Summary
The world is undergoing an unprecedented shift in demographics, with the number of individuals over the age of 60 years projected to reach 2 billion or more by 2050, representing 22% of the global population. Elderly people are at a higher risk for chronic disease and more susceptible to infection, due in part to age‐related dysfunction of the immune system resulting from low‐grade chronic inflammation known as ‘inflamm‐ageing’. The innate immune system of older individuals exhibits a diminished ability to respond to microbial threats and clear infections, resulting in a greater occurrence of many infectious diseases in elderly people. In particular, the incidence of and mortality from lung infections increase sharply with age, with such infections often leading to worse outcomes, prolonged hospital stays and life‐threatening complications, such as sepsis or acute respiratory distress syndrome. In this review, we highlight research on bacterial pneumonias and pulmonary viral infections and discuss age‐related changes in innate immunity that contribute to the higher rate of these infections in older populations. By understanding more clearly the innate immune defects in elderly individuals, we can design age‐specific therapies to address lung infections in such a vulnerable population.
Keywords: aging, inflammation, infection, lung, macrophages, neutrophils, pneumonia
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Immunosenescence: the importance of considering age in health and disease. Clinical and Experimental Immunology 2017, 187: 1–3.
The convergence of senescence and nutrient sensing during lymphocyte ageing. Clinical and Experimental Immunology 2017, 187: 4–5.
Immune senescence: significance of the stromal microenvironment. Clinical and Experimental Immunology 2017, 187: 6–15.
Age‐related alterations in immune responses to West Nile virus infection. Clinical and Experimental Immunology 2017, 187: 26–34.
Intracellular signalling pathways: targets to reverse immunosenescence. Clinical and Experimental Immunology 2017, 187: 35–43.
Ageing and inflammation in patients with HIV infection. Clinical and Experimental Immunology 2017, 187: 44–52.
Considerations for successful cancer immunotherapy in aged hosts. Clinical and Experimental Immunology 2017, 187: 53–63.
Ageing and obesity similarly impair antibody responses. Clinical and Experimental Immunology 2017, 187: 64–70.
The life cycle of a T cell after vaccination – where does immune ageing strike? Clinical and Experimental Immunology 2017, 187: 71–81.
Herpes zoster and the search for an effective vaccine. Clinical and Experimental Immunology 2017, 187: 82–92.
Adult vaccination against tetanus and diphtheria: the European perspective. Clinical and Experimental Immunology 2017, 187: 93–99.
Introduction
In elderly people, the environment of the lung is characterized by chronic low‐grade inflammation, an aspect of a systemic inflammatory state associated with ageing often referred to as ‘inflamm‐ageing’ 1, 2, 3. Many studies have found higher baseline levels of proinflammatory mediators, such as C‐reactive protein, tumour necrosis factor (TNF)‐α, interleukin (IL)−1β and IL‐6 in elderly individuals, and elevated levels of these mediators correlate with disease‐associated mortality in this population 4, 5, 6, 7, 8, 9, 10. Moreover, the heightened basal levels of proinflammatory mediators present in older subjects probably contributes to decreased pulmonary function and blunted immune responses to respiratory tract infections 11, 12, 13. Seniors, defined as those greater than 65 years of age, are at higher risk for developing lung infections and, once acquired, have more complications, longer hospital stays 14 and increased mortality 15. While seniors have higher rates of co‐morbidities that may worsen clinical outcomes after infection, baseline immune dysfunction plays a central role in their susceptibility to respiratory infections and higher mortality rates 16.
It is known that advanced age affects multiple aspects of pulmonary immunity, including the structure and function of the lung itself, and both the innate and adaptive arms of the immune system 11. Immunosenescence is thus one of the major factors underlying the increased incidence and severity of respiratory tract infections in elderly people. In this review, we will discuss studies examining age‐related changes in the response to lung infections, with a particular focus on cellular dysfunction and altered signalling in the innate immune system. By understanding the effects of ageing on the cells of the innate immune system in the context of respiratory infections, we can gain insight into the common deficits in innate immunity that predispose elderly people to these illnesses.
Pulmonary infections in elderly people
Pneumonia is a primary cause of morbidity, mortality and socioeconomic cost leading to >50 000 deaths 17 and costing more than $7 billion in medical costs annually in the United States alone 18. The incidence of pneumonia has been increasing in elderly people in recent years 19; in 2014, more than 83% of pneumonia deaths in the United States occurred in seniors 17. The leading cause of community‐acquired pneumonia among elderly individuals is streptococcal respiratory infections 20. Fewer studies have examined the ageing immune system in the context of Gram‐negative pneumonias, yet these infections also contribute to the overall increase in pneumonia‐related deaths in this population 20. Furthermore, rates of hospitalization, requirements for intensive care and mortality rates from respiratory tract infections increase drastically as seniors continue to age 17. Nosocomial pneumonias are common after hospitalization 21, particularly in geriatric trauma patients 22, 23, with bacterial infections as the most frequent cause of ventilator‐associated 24 and hospital‐acquired pneumonias 25.
Individuals aged 65 years and older also have higher mortality rates due to viral infections in the lung, the two most prevalent being influenza virus and respiratory syncytial virus (RSV) infections 26. Infection with respiratory syncytial virus (RSV) is a major cause of morbidity and mortality in individuals over the age of 65, with rates just below that of influenza virus infection 26, 27, 28, 29, 30, 31, 32. RSV, like influenza virus, infects cells of the respiratory tract 33. However, less is known about the host immune response to RSV compared to influenza virus, due mainly to a lack of decent animal models that recapitulate the response to infection in humans 34. Studies examining the impact of advanced age on the immune response to RSV infection have demonstrated that viral titres are higher and the virus persists longer in aged compared to younger hosts. Interestingly, some studies have shown an early delay in viral replication in older hosts, which the authors hypothesize is due possibly to changes in the pulmonary epithelium due to ageing 35, 36, 37, 38, 39. Contributing to the enhanced mortality is that elderly individuals do not respond to influenza virus vaccinations as well as younger individuals, and a Food and Drug Administration (FDA)‐approved RSV vaccination does not currently exist 40, 41. Underlying poor vaccination responses and higher prevalence of and mortality due to infection in older individuals is the reduced responsive capacity of the immune system of this population 42. However, this discussion will focus upon the innate arm of the immune system in the lungs, although a diverse body of detailed literature exists on changes in adaptive immunity with ageing. Furthermore, it should be noted that while similar responses to vaccination and natural infection by bacterial and viral pathogens are useful to examine general age‐related dysfunction in leucocytes and pulmonary immunity, such disparate models are not directly comparable and may yield different conclusions based on the pathogen and type of immunological challenge.
