, MA, USA: Blackwell; 2006. Provides overview of SCD and current diagnosis techniques. [Google Scholar]
- 166.Bain BJ, Wild B, Stephens A, Phelan L. Variant haemoglobins: a guide to identification. Oxford, UK: Wiley-Blackwell; 2011. [Google Scholar]
- 167.Sickle-cell disease: a strategy for the WHO African Region: Report of the Regional Director. AFR/RC60/8. World Health Organization Regional Office for Africa; 2010. [Google Scholar]
- 168.Ansong D, Akoto AO, Ocloo D, Ohene-Frempong K. Sickle cell disease: management options and challenges in developing countries. Mediterr J Hematol Infect Dis. 2013;5(1):e2013062. doi: 10.4084/MJHID.2013.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cober MP, Phelps SJ. Penicillin prophylaxis in children with sickle cell disease. J Pediatr Pharmacol Ther. 2010;15(3):152–159. [PMC free article] [PubMed] [Google Scholar]
- 170.McGann PT, Nero AC, Ware RE. Current management of sickle cell anemia. Cold Spring Harb Perspect Med. 2013;3(8) doi: 10.1101/cshperspect.a011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mulumba LL, Wilson L. Sickle cell disease among children in Africa: An integrative literature review and global recommendations. International Journal of Africa Nursing Sciences. 2015;3:56–64. [Google Scholar]
- 172.Berg AO. Sickle cell disease: screening, diagnosis, management, and counseling in newborns and infants. The Agency for Health Care Policy and Research. J Am Board Fam Pract. 1994;7(2):134–140. [PubMed] [Google Scholar]
- 173.Interpretation of Newborn Hemoglobin Screening Results. 2013. [Google Scholar]
- 174.Greene DN, Vaughn CP, Crews BO, Agarwal AM. Advances in detection of hemoglobinopathies. Clin Chim Acta. 2015;439:50–57. doi: 10.1016/j.cca.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 175.Makani J, Ofori-Acquah SF, Nnodu O, Wonkam A, Ohene-Frempong K. Sickle cell disease: new opportunities and challenges in Africa. ScientificWorldJournal. 2013;2013:193252. doi: 10.1155/2013/193252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Clark BE, Thein SL. Molecular diagnosis of haemoglobin disorders. Clin Lab Haematol. 2004;26(3):159–176. doi: 10.1111/j.1365-2257.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- 177.Yang X, Kanter J, Piety NZ, Benton MS, Vignes SM, Shevkoplyas SS. A simple, rapid, low-cost diagnostic test for sickle cell disease. Lab on a Chip. 2013;13(8):1464–1467. doi: 10.1039/c3lc41302k. [DOI] [PubMed] [Google Scholar]
- 178.Yang X, Piety NZ, Vignes SM, Benton MS, Kanter J, Shevkoplyas SS. Simple paper-based test for measuring blood hemoglobin concentration in resource-limited settings. Clin Chem. 2013;59(10):1506–1513. doi: 10.1373/clinchem.2013.204701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179**.Piety NZ, Yang X, Lezzar D, George A, Shevkoplyas SS. A rapid paper-based test for quantifying sickle hemoglobin in blood samples from patients with sickle cell disease. Am J Hematol. 2015;90(6):478–482. doi: 10.1002/ajh.23980. Describes a POC technology for SCD screening. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Piety NZ, Yang X, Kanter J, Vignes SM, George A, Shevkoplyas SS. Validation of a Low-Cost Paper-Based Screening Test for Sickle Cell Anemia. PLoS ONE. 2016;11(1):e0144901. doi: 10.1371/journal.pone.0144901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kanter J, Telen MJ, Hoppe C, Roberts CL, Kim JS, Yang X. Validation of a novel point of care testing device for sickle cell disease. BMC Med. 2015;13:225. doi: 10.1186/s12916-015-0473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182**.McGann PT, Schaefer BA, Paniagua M, Howard TA, Ware RE. Characteristics of a rapid, point-of-care lateral flow immunoassay for the diagnosis of sickle cell disease. Am J Hematol. 