Abstract
DNA barcoding has been used extensively to solve taxonomic questions and identify new species. Neotropical fishes are found in a wide variety of shapes and sizes, with a large number of species yet to be described, many of which are very difficult to identify. Characidae is the most species-rich family of the Characiformes, and many of its genera are affected by taxonomic uncertainties, including the widely-distributed, species-rich genus Astyanax. In this study, we present an extensive analysis of Astyanax covering almost its entire area of occurrence, based on DNA barcoding. The use of different approaches (ABGD, GMYC and BIN) to the clustering of the sequences revealed ample consistency in the results obtained by the initial cutoff value of 2% divergence for putative species in the Neighbor-Joining analysis using the Kimura-2-parameter model. The results indicate the existence of five Astyanax lineages. Some groups, such as that composed by the trans-Andean forms, are mostly composed of well-defined species, and in others a number of nominal species are clustered together, hampering the delimitation of species, which in many cases proved impossible. The results confirm the extreme complexity of the systematics of the genus Astyanax and show that DNA barcoding can be an useful tool to address these complexes questions.
Introduction
Enormously diverse, Neotropical freshwater fish can be found from South America to southern North America, with more than 7000 recognized species [1] representing 71 families [2] mainly arranged in two major groups, the Characiformes and the Siluriformes [3]. The characiforms are the most diverse group, with a huge variety of body shapes and sizes, found in the lakes and rivers of the Neotropical region, as well as Africa [4]. The most diverse family of this order is the Characidae, which recently reached a total of more than 1100 recognized species [3].
Given its species diversity, the Characidae is also the Neotropical fish family with the most taxonomic problems [5]. The relationships within the family are largely uncertain and many genera are not monophyletic, including Astyanax, which is the one of the most species-rich characid genus [5, 6]. Eigenmann [7, 8] presented the first substantial review of the genus, recognizing 74 species and subspecies, many of which have now been re-assigned to other genera, such as Jupiaba Zanata, 1997. The second major review of the twentieth century, Géry [4], differed little from Eigenmann’s original proposal. By 2003, the genus had 86 valid species [9], although this number has now increased to 147, of which, approximately 20% were described in the past 10 years [3].
Many Astyanax species are currently identified at genus level [10]. A number of authors have also proposed the existence of species complexes, such as those of Astyanax bimaculatus Linnaeus, 1758 (the A. bimaculatus species complex) [11, 12], Astyanax fasciatus Cuvier, 1819 (the A. fasciatus species complex) [13] and Astyanax scabripinnis Jennys, 1842 (the A. scabripinnis species complex) [14]. These groups, together with the large number of other Astyanax species, reflect the highly complex nature of the identification of Neotropical characiform species, due to the phenotypic plasticity of the morphological characters traditionally used for species determination, resulting in many identification errors.
One potential solution for these taxonomic problems is the use of molecular species identification. In this context, DNA barcoding has been extensively and successfully used for fish species identification and the resolution of many taxonomic problems. The first study of marine fishes, for example, obtained a 100% success rate in species identification with no overlap found [15], and a recent study also identified correctly almost all the species in a study of the coastal fish fauna in India [16]. Success rates have been lower for freshwater groups, however, with a correct identification rate of 93% being recorded in Mexico and Guatemala [17] and Canada [18], although another study did obtain a success rate of 99.2% in the upper Paraná Basin in Brazil [19].