Changes in the ageing lung environment
There are many alterations in the ageing lung environment that impact innate immune function and host defences against lung infections. For example, the mucociliary barrier is an important defence against pathogens in the upper respiratory tract and bronchioles of the lung, both providing a physical barrier as well as sweeping microbes and debris upwards out of the airways. It has been shown that elderly individuals exhibit reduced mucociliary clearance 43, 44, contributing to microbial invasion of the lower airways and alveoli. The chronic inflammation present in aged mice also causes an up‐regulation of two proteins implicit in the attachment and infiltration of bacteria in the lung in epithelial cells: polymeric immunoglobulin receptor and platelet‐activating factor receptor (PAFr) 45, 46. Shivshankar et al. demonstrated the importance of these findings for host survival, in that increased expression of bacterial adhesion ligands in the lungs, including PAFr, correlated with mortality after pulmonary infection. Furthermore, up‐regulation of such proteins and other markers of cellular senescence were identified in the lung tissue from both elderly humans and aged mice, demonstrating that immunosenescence probably plays an important role in the increased susceptibility to infection in ageing populations 47. Other factors in the immune environment of the lung are also susceptible to age‐related changes. Pulmonary levels of complement proteins and surfactant proteins, important anti‐microbial factors in the lung, have been found to increase with age 48. While antibodies from aged individuals opsonize bacteria adequately, research suggests that serum levels in seniors are insufficient to facilitate antibody‐mediated phagocytosis of microbes by innate immune cells 49, 50. As such, host defence in the ageing lung is impaired not only by leucocyte dysfunction, but also by other changes in innate immunity and the local tissue environment resulting from inflamm‐ageing.
There is recent evidence that the age‐related changes in resident gut and lung microbiota may also be involved in regulating overall immunity to respiratory infections. Microbiome studies have revealed an age‐related shift in the composition and diversity of the respiratory tract microbiome 51. Endogenous bacteria of the murine gut microbiome are protective against both Pseudomonas aeruginosa and Staphylococcus aureus pneumonia 52, 53, and alterations in the lung microbiome of aged mice may also play a role during the host response to these lung infections. For example, dysbiosis of the respiratory tract is observed in elderly pneumonia patients 54 as well as aged mice colonized with Streptococcus pneumoniae 55, 56, 57. Although these results hint that age‐related changes in microbiota correspond with alterations in immune function, there is currently no evidence directly connecting age‐related alterations in respiratory microbiome with innate immunity.
Age‐related defects in innate immune receptors
Infection by microbial pathogens activates multiple pathogen recognition receptors (PRRs) in both respiratory epithelial cells and haematopoietic innate immune cells, including Toll‐like receptors (TLRs), retinoic acid inducible gene (RIG)‐I‐like receptors (RLRs) and nuclear oligomerization domain‐like receptors (NLRs) 58, 59. Triggering of these receptors leads to the induction of cytokine and chemokine production and maturation of some cell types, such as dendritic cells (DCs) 60, 61, 62, 63, 64. Several studies have shown that ageing leads to reductions in TLR expression (both mRNA and protein), signalling and downstream cytokine production in some cell types and models 59, 65, 66. Unfortunately, there is a paucity of information regarding changes due to advanced age in PRR‐mediated signalling after influenza virus infection. However, a recent study of influenza virus infection in mice suggests that monocytes from aged animals have diminished anti‐viral interferon production but intact inflammasome responses 67. RSV is thought to be detected by various TLRs, RLRs and NLRs, but the exact role of each type of receptor in host immunity to this virus has not been studied extensively 33. Activation of these PRRs in lung epithelial cells and other innate immune cells initiates a signalling cascade that results in the secretion of important proinflammatory cytokines, such as IL‐1β and IL‐6 33, 68. In response to RSV infection, advanced age alters cytokine production such that there are decreased levels of type I and II interferons (IFNs) and TNF‐α, but elevated levels of IL‐1β and IL‐4 35, 37, 38, 69, 70, 71. As a result, older animals exhibit increased bronchopulmonary inflammation after RSV infection compared to younger animals. Infiltrating cells are comprised of granulocytes, with the large majority being neutrophils 36, 37, 71. Tissue damage caused by immune cells may contribute to the elevated rate of RSV‐induced mortality in elderly people.
Similarly, peripheral blood mononuclear cells isolated from elderly individuals exhibit reduced and delayed production of TNF‐α, IL‐6, IL‐1b, IFN‐α, IFNc, C‐C motif chemokine ligand (CCL)2 and CCL7 after stimulation with TLR‐4, TLR‐7/8 and RIG‐1 agonists, subsequently hindering the ability of stimulated cells to induce T cell proliferation in vitro 72. Hinojosa and colleagues demonstrated that the chronic, low‐grade inflammation in the lungs of aged mice up‐regulates regulators of immune signalling such as A20, a de‐ubiquitinase that inhibits TLR signalling and downstream nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB) activation, showing that not only is TLR signalling itself dampened by ageing, but negative feedback loops associated with TLR signalling are up‐regulated with advanced age 45, 73. Constitutive expression of such negative regulators impede the ability of epithelial and immune cells to sense and respond to microbes, decreasing the host's ability to mount an immune response to microbial challenge. Thus, age‐mediated deficiency in proper TLR (and probably other PRR) function by epithelial cells and leucocytes probably contributes to dysregulated inflammation and worsened outcomes in elderly people infected with respiratory infections.
Ageing and alveolar macrophages
Alveolar macrophages, the resident innate immune cells of the airways, stand as the first line of defence against microbes, including those that cause pneumonia, and play central roles in the initiation and resolution of inflammation (Fig. 1). The initial response of macrophages to microbes and other inflammatory stimuli is reduced in aged hosts, which has been attributed to inflamm‐ageing 45, 72, 74, 75, 76, 77. This reduction in pathogen detection is probably a result of changes in TLR signalling pathways and constitutively elevated negative feedback signalling due to chronic inflammation present in older individuals 73. Specifically, studies of macrophages isolated from aged mice show a diminished response to TLR‐1, TLR‐2 or TLR‐4 stimulation with peptidoglycans, zymosan or lipopolysaccharide (LPS), respectively. Specifically, these cells produced less TNF‐α and IL‐6 due to attenuated activation of proinflammatory signal transduction in the NF‐κB, p38 and c‐jun NH2 terminal kinase (JNK) pathways 45, 75, 76, 77, 78, 79. Furthermore, the same decreases in proinflammatory cytokine (TNF‐α, IL‐1β and IL‐6) production by macrophages from aged mice have been observed after pulmonary infection with S. pneumoniae, suggesting that the reduction in TLR signalling occurs in vivo during an active infection 45, 73, 77. These alterations in TLR signalling and associated downstream events are highlighted in Fig. 2. Furthermore, the ability of macrophages to activate CD4+ T cells is also probably impaired due to ageing, as macrophages from aged mice do not express the same levels of major histocompatibility (MHC) class II molecules (required for antigen presentation to CD4+ T cells) as macrophages from younger animals 80.