2016;91(2):205–210. doi: 10.1002/ajh.24232. Describes a POC technology for SCD screening. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183**.Quinn CT, Paniagua MC, DiNello RK, Panchal A, Geisberg M. A rapid, inexpensive and disposable point-of-care blood test for sickle cell disease using novel, highly specific monoclonal antibodies. Br J Haematol. :2016. doi: 10.1111/bjh.14298. Describes a POC technology for SCD screening. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184**.Kumar AA, Patton MR, Hennek JW, Lee SY, D’Alesio-Spina G, Yang X, Kanter J, Shevkoplyas SS, Brugnara C, Whitesides GM. Density-based separation in multiphase systems provides a simple method to identify sickle cell disease. Proc Natl Acad Sci U S A. 2014;111(41):14864–14869. doi: 10.1073/pnas.1414739111. Describes a POC technology for SCD screening. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185**.Ung R, Alapan Y, Hasan MN, Romelfanger M, He P, Tam A, Rosanwo T, Akkus A, Cakar MA, Icoz K, Piccone CM, Little JA, Gurkan UA. Point-of-Care Screening for Sickle Cell Disease By a Mobile Micro-Electrophoresis Platform. Blood. 2015;126(23):3379–3379. Describes a POC technology for SCD screening. [Google Scholar]
- 186.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84(6):363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 187.Nouraie M, Lee JS, Zhang Y, Kanias T, Zhao X, Xiong Z, Oriss TB, Zeng Q, Kato GJ, Gibbs JS, Hildesheim ME, Sachdev V, Barst RJ, Machado RF, Hassell KL, Little JA, Schraufnagel DE, Krishnamurti L, Novelli E, Girgis RE, Morris CR, Rosenzweig EB, Badesch DB, Lanzkron S, Castro OL, Goldsmith JC, Gordeuk VR, Gladwin MT. The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica. 2013;98(3):464–472. doi: 10.3324/haematol.2012.068965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, Wethers DL, Pegelow CH, Gill FM. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–294. [PubMed] [Google Scholar]
- 189.West MS, Wethers D, Smith J, Steinberg M. Laboratory profile of sickle cell disease: a cross-sectional analysis. The Cooperative Study of Sickle Cell Disease. J Clin Epidemiol. 1992;45(8):893–909. doi: 10.1016/0895-4356(92)90073-v. [DOI] [PubMed] [Google Scholar]
- 190.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia [see comments] N Engl J Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 191.Lorch D, Spevack D, Little J. An elevated estimated pulmonary arterial systolic pressure, whenever measured, is associated with excess mortality in adults with sickle cell disease. Acta Haematol. 2011;125(4):225–229. doi: 10.1159/000323464. [DOI] [PubMed] [Google Scholar]
- 192.Lettre G, Sankaran VG, Bezerra MA, Araujo AS, Uda M, Sanna S, Cao A, Schlessinger D, Costa FF, Hirschhorn JN, Orkin SH. DNA polymorphisms at the BCL11A, HBS1L- MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci U S A. 2008;105(33):11869–11874. doi: 10.1073/pnas.0804799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Milton JN, Rooks H, Drasar E, McCabe EL, Baldwin CT, Melista E, Gordeuk VR, Nouraie M, Kato GR, Minniti C, Taylor J, Campbell A, Luchtman-Jones L, Rana S, Castro O, Zhang Y, Thein SL, Sebastiani P, Gladwin MT, Steinberg MH. Genetic determinants of haemolysis in sickle cell anaemia. Br J Haematol. 