More than a decade after the molecular identification system was first proposed [20], a variety of different cutoff values for species based on genetic distances have been tested. While some use a cutoff of 1%, for example [21], values of 2% and 3% are most common in studies of Neotropical fish [19, 22–26]. Other criteria include one order of magnitude (10 x) greater than the mean intraspecific divergence [27] and the barcoding gap [28]. More recently, new methods have been proposed for the automatic species identification, such as the Automatic Barcode Gap Discovery (ABGD), the Barcode Index Number (BIN) and the Generalized Mixed Yule Coalescent model (GMYC). The ABGD is an automatic identification procedure that forms clusters of sequences of possible species, based on the distances and differences between intra- and interspecific levels of variation, detecting boundaries even when the distribution overlaps [29, 30]. The BIN is also based on distance methods, clustering sequences with a 2.2% threshold, followed by a Markov analysis [31]. Other approaches are not based only on distance as the criterion for species discrimination [32–34]. The GMYC analysis uses an ultrametric tree to establish species limits, based on a mixture of the Yule (pure-birth) [35] and Kingman models (coalescence) [36], where the algorithm computes the probability of splits in a lineage based on speciation rates, thus identifying a cutoff value which enables the identification of the point at which species or populations split [37].
Given the considerable difficulties for the identification of Astyanax species based on morphological traits, and the potential existence of species complexes, the present study investigated the genetic diversity of the genus based on a DNA barcoding approach. With this, we hope to expand our knowledge of one of the most species diverse Neotropical fish genera.
Material and Methods
Ethical statement
We declare that the fish under study are not protected under wildlife conservation, and no experimentation was conducted on live specimens. All specimens used were collected in accordance local laws, and in Brazil the sampling was approved by the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA) and Sistema de Autorização e Informação em Biodiversidade (SISBIO) under a license issued in the name of Dr. Claudio Oliveira (SISBIO number 13843–1). After collection, the animals were anesthetized and sacrificed using 1% benzocaine in water as approved by the Bioscience Institute/UNESP Ethics Committee on the Use of Animals (CEUA; protocol 405) and recommended by the National Council for the Control of Animal Experimentation and the Federal Board of Veterinary Medicine.
Specimen collection
Astyanax specimens, a total of 1309 fishes, were collected in a number of different river basins in Argentina, Brazil, Colombia, Guyana, Peru and Venezuela (S1 Table). Additionally, 366 samples were obtained from GenBank. For Astyanax sp. we followed the previously existing identification system from Ornelas-Garcia et al. (2008; Astyanax sp. 1 to 9 from Mesoamerica). All the other Astyanax sp. are named in sequential order from this study. Tissue samples from Argentina were provided by the fish collection of the Coastal and Marine Research Institute (IIMyC) at Universidad Nacional de Mar del Plata in Mar del Plata, Argentina. The tissue samples used for the molecular analyses were preserved in absolute ethanol and stored at -20°C. The voucher specimens were fixed in a 10% formalin solution and are preserved in 70% ethanol. The morphological vouchers were deposited in the fish collection of the Fish Biology and Genetics Laboratory (LBP) at Paulista State University (Universidade Estadual Paulista) in Botucatu, Brazil or Universidad Nacional de Mar del Plata, Argentina. Species identification was based on morphological traits as meristic and morphometric data, color pattern and teeth morphology which are arranged or presented on dichotomic keys, original descriptions, redescriptions and taxonomic reviews [4, 7, 11–13, 38–64]. Consensus sequences were deposited in the BOLD database in the dataset named "BAST- Barcoding Astyanax".
DNA extraction, PCR amplification and sequencing
Total DNA was extracted from muscle fragments following the protocol of the Canadian Center for DNA-Barcoding (CCDB), available at http://www.ccdb.ca. A segment of the 5' region of the mitochondrial COI gene was amplified using different combinations of primers, including L5698-Asn [65], FishF1, FishF2, FishR1 and FishR2 [15], C_FishF1t1–C_FishR1t1 cocktail [66], and H7271-COI [67]. Polymerase chain reactions (PCR) were run in a 12.5 μl volume containing: 1 μl DNA (concentration 50 ng/μl), 0.25 μl each of the forward and reverse primers (concentration 10 mM), 1.25 μl of reaction buffer, 0.2 μl of 200 mM dNTPs mix, 0.37 μl of MgCl2 and 0.0625 μl (5 units/μl) of Platinum Taq DNA polymerase (Invitrogen).