Figure 1.
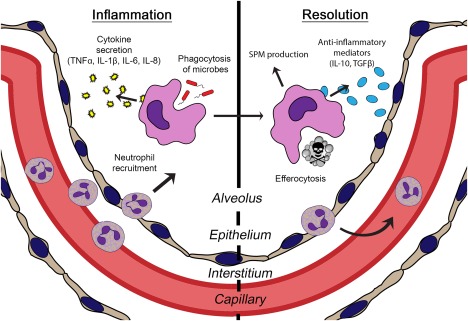
Innate immune functions of alveolar macrophages. As the resident innate immune cell of the pulmonary airspace, alveolar macrophages stand at the forefront of host defence against microbial invaders in the lung. Along with their role in effecting and propagating the inflammatory response by phagocytosing microbes and secreting proinflammatory mediators, alveolar macrophages also facilitate resolution by clearing away dead cells (efferocytosis) and producing anti‐inflammatory mediators.
Figure 2.
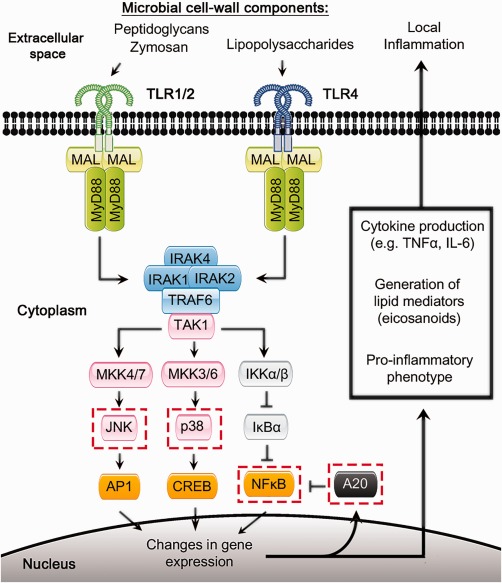
Dysregulated Toll‐like receptor signalling associated with advanced age. This figure depicts signalling pathways downstream of Toll‐like receptor signalling, some of which have been shown to be disrupted or altered with age. Dashed red boxes indicate specific pathway components known to be affected by ageing, as discussed in this review 29, 30, 31, 32, 33, 34.
In addition to initiating and sustaining the innate immune response to bacterial infection, macrophages promote resolution of inflammation caused by infection by removing extracellular debris and clearing apoptotic cells from the airways, a process known as efferocytosis. Advanced age disrupts these functions, reducing the ability of macrophages to remove apoptotic cells and resulting in prolonged inflammation after infection, even after the pathogen is cleared 81, 82. Monocytes and macrophages from aged individuals and mice also exhibit reduced phagocytic capacity 83, 84, 85, impairing their ability to remove microbes from the host in the inflammatory response to infection. Macrophages further play an essential role in controlling inflammation and restoring tissue homeostasis after infection by producing signalling molecules such as the anti‐inflammatory cytokines IL‐10 and transforming growth factor beta (TGF‐β), and pro‐resolving lipid mediators 86. In response to pneumococcal pneumonia, aged mice produce less IL‐10 but higher levels of chemokines chemokine (C‐X‐C motif) ligand (CXCL)9, CXCL12, chemokine (C‐C motif) ligand 3 (CCL3), CCL4, CCL5, CCL11 and CCL17, suggesting a defect in anti‐inflammatory cytokine production by immune cells in the lung 87. Similarly, others have found a decline in IL‐10‐producing macrophages with ageing in a murine model of spinal cord injury 88.
The effect of advanced age on the production of lipid mediators by alveolar macrophage has not been studied. However, evidence from infections at other sites suggests that this probably also occurs in the lung. For example, in a model of self‐resolving peritonitis, macrophages from aged mice produced more proinflammatory eicosanoids and less specialized pro‐resolving mediators (SPMs), contributing to delayed resolution of acute inflammation 82. Moreover, SPMs may serve as potential therapeutics in the context of prolonged inflammation due to respiratory infection, as nanoparticles loaded with leucocyte‐derived SPMs (resolvins D1 and D3) were able to correct age‐related decline in efferocytosis by macrophages. Although there is still much to be learned about the resolution of acute inflammation in the lung, these data hint at a significant impairment in the ability of macrophages from aged subjects to promote resolution, further adding to the inability of the aged immune system to properly clear pulmonary infections with excessive inflammation and tissue damage.
DCs in the ageing lung
DCs are another immune cell subset residing in the lungs that are affected by the detrimental effects of inflamm‐ageing (reviewed in 89). Macrophages and DCs both carry influenza virus antigen, and upon activation of PRRs they can traffic to the draining lymph node to present antigen and activate virus‐specific T cells 90. Similarly, upon detection of RSV by PRRs and in response to proinflammatory cytokines, DCs traffic to the lung draining lymph nodes, where they activate CD4+ and CD8+ T cells 68, 91. DCs from aged subjects have impaired phagocytosis and pinocytosis in vitro 92, and the migratory capacity of DCs is reduced in aged mice, decreasing the number available to stimulate T cells in the lymph node after influenza or RSV infection 93, 94. Ageing reduces the up‐regulation of co‐stimulatory molecules critical for T cell priming and diminishes cytokine production by alveolar macrophages and DCs after exposure to influenza virus 94, 95, 96. These age‐mediated alterations in macrophages and DCs are sufficient to cause a reduction in the ability of these antigen‐presenting cells (APCs) to activate CD8+ T cells 93, 95. There is mounting evidence that these defects in APC function, in combination with intrinsic changes in T cells, are responsible for the blunted adaptive immune responses that occur in elderly people 97, 98, 99. The reduction in DC and macrophage function due to ageing leads to poor viral clearance, ultimately causing increased mortality after influenza virus infection 93, 94, 100. While the exact underlying mechanism by which ageing alters DC function has not been determined, one study suggests that age‐mediated changes in histone modifications might contribute 96. Others have implicated age‐related mitochondrial dysfunction as resulting in impaired phagocytosis and antigen presentation by DCs 101. However, further work is necessary to elucidate the intrinsic and extrinsic factors that drive aberrant DC function in ageing, particularly in the context of bacterial infections.