2013;161(2):270–278. doi: 10.1111/bjh.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bae HT, Baldwin CT, Sebastiani P, Telen MJ, Ashley-Koch A, Garrett M, Hooper WC, Bean CJ, Debaun MR, Arking DE, Bhatnagar P, Casella JF, Keefer JR, Barron-Casella E, Gordeuk V, Kato GJ, Minniti C, Taylor J, Campbell A, Luchtman-Jones L, Hoppe C, Gladwin MT, Zhang Y, Steinberg MH. Meta-analysis of 2040 sickle cell anemia patients: BCL11A and HBS1L-MYB are the major modifiers of HbF in African Americans. Blood. 2012;120(9):1961–1962. doi: 10.1182/blood-2012-06-432849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Milton JN, Sebastiani P, Solovieff N, Hartley SW, Bhatnagar P, Arking DE, Dworkis DA, Casella JF, Barron-Casella E, Bean CJ, Hooper WC, DeBaun MR, Garrett ME, Soldano K, Telen MJ, Ashley-Koch A, Gladwin MT, Baldwin CT, Steinberg MH, Klings ES. A genome-wide association study of total bilirubin and cholelithiasis risk in sickle cell anemia. PLoS One. 2012;7(4):e34741. doi: 10.1371/journal.pone.0034741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Manwani D, Chen G, Carullo V, Serban S, Olowokure O, Jang J, Huggins M, Cohen HW, Billett H, Atweh GF, Frenette PS, Shi PA. Single-dose intravenous gammaglobulin can stabilize neutrophil Mac-1 activation in sickle cell pain crisis. Am J Hematol. 2015;90(5):381–385. doi: 10.1002/ajh.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Manwani D, Chen G, Carullo V, Cohen HW, Serban S, Olowukere O, Jang J, Huggins M, Billett HH, Atweh GF, Frenette PS, Shi PA. Vaso-Occlusion-Promoting Neutrophil Mac-1 Integrin Activation in Human Sickle Cell Crises Is Stabilized By a Single Dose of Intravenous Gammaglobulin. Blood. 2014;124(21):4089–4089. [Google Scholar]
- 198.Field JJ, Lin G, Okam MM, Majerus E, Keefer J, Onyekwere O, Ross A, Campigotto F, Neuberg D, Linden J, Nathan DG. Sickle cell vaso-occlusion causes activation of iNKT cells that is decreased by the adenosine A2A receptor agonist regadenoson. Blood. 2013;121(17):3329–3334. doi: 10.1182/blood-2012-11-465963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lanzkron S, Strouse JJ, Wilson R, Beach MC, Haywood C, Park H, Witkop C, Bass EB, Segal JB. Systematic review: Hydroxyurea for the treatment of adults with sickle cell disease. Annals of Internal Medicine. 2008;148(12):939. doi: 10.7326/0003-4819-148-12-200806170-00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zimmerman SA, Schultz WH, Davis JS, Pickens CV, Mortier NA, Howard TA, Ware RE. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103(6):2039–2045. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- 201.Hydroxyurea for the Treatment of Sickle Cell Disease. Agency for Healthcare Research and Quality; Feb, 2008. [Google Scholar]
- 202.Rodgers GP, Dover GJ, Noguchi CT, Schechter AN, Nienhuis AW. Hematologic Responses of Patients with Sickle-Cell Disease to Treatment with Hydroxyurea. New England Journal of Medicine. 1990;322(15):1037–1045. doi: 10.1056/NEJM199004123221504. [DOI] [PubMed] [Google Scholar]
- 203.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 204.McGann PT, Ware RE. Hydroxyurea for sickle cell anemia: what have we learned and what questions still remain? Current Opinion in Hematology. 2011;18(3):158–165. doi: 10.1097/MOH.0b013e32834521dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ware RE, Eggleston B, Redding-Lallinger R, Wang WC, Smith-Whitley K, Daeschner C, Gee B, Styles LA, Helms RW, Kinney TR, Ohene-Frempong K. Predictors of fetal hemoglobin response in children with sickle cell anemia receiving hydroxyurea therapy. Blood. 2002;99(1):10–14. doi: 10.1182/blood.v99.1.10. [DOI] [PubMed] [Google Scholar]
- 206.Halsey C, Roberts IAG. The role of hydroxyurea in sickle cell disease. British Journal of Haematology. 2003;120(2):177–186. doi: 10.1046/j.1365-2141.2003.03849.x. [DOI] [PubMed] [Google Scholar]
- 207.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood. 2010;115(26):5300–5311. doi: 10.1182/blood-2009-04-146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Heeney MM, Ware RE. Hydroxyurea for Children with Sickle Cell Disease. Hematology-Oncology Clinics of North America. 2010;24(1):199. doi: 10.1016/j.hoc.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Maier-Redelsperger M, de Montalembert M, Flahault A, Neonato MG, Ducrocq R, Masson MP, Girot R, Elion J, Dis FSGSC. Fetal hemoglobin and F-cell responses to long-term hydroxyurea treatment in young sickle cell patients. Blood. 1998;91(12):4472–4479. [PubMed] [Google Scholar]
- 210.Al-Anazi K. Hydroxyurea therapy in patients with sickle cell disease. Translational Medicine. 2015;5:145–150. [Google Scholar]
- 211.Steinberg MH. Sickle Cell Anemia, the First Molecular Disease: Overview of Molecular Etiology, Pathophysiology, and Therapeutic Approaches. Thescientificworldjournal. 2008;8:1295–1324. doi: 10.1100/tsw.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kohne E. Hemoglobinopathies Clinical Manifestations, Diagnosis, and Treatment. Deutsches Arzteblatt International. 2011;108(31–32):532–U521. doi: 10.3238/arztebl.2011.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Harmatz P, Butensky E, Quirolo K, Williams R, Ferrell L, Moyer T, Golden D, Neumayr L, Vichinsky E. Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood. 2000;96(1):76–79. [PubMed] [Google Scholar]
- 214.Stuart MJ, Nagel RL. Sickle-cell disease. The Lancet. 2004;364(9442):1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 215.Yang X, Reavis HD, Roberts CL, Kim JS. Quantitative, Point-of-Care Immunoassay Platform to Guide and Monitor Sickle Cell Disease Therapy. Analytical Chemistry. 2016;88(16):7904–7909. doi: 10.1021/acs.analchem.6b01649. [DOI] [PubMed] [Google Scholar]
- 216.Adams RJ, McKie VC, Brambilla D, Carl E, Gallagher D, Nichols FT, Roach S, Abboud M, Berman B, Driscoll C, Files B, Hsu L, Hurlet A, Miller S, Olivieri N, Pegelow C, Scher C, Vichinsky E, Wang W, Woods G, Kutlar A, Wright E, Hagner S, Tighe F, Lewin J, Cure J, Zimmerman RA, Waclawiw MA. Stroke prevention trial in sickle cell anemia. Controlled Clinical Trials. 1998;19(1):110–129. doi: 10.1016/s0197-2456(97)00099-8. [DOI] [PubMed] [Google Scholar]
- 217.Nagel RL, Vichinsky E, Shah M, Johnson R, Spadacino E, Fabry ME, Mangahas L, Abel R, Stamatoyannopoulos G. F-Reticulocyte Response in Sickle-Cell-Anemia Treated with Recombinant-Human-Erythropoietin - a Double-Blind-Study. Blood. 1993;81(1):9–14. [PubMed] [Google Scholar]
- 218.Strouse JJ, Heeney MM. Hydroxyurea for the treatment of sickle cell disease: Efficacy, barriers, toxicity, and management in children. Pediatric Blood & Cancer. 2012;59(2):365–371. doi: 10.1002/pbc.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Howard J, Oteng-Ntim E. The obstetric management of sickle cell disease. Best Pract Res Clin Obstet Gynaecol. 2012;26(1):25–36. doi: 10.1016/j.bpobgyn.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 220.Tuchin VV, Tarnok A, Zharov VP. In vivo flow cytometry: a horizon of opportunities. Cytometry A. 2011;79(10):737–745. doi: 10.1002/cyto.a.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Morgan SP. Can New Optical Techniques for In Vivo Imaging and Flow Cytometry of the Microcirculation Benefit Sickle Cell Disease Research? Cytometry Part A. 2011;79A(10):766–774. doi: 10.1002/cyto.a.21101. [DOI] [PubMed] [Google Scholar]