Age‐related changes in neutrophil function
Neutrophils are another key effector cell in the innate immune response to pathogens, employing a wide range of microbicidal functions to clear pathogens from tissues in the early stages of lung infections. Neutrophils migrate into infected tissues soon after a pathogen is detected, and they work together with macrophages to contain and clear infections 102, 103. However, neutrophil functions decline with age in many different models, as summarized in Table 1. As such, these granulocytes are impaired in their ability to eliminate bacteria and other microbes. When considering the sometimes conflicting results of in‐vitro and in‐vivo studies on neutrophil function in elderly people, it is important to note that many of the studies cited in this review used different stimuli to examine neutrophil function, e.g. particles versus microbes for experiments on phagocytosis, with resulting discrepancies in results. For instance, neutrophils from aged individuals exhibit reduced ROS generation in response to S. aureus but not Escherichia coli 104. Such a disparity in the response between Gram‐positive and Gram‐negative organisms illustrates the different ways in which ageing affects neutrophil responses to distinct stimuli. Additionally, no reports to date have examined the impact of ageing on the response of neutrophils during viral infection. None the less, the impaired functions observed in neutrophils from aged subjects can be taken collectively as a consensus acknowledging general decline in cell‐based immunity, often with severe ramifications. One such example comes from research by Tseng et al., suggesting that impaired neutrophil extracellular trap (NET) formation permits the systemic dissemination of bacteria from the lungs of aged mice, demonstrating how such age‐related defects in neutrophil function may lead to dire outcomes 105.
Table 1.
Anti‐microbial neutrophil functions altered by ageing
| Function | Species | Reference |
|---|---|---|
| Phagocytosis | Human, mouse | 22, 104, 125, 126, 127, 128, 129 |
| Cytokine production | Human | 130 |
| ROS generation | Human, mouse, rat | 104, 126, 127, 128, 130, 131, 132, 133 |
| Chemotaxis | Human | 114, 126, 127, 131, 134 |
| NET formation | Human, mouse | 105, 135 |
| Degranulation | Human | 134 |
ROS = reactive oxygen species; NET = neutrophil extracellular trap.
Along with deficiencies in anti‐microbial functions, there is also evidence that neutrophil recruitment and in‐vivo chemotaxis are dysregulated in the lungs of aged mice and elderly patients. In some infection models neutrophil recruitment is impaired at early time‐points, while in others too many neutrophils accumulate at the site of infection and fail to disperse later. For example, in a murine model of pulmonary infection with Francisella tularensis, older mice exhibited delayed production of neutrophil‐attracting chemokines and diminished neutrophil infiltration in the early stages of infection 106. Conversely, elderly patients with S. pneumoniae respiratory tract infections and aged mice infected with P. aeruginosa had increased and prolonged neutrophil accumulation in the lung parenchyma relative to young controls 107, 108, 109.
The deregulated recruitment of neutrophils is not infection‐specific; studies examining the role of ageing in burn‐induced pulmonary inflammation showed dysfunctional neutrophil migration and chemotaxis in the lungs of aged mice due to altered chemokine signalling through CXCR2 110, 111. Additionally, other pulmonary inflammatory stimuli resulted in heightened neutrophil‐attracting chemokine levels and prolonged neutrophilia in aged mice 112, 113. Therefore, inflammatory signalling in the lung is altered markedly due to ageing, contributing to aberrant neutrophil trafficking observed after infection or other pulmonary insults. Few studies have examined the mechanism by which this occurs, but one study suggests that constitutive phosphoinositide‐3‐kinase (PI3K) signalling contributes to the abnormal chemotaxis by neutrophils from older subjects, finding that inhibition of PI3K γ or δ isoforms restored accuracy to neutrophil migration 114. Together, these studies show that ageing alters neutrophil recruitment, the direction of which is potentially pathogen‐ or insult‐dependent. Age‐related changes in normally tightly regulated neutrophil chemotaxis can result in delayed pathogen clearance 115, 116 and contribute to prolonged inflammation and pulmonary tissue damage 114. Thus, it is evident that neutrophil dysfunction plays an important role in the inability of older individuals to mount an effective response to bacterial pathogens, and to properly resolve neutrophil‐mediated pulmonary inflammation.
Ageing and natural killer (NK) cells in the lung
NK cells are responsible for killing infected or transformed cells and they secrete important cytokines for host defence, including IFN‐γ 68, 117. Advanced age leads to a reduction in the frequency of NK cells present in the lung after influenza virus infection, and cytokine production by NK cells is diminished in older animals 94, 118, 119. Both human and animal studies have shown that ageing also reduces the NK cell cytotoxicity in response to influenza virus or RSV 35, 71, 118, 120. Exactly how advanced age reduces the frequency and functional capacity of NK cells is not known, although the basal low level of inflammation in older individuals is thought to play a role 119. Furthermore, one report suggests that age‐related alterations in non‐haematopoietic cells drive the functional deficits in NK cells in aged mice 121. In summary, the functional capacity of NK cells is reduced by advanced age, due probably to changes in the pulmonary microenvironment of older individuals.
Outlook and future directions
While a commendable effort has been made to prevent viral and bacterial pneumonias, both community and hospital‐acquired infections continue to be a significant burden of morbidity, mortality and socioeconomic cost. Furthermore, few therapeutic strategies or treatments designed specifically for elderly patients with lung infections currently exist, despite the profound, age‐dependent changes in innate immune function discussed above. Primary data on elderly individuals' innate immune response during lung infections is still scarce, despite the growing need for more knowledge regarding the physiological changes due to ageing. Therefore, understanding how advanced age alters innate immune cell populations, including the changes in APCs that result in blunted adaptive immune responses and the enhanced pulmonary neutrophilia present in older individuals, is critical in determining why mortality rates due to respiratory infection are higher in elderly people. There have been advances in our understanding of the biology of ageing that give hope for improved care and treatment of elderly people, such as age‐specific vaccines and adjuvants 40, 122, 123, 124. As a greater proportion of the population become seniors, it is increasingly important to identify reversible causes of immunosenescence and inflamm‐ageing in the lungs in order to develop targeted therapies for this at‐risk and quickly growing patient population.
Disclosure
The authors have no competing interests to report.