- 222.Galanzha EI, Zharov VP. In Vivo Photoacoustic and Photothermal Cytometry for Monitoring Multiple Blood Rheology Parameters. Cytometry Part A. 2011;79A(10):746–757. doi: 10.1002/cyto.a.21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Barabino GA, Platt MO, Kaul DK. Sickle Cell Biomechanics. Annual Review of Biomedical Engineering. 2010;12:345–367. doi: 10.1146/annurev-bioeng-070909-105339. [DOI] [PubMed] [Google Scholar]
- 224.Silva DGH, Belini E, Torres LD, Ricci O, Lobo CD, Bonini-Domingos CR, de Almeida EA. Relationship between oxidative stress, glutathione S-transferase polymorphisms and hydroxyurea treatment in sickle cell anemia. Blood Cells Molecules and Diseases. 2011;47(1):23–28. doi: 10.1016/j.bcmd.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 225.Ware RE, Despotovic JM, Mortier NA, Flanagan JM, He J, Smeltzer MP, Kimble AC, Aygun B, Wu S, Howard T, Sparreboom A. Pharmacokinetics, pharmacodynamics, and pharmacogenetics of hydroxyurea treatment for children with sickle cell anemia. Blood. 2011;118(18):4985–4991. doi: 10.1182/blood-2011-07-364190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sakhalkar VS, Rao SP, Weedon J, Miller ST. Elevated plasma sVCAM-1 levels in children with sickle cell disease: impact of chronic transfusion therapy. Am J Hematol. 2004;76(1):57–60. doi: 10.1002/ajh.20016. [DOI] [PubMed] [Google Scholar]
- 227.Kato GJ, Martyr S, Blackwelder WC, Nichols JS, Coles WA, Hunter LA, Brennan ML, Hazen SL, Gladwin MT. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br J Haematol. 2005;130(6):943–953. doi: 10.1111/j.1365-2141.2005.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Picot J, Goudot C, Berkenou J, Galacteros F, Colin Y, Bartolucci P, le van Kim C. Flow cytometry analyses reveal association between Lu/BCAM adhesion molecule and osteonecrosis in sickle cell disease. Am J Hematol. 2014;89(1):115–117. doi: 10.1002/ajh.23597. [DOI] [PubMed] [Google Scholar]
- 229.Chaar V, Laurance S, Lapoumeroulie C, Cochet S, De Grandis M, Colin Y, Elion J, Le Van Kim C, El Nemer W. Hydroxycarbamide decreases sickle reticulocyte adhesion to resting endothelium by inhibiting endothelial lutheran/basal cell adhesion molecule (Lu/BCAM) through phosphodiesterase 4A activation. J Biol Chem. 2014;289(16):11512–11521. doi: 10.1074/jbc.M113.506121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kim Y, Kim K, Park Y. Measurement Techniques for Red Blood Cell Deformability: Recent Advances. 2012. [Google Scholar]
- 231.Bacabac RG, Smit TH, Cowin SC, Van Loon JJ, Nieuwstadt F, Heethaar R, Klein-Nulend J. Dynamic shear stress in parallel-plate flow chambers. Journal of biomechanics. 2005;38(1):159–167. doi: 10.1016/j.jbiomech.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 232.Yang Y, Koo S, Lin CS, Neu B. Specific Binding of Red Blood Cells to Endothelial Cells Is Regulated by Nonadsorbing Macromolecules. J Biol Chem. 2010;285(52):40489–40495. doi: 10.1074/jbc.M110.116608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tasoglu S, Safaee H, Zhang X, Kingsley JL, Catalano PN, Gurkan UA, Nureddin A, Kayaalp E, Anchan RM, Maas RL, Tuzel E, Demirci U. Exhaustion of racing sperm in nature-mimicking microfluidic channels during sorting. Small. 