Acknowledgements
This work was supported in part by NIH R01 AG018859 (EJK). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
References
- 1. Franceschi C, Bonafe M, Valensin S et al Inflamm‐aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci 2000; 908:244–54. [DOI] [PubMed] [Google Scholar]
- 2. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age‐associated diseases. J Gerontol A 2014; 69:S4–9. [DOI] [PubMed] [Google Scholar]
- 3. Franceschi C, Capri M, Monti D et al Inflammaging and anti‐inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 2007; 128:92–105. [DOI] [PubMed] [Google Scholar]
- 4. Adriaensen W, Mathei C, Vaes B, van Pottelbergh G, Wallemacq P, Degryse JM. Interleukin‐6 predicts short‐term global functional decline in the oldest old: results from the BELFRAIL study. Age 2014; 36:9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adriaensen W, Mathei C, van Pottelbergh G et al Significance of serum immune markers in identification of global functional impairment in the oldest old: cross‐sectional results from the BELFRAIL study. Age 2014; 36:457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arai Y, Martin‐Ruiz CM, Takayama M et al Inflammation, but not telomere length, predicts successful ageing at extreme old age: a longitudinal study of semi‐supercentenarians. EBioMedicine 2015; 2:1549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giovannini S, Onder G, Liperoti R et al Interleukin‐6, C‐reactive protein, and tumor necrosis factor‐alpha as predictors of mortality in frail, community‐living elderly individuals. J Am Geriatr Soc 2011; 59:1679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jylha M, Paavilainen P, Lehtimaki T et al Interleukin‐1 receptor antagonist, interleukin‐6, and C‐reactive protein as predictors of mortality in nonagenarians: the vitality 90+ study. J Gerontol A 2007; 62:1016–21. [DOI] [PubMed] [Google Scholar]
- 9. Kim HO, Kim HS, Youn JC, Shin EC, Park S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead‐based immunoassays. J Transl Med 2011; 9:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Puzianowska‐Kuznicka M, Owczarz M, Wieczorowska‐Tobis K et al Interleukin‐6 and C‐reactive protein, successful aging, and mortality: the PolSenior study. Immun Ageing 2016; 13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lowery EM, Brubaker AL, Kuhlmann E, Kovacs EJ. The aging lung. Clini Interv Aging 2013; 8:1489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boyd AR, Orihuela CJ. Dysregulated inflammation as a risk factor for pneumonia in the elderly. Aging Dis 2011; 2:487–500. [PMC free article] [PubMed] [Google Scholar]
- 13. United Nations . World Population Ageing Report 2015. New York: United Nations; 2015. [Google Scholar]
- 14. Park H, Adeyemi AO, Rascati KL. Direct medical costs and utilization of health care services to treat pneumonia in the United States: an analysis of the 2007‐2011 Medical Expenditure Panel Survey. Clin Ther 2015; 37:1466–76.e1. [DOI] [PubMed] [Google Scholar]
- 15. Fedullo AJ, Swinburne AJ. Relationship of patient age to clinical features and outcome for in‐hospital treatment of pneumonia. J Gerontol 1985; 40:29–33. [DOI] [PubMed] [Google Scholar]
- 16. Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukoc Biol 2004; 76:291–9. [DOI] [PubMed] [Google Scholar]
- 17. Kochanek KM, Murphy SL, Xu J, Tejada‐Vera B. National Vital Statistics Reports. US Department of Health and Human Services, Statistics NCfH; 30 June 2016. Report no.: Contract no. 4. Hyattsville, MD: National Center for Health Statistics; 2016.
- 18. Thomas CP, Ryan M, Chapman JD et al Incidence and cost of pneumonia in Medicare beneficiaries. Chest 2012; 142:973–81. [DOI] [PubMed] [Google Scholar]
- 19. Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988–2002. JAMA 2005; 294:2712–9. [DOI] [PubMed] [Google Scholar]
- 20. Stupka JE, Mortensen EM, Anzueto A, Restrepo MI. Community‐acquired pneumonia in elderly patients. Aging Health 2009; 5:763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saviteer SM, Samsa GP, Rutala WA. Nosocomial infections in the elderly. Increased risk per hospital day. Am J Med 1988; 84:661–6. [DOI] [PubMed] [Google Scholar]
- 22. Butcher SK, Killampalli V, Chahal H, Kaya Alpar E, Lord JM. Effect of age on susceptibility to post‐traumatic infection in the elderly. Biochem Soc Trans 2003; 31:449–51. [DOI] [PubMed] [Google Scholar]
- 23. Vanzant EL, Hilton RE, Lopez CM et al Advanced age is associated with worsened outcomes and a unique genomic response in severely injured patients with hemorrhagic shock. Crit Care (London, UK) 2015; 19:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kollef MH, Morrow LE, Niederman MS et al Clinical characteristics and treatment patterns among patients with ventilator‐associated pneumonia. Chest 2006; 129:1210–8. [DOI] [PubMed] [Google Scholar]
- 25. Weber DJ, Rutala WA, Sickbert‐Bennett EE, Samsa GP, Brown V, Niederman MS. Microbiology of ventilator‐associated pneumonia compared with that of hospital‐acquired pneumonia. Infect Control Hosp Epidemiol 2007; 28:825–31. [DOI] [PubMed] [Google Scholar]
- 26. Thompson WW, Shay DK, Weintraub E et al Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–86. [DOI] [PubMed] [Google Scholar]
- 27. Nicholson KG, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ 1997; 315:1060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Falsey AR. Respiratory syncytial virus infection in elderly and high‐risk adults. Exp Lung Res 2005; 31:77. [PubMed] [Google Scholar]
- 29. Falsey AR, Walsh EE. Respiratory syncytial virus infection in elderly adults. Drugs Aging 2005; 22:577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high‐risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 31. Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis 2004; 189:233–8. [DOI] [PubMed] [Google Scholar]
- 32. Branche AR, Falsey AR. Respiratory syncytial virus infection in older adults: an under‐recognized problem. Drugs Aging 2015; 32:261–9. [DOI] [PubMed] [Google Scholar]
- 33. Kim TH, Lee HK. Innate immune recognition of respiratory syncytial virus infection. BMB Rep 2014; 47:184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sacco RE, Durbin RK, Durbin JE. Animal models of respiratory syncytial virus infection and disease. Curr Opin Virol 2015; 13:117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Y, Wang Y, Gilmore X, Xu K, Wyde PR, Mbawuike IN. An aged mouse model for RSV infection and diminished CD8(+) CTL responses. Exp Biol Med 2002; 227:133–40. [DOI] [PubMed] [Google Scholar]
- 36. Curtis SJ, Ottolini MG, Porter DD, Prince GA. Age‐dependent replication of respiratory syncytial virus in the cotton rat. Exp Biol Med 2002; 227:799–802. [DOI] [PubMed] [Google Scholar]
- 37. Wong TM, Boyapalle S, Sampayo V et al Respiratory syncytial virus (RSV) infection in elderly mice results in altered antiviral gene expression and enhanced pathology. PLOS ONE 2014; 9:e88764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boukhvalova MS, Yim KC, Kuhn KH et al Age‐related differences in pulmonary cytokine response to respiratory syncytial virus infection: modulation by anti‐inflammatory and antiviral treatment. J Infect Dis 2007; 195:511–8. [DOI] [PubMed] [Google Scholar]
- 39. Guichelaar T, Hoeboer J, Widjojoatmodjo MN et al Impaired immune response to vaccination against infection with human respiratory syncytial virus at advanced age. J Virol 2014; 88:9744–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oviedo‐Orta E, Li CK, Rappuoli R. Perspectives on vaccine development for the elderly. Curr Opin Immunol 2013; 25:529–34. [DOI] [PubMed] [Google Scholar]
- 41. Fink AL, Klein SL. Sex and gender impact immune responses to vaccines among the elderly. Physiology 2015; 30:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology 2016; 17:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ho JC, Chan KN, Hu WH et al The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med 2001; 163:983–8. [DOI] [PubMed] [Google Scholar]
- 44. Svartengren M, Falk R, Philipson K. Long‐term clearance from small airways decreases with age. Eur Respir J 2005; 26:609–15. [DOI] [PubMed] [Google Scholar]
- 45. Hinojosa E, Boyd AR, Orihuela CJ. Age‐associated inflammation and toll‐like receptor dysfunction prime the lungs for pneumococcal pneumonia. J Infect Dis 2009; 200:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hinojosa CA, Mgbemena V, Van Roekel S et al Enteric‐delivered rapamycin enhances resistance of aged mice to pneumococcal pneumonia through reduced cellular senescence. Exp Gerontol 2012; 47:958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shivshankar P, Boyd AR, Le Saux CJ, Yeh IT, Orihuela CJ. Cellular senescence increases expression of bacterial ligands in the lungs and is positively correlated with increased susceptibility to pneumococcal pneumonia. Aging Cell 2011; 10:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moliva JI, Rajaram MV, Sidiki S et al Molecular composition of the alveolar lining fluid in the aging lung. Age 2014; 36:9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Simell B, Vuorela A, Ekstrom N et al Aging reduces the functionality of anti‐pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine 2011; 29:1929–34. [DOI] [PubMed] [Google Scholar]
- 50. Park S, Nahm MH. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infect Immun 2011; 79:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stearns JC, Davidson CJ, McKeon S et al Culture and molecular‐based profiles show shifts in bacterial communities of the upper respiratory tract that occur with age. ISME J 2015; 9:1246–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fox AC, McConnell KW, Yoseph BP et al The endogenous bacteria alter gut epithelial apoptosis and decrease mortality following Pseudomonas aeruginosa pneumonia. Shock 2012; 38:508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gauguet S, D'Ortona S, Ahnger‐Pier K et al Intestinal microbiota of mice influences resistance to Staphylococcus aureus pneumonia. Infect Immun 2015; 83:4003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de Steenhuijsen Piters WA, Huijskens EG, Wyllie AL et al Dysbiosis of upper respiratory tract microbiota in elderly pneumonia patients. ISME J 2016; 10:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Krone CL, Biesbroek G, Trzcinski K, Sanders EA, Bogaert D. Respiratory microbiota dynamics following Streptococcus pneumoniae acquisition in young and elderly mice. Infect Immun 2014; 82:1725–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Krone CL, Trzcinski K, Zborowski T, Sanders EA, Bogaert D. Impaired innate mucosal immunity in aged mice permits prolonged Streptococcus pneumoniae colonization. Infect Immun 2013; 81:4615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thevaranjan N, Whelan FJ, Puchta A et al Streptococcus pneumoniae colonization disrupts the microbial community within the upper respiratory tract of aging mice. Infect Immun 2016; 84:906–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tripathi S, White MR, Hartshorn KL. The amazing innate immune response to influenza A virus infection. Innate Immun 2015; 21:73–98. [DOI] [PubMed] [Google Scholar]
- 59. Shaw AC, Panda A, Joshi SR, Qian F, Allore HG, Montgomery RR. Dysregulation of human toll‐like receptor function in aging. Ageing Res Rev 2011; 10:346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. De Nardo D. Toll‐like receptors: activation, signalling and transcriptional modulation. Cytokine 2015; 74:181–9. [DOI] [PubMed] [Google Scholar]
- 61. Kaparakis M, Philpott DJ, Ferrero RL. Mammalian NLR proteins; discriminating foe from friend. Immunol Cell Biol 2007; 85:495–502. [DOI] [PubMed] [Google Scholar]
- 62. Mikkelsen SS, Jensen SB, Chiliveru S et al RIG‐I‐mediated activation of p38 MAPK is essential for viral induction of interferon and activation of dendritic cells: dependence on TRAF2 and TAK1. J Biol Chem 2009; 284:10774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pichlmair A, Schulz O, Tan CP et al RIG‐I‐mediated antiviral responses to single‐stranded RNA bearing 5'‐phosphates. Science 2006; 314:997–1001. [DOI] [PubMed] [Google Scholar]
- 64. Pothlichet J, Meunier I, Davis BK et al Type I IFN triggers RIG‐I/TLR3/NLRP3‐dependent inflammasome activation in influenza A virus infected cells. PLOS Pathog 2013; 9:e1003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dunston CR, Griffiths HR. The effect of ageing on macrophage toll‐like receptor‐mediated responses in the fight against pathogens. Clin Exp Immunol 2010; 161:407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Volkova M, Zhang Y, Shaw AC, Lee PJ. The role of toll‐like receptors in age‐associated lung diseases. J Gerontol A 2012; 67:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pillai PS, Molony RD, Martinod K et al Mx1 reveals innate pathways to antiviral resistance and lethal influenza disease. Science (New York) 2016; 352:463–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Arruvito L, Raiden S, Geffner J. Host response to respiratory syncytial virus infection. Curr Opin Infect Dis 2015; 28:259–66. [DOI] [PubMed] [Google Scholar]