2013;9(20):3374–3384. doi: 10.1002/smll.201300020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rizvi I, Gurkan UA, Tasoglu S, Alagic N, Celli JP, Mensah LB, Mai Z, Demirci U, Hasan T. Flow induces epithelial-mesenchymal transition, cellular heterogeneity and biomarker modulation in 3D ovarian cancer nodules. Proc Natl Acad Sci U S A. 2013;110(22):E1974–1983. doi: 10.1073/pnas.1216989110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gurkan UA, Moon S, Geckil H, Xu F, Wang S, Lu TJ, Demirci U. Miniaturized lensless imaging systems for cell and microorganism visualization in point-of-care testing. Biotechnol J. 2011;6(2):138–149. doi: 10.1002/biot.201000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhang X, Khimji I, Gurkan UA, Safaee H, Catalano PN, Keles HO, Kayaalp E, Demirci U. Lensless imaging for simultaneous microfluidic sperm monitoring and sorting. Lab Chip. 2011;11(15):2535–2540. doi: 10.1039/c1lc20236g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Moon S, Gurkan UA, Blander J, Fawzi WW, Aboud S, Mugusi F, Kuritzkes DR, Demirci U. Enumeration of CD4+ T-cells using a portable microchip count platform in Tanzanian HIV-infected patients. PLoS ONE. 2011;6(7):e21409. doi: 10.1371/journal.pone.0021409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gurkan UA, Anand T, Tas H, Elkan D, Akay A, Keles HO, Demirci U. Controlled viable release of selectively captured label-free cells in microchannels. Lab Chip. 2011;11(23):3979–3989. doi: 10.1039/c1lc20487d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gurkan UA, Tasoglu S, Akkaynak D, Avci O, Unluisler S, Canikyan S, Maccallum N, Demirci U. Smart interface materials integrated with microfluidics for on-demand local capture and release of cells. Adv Healthc Mater. 2012;1(5):661–668. doi: 10.1002/adhm.201200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Du E, Diez-Silva M, Kato GJ, Dao M, Suresh S. Kinetics of sickle cell biorheology and implications for painful vasoocclusive crisis. Proc Natl Acad Sci U S A. 2015;112(5):1422–1427. doi: 10.1073/pnas.1424111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Cohen SI, Mahadevan L. Hydrodynamics of hemostasis in sickle-cell disease. Phys Rev Lett. 2013;110(13):138104. doi: 10.1103/PhysRevLett.110.138104. [DOI] [PubMed] [Google Scholar]
- 242.Higgins JM, Eddington DT, Bhatia SN, Mahadevan L. Sickle cell vasoocclusion and rescue in a microfluidic device. Proc Natl Acad Sci U S A. 2007;104(51):20496–20500. doi: 10.1073/pnas.0707122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wood DK, Soriano A, Mahadevan L, Higgins JM, Bhatia SN. A biophysical indicator of vaso-occlusive risk in sickle cell disease. Sci Transl Med. 2012;4(123):123ra126. doi: 10.1126/scitranslmed.3002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tsai M, Kita A, Leach J, Rounsevell R, Huang JN, Moake J, Ware RE, Fletcher DA, Lam WA. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest. 2012;122(1):408–418. doi: 10.1172/JCI58753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Solovey AA, Solovey AN, Harkness J, Hebbel RP. Modulation of endothelial cell activation in sickle cell disease: a pilot study. Blood. 2001;97(7):1937–1941. doi: 10.1182/blood.v97.7.1937. [DOI] [PubMed] [Google Scholar]