- 69. Ditt V, Lusebrink J, Tillmann RL, Schildgen V, Schildgen O. Respiratory infections by HMPV and RSV are clinically indistinguishable but induce different host response in aged individuals. PLOS ONE 2011; 6:e16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Looney RJ, Falsey AR, Walsh E, Campbell D. Effect of aging on cytokine production in response to respiratory syncytial virus infection. J Infect Dis 2002; 185:682–5. [DOI] [PubMed] [Google Scholar]
- 71. Liu B, Kimura Y. Local immune response to respiratory syncytial virus infection is diminished in senescence‐accelerated mice. J Gen Virol 2007; 88:2552–8. [DOI] [PubMed] [Google Scholar]
- 72. Metcalf TU, Cubas RA, Ghneim K et al Global analyses revealed age‐related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell 2015; 14:421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hinojosa CA, Akula Suresh Babu R, Rahman MM, Fernandes G, Boyd AR, Orihuela CJ. Elevated A20 contributes to age‐dependent macrophage dysfunction in the lungs. Exp Gerontol 2014; 54:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Albright JM, Dunn RC, Shults JA, Boe DM, Afshar M, Kovacs EJ. Advanced age alters monocyte and macrophage responses. Antioxid Redox Signal 2016; in press. doi: 10.1089/ars.2016.6691 [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age‐dependent decrease in Toll‐like receptor 4‐mediated proinflammatory cytokine production and mitogen‐activated protein kinase expression. J Leukoc Biol 2004; 75:342–9. [DOI] [PubMed] [Google Scholar]
- 76. Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage TLR2‐ and TLR4‐mediated pro‐inflammatory responses without affecting the IL‐2‐stimulated pathway. Mech Ageing Dev 2005; 126:1305–13. [DOI] [PubMed] [Google Scholar]
- 77. Boyd AR, Shivshankar P, Jiang S, Berton MT, Orihuela CJ. Age‐related defects in TLR2 signaling diminish the cytokine response by alveolar macrophages during murine pneumococcal pneumonia. Exp Gerontol 2012; 47:507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sun Y, Li H, Yang MF, Shu W, Sun MJ, Xu Y. Effects of aging on endotoxin tolerance induced by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli . PLOS ONE 2012; 7:e39224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. van Duin D, Mohanty S, Thomas V et al Age‐associated defect in human TLR‐1/2 function. J Immunol 2007; 178:970–5. [DOI] [PubMed] [Google Scholar]
- 80. Herrero C, Marques L, Lloberas J, Celada A. IFN‐gamma‐dependent transcription of MHC class II IA is impaired in macrophages from aged mice. J Clin Invest 2001; 107:485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Aprahamian T, Takemura Y, Goukassian D, Walsh K. Ageing is associated with diminished apoptotic cell clearance in vivo . Clin Exp Immunol 2008; 152:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Arnardottir HH, Dalli J, Colas RA, Shinohara M, Serhan CN. Aging delays resolution of acute inflammation in mice: reprogramming the host response with novel nano‐proresolving medicines. J Immunol 2014; 193:4235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hearps AC, Martin GE, Angelovich TA et al Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 2012; 11:867–75. [DOI] [PubMed] [Google Scholar]
- 84. De La Fuente M. Changes in the macrophage function with aging. Comp Biochem Physiol A 1985; 81:935–8. [DOI] [PubMed] [Google Scholar]
- 85. Linehan E, Dombrowski Y, Snoddy R, Fallon PG, Kissenpfennig A, Fitzgerald DC. Aging impairs peritoneal but not bone marrow‐derived macrophage phagocytosis. Aging Cell 2014; 13:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014; 40:315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Williams AE, Jose RJ, Brown JS, Chambers RC. Enhanced inflammation in aged mice following infection with Streptococcus pneumoniae is associated with decreased IL‐10 and augmented chemokine production. Am J Physiol Lung Cell Mol Physiol 2015; 308:L539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhang B, Bailey WM, Braun KJ, Gensel JC. Age decreases macrophage IL‐10 expression: implications for functional recovery and tissue repair in spinal cord injury. Exp Neurol 2015; 273:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Agrawal A, Gupta S. Impact of aging on dendritic cell functions in humans. Ageing Res Rev 2011; 10:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kohlmeier JE, Woodland DL. Immunity to respiratory viruses. Annu Rev Immunol 2009; 27:61–82. [DOI] [PubMed] [Google Scholar]
- 91. Garg R, Shrivastava P, van Drunen Littel‐van den Hurk S. The role of dendritic cells in innate and adaptive immunity to respiratory syncytial virus, and implications for vaccine development. Expert Rev Vaccines 2012; 11:1441–57. [DOI] [PubMed] [Google Scholar]
- 92. Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3‐kinase‐signaling pathway. J Immunol 2007; 178:6912–22. [DOI] [PubMed] [Google Scholar]
- 93. Zhao J, Zhao J, Legge K, Perlman S. Age‐related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest 2011; 121:4921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res 2009; 10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Plowden J, Renshaw‐Hoelscher M, Gangappa S, Engleman C, Katz JM, Sambhara S. Impaired antigen‐induced CD8+ T cell clonal expansion in aging is due to defects in antigen presenting cell function. Cell Immunol 2004; 229:86–92. [DOI] [PubMed] [Google Scholar]
- 96. Prakash S, Agrawal S, Cao JN, Gupta S, Agrawal A. Impaired secretion of interferons by dendritic cells from aged subjects to influenza: role of histone modifications. Age 2013; 35:1785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Linton PJ, Li SP, Zhang Y, Bautista B, Huynh Q, Trinh T. Intrinsic versus environmental influences on T‐cell responses in aging. Immunol Rev 2005; 205:207–19. [DOI] [PubMed] [Google Scholar]
- 98. Ely KH, Roberts AD, Kohlmeier JE, Blackman MA, Woodland DL. Aging and CD8+ T cell immunity to respiratory virus infections. Exp Gerontol 2007; 42:427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang W, Brahmakshatriya V, Swain SL. CD4 T cell defects in the aged: causes, consequences and strategies to circumvent. Exp Gerontol 2014; 54:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Josset L, Engelmann F, Haberthur K et al Increased viral loads and exacerbated innate host responses in aged macaques infected with the 2009 pandemic H1N1 influenza A virus. J Virol 2012; 86:11115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chougnet CA, Thacker RI, Shehata HM et al Loss of phagocytic and antigen cross‐presenting capacity in aging dendritic cells is associated with mitochondrial dysfunction. J Immunol 2015; 195:2624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Silva MT. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J Leukoc Biol 2010; 87:93–106. [DOI] [PubMed] [Google Scholar]