- 246.Fiddes LK, Raz N, Srigunapalan S, Tumarkan E, Simmons CA, Wheeler AR, Kumacheva E. A circular cross-section PDMS microfluidics system for replication of cardiovascular flow conditions. Biomaterials. 2010;31(13):3459–3464. doi: 10.1016/j.biomaterials.2010.01.082. [DOI] [PubMed] [Google Scholar]
- 247.Myers DR, Sakurai Y, Tran R, Ahn B, Hardy ET, Mannino R, Kita A, Tsai M, Lam WA. Endothelialized microfluidics for studying microvascular interactions in hematologic diseases. J Vis Exp. 2012;(64) doi: 10.3791/3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Campo-Deano L, Dullens RP, Aarts DG, Pinho FT, Oliveira MS. Viscoelasticity of blood and viscoelastic blood analogues for use in polydymethylsiloxane in vitro models of the circulatory system. Biomicrofluidics. 2013;7(3):34102. doi: 10.1063/1.4804649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Little JA, Alapan Y, Gray KE, Gurkan UA. SCD-Biochip: A Functional Assay for Red Cell Adhesion in Sickle Cell Disease. Blood. 2014;124(21):4053–4053. [Google Scholar]
- 250.Ballas SK, Marcolina MJ. Hyperhemolysis during the evolution of uncomplicated acute painful episodes in patients with sickle cell anemia. Transfusion. 2006;46(1):105–110. doi: 10.1111/j.1537-2995.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 251.Stankovic Stojanovic K, Steichen O, Lefevre G, Bachmeyer C, Avellino V, Grateau G, Girot R, Lionnet F. High lactate dehydrogenase levels at admission for painful vaso-occlusive crisis is associated with severe outcome in adult SCD patients. Clin Biochem. 2012;45(18):1578–1582. doi: 10.1016/j.clinbiochem.2012.07.114. [DOI] [PubMed] [Google Scholar]
- 252.Hierso R, Waltz X, Mora P, Romana M, Lemonne N, Connes P, Hardy-Dessources MD. Effects of oxidative stress on red blood cell rheology in sickle cell patients. Br J Haematol. 2014;166(4):601–606. doi: 10.1111/bjh.12912. [DOI] [PubMed] [Google Scholar]
- 253.Eaton WA, Hofrichter J, Ross PD. Editorial: Delay time of gelation: a possible determinant of clinical severity in sickle cell disease. Blood. 1976;47(4):621–627. [PubMed] [Google Scholar]
- 254.Powars DR, Weiss JN, Chan LS, Schroeder WA. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood. 1984;63(4):921–926. [PubMed] [Google Scholar]
- 255.Brittenham GM, Schechter AN, Noguchi CT. Hemoglobin S polymerization: primary determinant of the hemolytic and clinical severity of the sickling syndromes. Blood. 1985;65(1):183–189. [PubMed] [Google Scholar]
- 256.Sewchand LS, Johnson CS, Meiselman HJ. The effect of fetal hemoglobin on the sickling dynamics of SS erythrocytes. Blood Cells. 1983;9:147–159. [PubMed] [Google Scholar]
- 257.Sickle Cell Disease and other Hemoglobin Disorders Fact Sheet. World Health Organization; 2011. [Google Scholar]
- 258.Makani J, Cox SE, Soka D, Komba AN, Oruo J, Mwamtemi H, Magesa P, Rwezaula S, Meda E, Mgaya J, Lowe B, Muturi D, Roberts DJ, Williams TN, Pallangyo K, Kitundu J, Fegan G, Kirkham FJ, Marsh K, Newton CR. Mortality in sickle cell anemia in Africa: a prospective cohort study in Tanzania. PLoS One. 2011;6(2):e14699. doi: 10.1371/journal.pone.0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Okpala I. Investigational selectin-targeted therapy of sickle cell disease. Expert Opin Investig Drugs. 2015;24(2):229–238. doi: 10.1517/13543784.2015.963552. [DOI] [PubMed] [Google Scholar]
- 260.Telen MJ. Cellular adhesion and the endothelium: E-selectin, L-selectin, and pan-selectin inhibitors. Hematol Oncol Clin North Am. 2014;28(2):341–354. doi: 10.1016/j.hoc.2013.11.010. [DOI] [PubMed] [Google Scholar]