- 103. Silva MT, Correia‐Neves M. Neutrophils and macrophages: the main partners of phagocyte cell systems. Front Immunol 2012; 3:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wenisch C, Patruta S, Daxbock F, Krause R, Horl W. Effect of age on human neutrophil function. J Leukoc Biol 2000; 67:40–5. [DOI] [PubMed] [Google Scholar]
- 105. Tseng CW, Kyme PA, Arruda A, Ramanujan VK, Tawackoli W, Liu GY. Innate immune dysfunctions in aged mice facilitate the systemic dissemination of methicillin‐resistant S. aureus . PLOS ONE 2012; 7:e41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mares CA, Sharma J, Ojeda SS et al Attenuated response of aged mice to respiratory Francisella novicida is characterized by reduced cell death and absence of subsequent hypercytokinemia. PLOS ONE 2010; 5:e14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Menter T, Giefing‐Kroell C, Grubeck‐Loebenstein B, Tzankov A. Characterization of the inflammatory infiltrate in Streptococcus pneumoniae pneumonia in young and elderly patients. Pathobiology 2014; 81:160–7. [DOI] [PubMed] [Google Scholar]
- 108. Chen MM, Palmer JL, Plackett TP, Deburghgraeve CR, Kovacs EJ. Age‐related differences in the neutrophil response to pulmonary pseudomonas infection. Exp Gerontol 2014; 54:42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bruunsgaard H, Skinhoj P, Qvist J, Pedersen BK. Elderly humans show prolonged in vivo inflammatory activity during pneumococcal infections. J Infect Dis 1999; 180:551–4. [DOI] [PubMed] [Google Scholar]
- 110. Nomellini V, Brubaker AL, Mahbub S, Palmer JL, Gomez CR, Kovacs EJ. Dysregulation of neutrophil CXCR2 and pulmonary endothelial ICAM‐1 promotes age‐related pulmonary inflammation. Aging Dis 2012; 3:234–47. [PMC free article] [PubMed] [Google Scholar]
- 111. Nomellini V, Faunce DE, Gomez CR, Kovacs EJ. An age‐associated increase in pulmonary inflammation after burn injury is abrogated by CXCR2 inhibition. J Leukoc Biol 2008; 83:1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ito Y, Betsuyaku T, Nasuhara Y, Nishimura M. Lipopolysaccharide‐induced neutrophilic inflammation in the lungs differs with age. Exp Lung Res 2007; 33:375–84. [DOI] [PubMed] [Google Scholar]
- 113. Moriyama C, Betsuyaku T, Ito Y et al Aging enhances susceptibility to cigarette smoke‐induced inflammation through bronchiolar chemokines. Am J Respir Cell Mol Biol 2010; 42:304–11. [DOI] [PubMed] [Google Scholar]
- 114. Sapey E, Greenwood H, Walton G et al Phosphoinositide 3‐kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood 2014; 123:239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Brubaker AL, Kovacs EJ. G‐CSF enhances resolution of Staphylococcus aureus wound infection in an age‐dependent manner. Shock 2013; 40:327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Brubaker AL, Rendon JL, Ramirez L, Choudhry MA, Kovacs EJ. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. J Immunol 2013; 190:1746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Schultz‐Cherry S. Role of NK cells in influenza infection. Curr Top Microbiol Immunol 2015; 386:109–20. [DOI] [PubMed] [Google Scholar]
- 118. Nogusa S, Ritz BW, Kassim SH, Jennings SR, Gardner EM. Characterization of age‐related changes in natural killer cells during primary influenza infection in mice. Mech Ageing Dev 2008; 129:223–30. [DOI] [PubMed] [Google Scholar]
- 119. Beli E, Clinthorne JF, Duriancik DM, Hwang I, Kim S, Gardner EM. Natural killer cell function is altered during the primary response of aged mice to influenza infection. Mech Ageing Dev 2011; 132:503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kutza J, Gross P, Kaye D, Murasko DM. Natural killer cell cytotoxicity in elderly humans after influenza immunization. Clin Diagn Lab Immunol 1996; 3:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Shehata HM, Hoebe K, Chougnet CA. The aged nonhematopoietic environment impairs natural killer cell maturation and function. Aging Cell 2015; 14:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Del Giudice G, Weinberger B, Grubeck‐Loebenstein B. Vaccines for the elderly. Gerontology 2015; 61:203–10. [DOI] [PubMed] [Google Scholar]
- 123. Boraschi D, Italiani P. Immunosenescence and vaccine failure in the elderly: strategies for improving response. Immunol Lett 2014; 162:346–53. [DOI] [PubMed] [Google Scholar]
- 124. Lefebvre JS, Haynes L. Vaccine strategies to enhance immune responses in the aged. Curr Opin Immunol 2013; 25:523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Butcher SK, Chahal H, Nayak L et al Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol 2001; 70:881–6. [PubMed] [Google Scholar]
- 126. Polignano A, Tortorella C, Venezia A, Jirillo E, Antonaci S. Age‐associated changes of neutrophil responsiveness in a human healthy elderly population. Cytobios 1994; 80:145–53. [PubMed] [Google Scholar]
- 127. Antonaci S, Jirillo E, Ventura MT, Garofalo AR, Bonomo L. Non‐specific immunity in aging: deficiency of monocyte and polymorphonuclear cell‐mediated functions. Mech Ageing Dev 1984; 24:367–75. [DOI] [PubMed] [Google Scholar]
- 128. Fulop T Jr, Foris G, Worum I, Leovey A. Age‐dependent alterations of Fc gamma receptor‐mediated effector functions of human polymorphonuclear leucocytes. Clin Exp Immunol 1985; 61:425–32. [PMC free article] [PubMed] [Google Scholar]
- 129. Amaya RA, Baker CJ, Keitel WA, Edwards MS. Healthy elderly people lack neutrophil‐mediated functional activity to type V group B Streptococcus. J Am Geriatr Soc 2004; 52:46–50. [DOI] [PubMed] [Google Scholar]
- 130. Dalboni TM, Abe AE, de Oliveira CE et al Activation profile of CXCL8‐stimulated neutrophils and aging. Cytokine 2013; 61:716–9. [DOI] [PubMed] [Google Scholar]
- 131. Di Lorenzo G, Balistreri CR, Candore G et al Granulocyte and natural killer activity in the elderly. Mech Ageing Dev 1999; 108:25–38. [DOI] [PubMed] [Google Scholar]
- 132. Tortorella C, Ottolenghi A, Pugliese P, Jirillo E, Antonaci S. Relationship between respiratory burst and adhesiveness capacity in elderly polymorphonuclear cells. Mech Ageing Dev 1993; 69:53–63. [DOI] [PubMed] [Google Scholar]
- 133. Fu YK, Arkins S, Li YM, Dantzer R, Kelley KW. Reduction in superoxide anion secretion and bactericidal activity of neutrophils from aged rats: reversal by the combination of gamma interferon and growth hormone. Infect Immun 1994; 62:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. McLaughlin B, O'Malley K, Cotter TG. Age‐related differences in granulocyte chemotaxis and degranulation. Clin Sci 1986; 70:59–62. [DOI] [PubMed] [Google Scholar]
- 135. Hazeldine J, Harris P, Chapple IL et al Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell 2014; 13:690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