The samples were amplified in a Veriti® 96-well thermocycler (Applied Biosystems), with initial denaturation of 5 minutes at 96°C followed by 35 cycles at 96°C for 45 seconds, 54°C for 45 seconds, 72°C for 1 minute, and final extension at 72°C for 1 minute. The amplified PCR products were cleaned up with ExoSAP-IT (USB Corporation) and sequenced in both directions using the BigDye Terminator v3.1 Cycle Sequencing kit (Life Technologies) in an ABI3130 Genetic Analyzer automated sequencer (Applied Biosystems).
Data analysis
The sequences were edited in BioEdit 7.0.9.0 [68] and aligned in MUSCLE (Multiple Sequence Comparison by Log-Expectation) [69]. The first analysis were conduct based on genetic distances calculated in MEGA 5 [70], using the Kimura-2-parameter (K2P) substitution model [71] to estimate the Neighbor-Joining (NJ) tree [72], based on a 2% cutoff value. This cutoff was used to define the initial clusters and then we tested all the NJ clusters defined by the 2% cutoff criterion using alternative clustering methods as proposed by other studies (see section Identification of Operational Taxonomic Units—OTUs). The first analyses was run in the ABGD program via a command line based using the K2P model. To maximize the potential species discovery the parameters were modified (relative value gap X = 0.1, Pmin = 0.005 and Pmax = 0.1) [31]. The BIN approach focused only on the BAST dataset in the BOLD database and the sequences from Argentina, and necessarily excluded the records from GenBank. Finally, for the GMYC analysis, ultrametric trees were generated in Beast v1.8.0 [73] using the Yule speciation and the GTR+G+I nucleotide substitution models (selected by MEGA 5 under BIC criteria), starting from a random tree, with 50 million generations, with the results being recorded every 5000 generations. The convergence of the values was checked in TRACER v1.6 [74]. The GMYC analysis [32] was implemented in the 'splits' (SPecies' LImits by Threshold Statistics) [75] package in R, with the "single threshold" option. Only unique haplotypes were used for this analysis, given problems arising from the analysis of redundant data, as previously reported [76]. For this, the repeated identical sequences were removed using the ElimDupes tool (available at http://hcv.lanl.gov/content/sequence/ELIMDUPES/elimdupes.html).
Identification of Operational Taxonomic Units (OTUs)
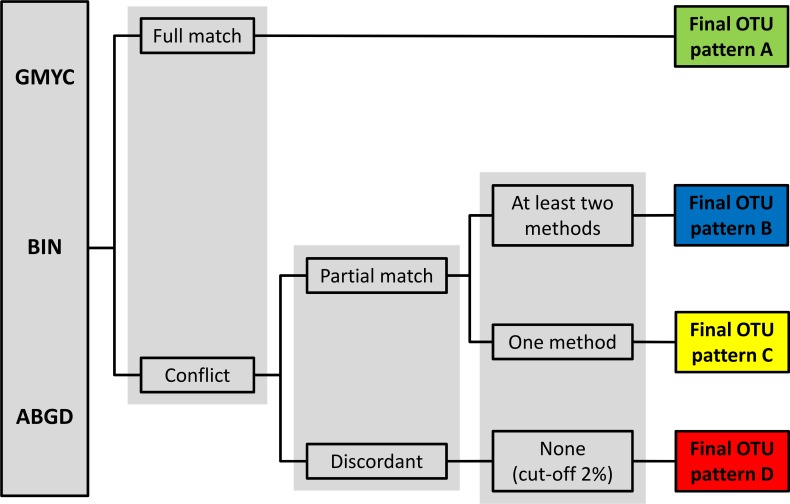
Several studies propose integrative clustering delimitation methods to check for congruence in the results of the different species clustering methods into operational taxonomic units, referring to the recognition of genetic patterns within groups that supports traditional taxonomic studies [77, 78]. We used a similar approach to Costa-Silva et al. [78], where the final OTUs were classified in four categories according to the degree of correspondence with the initial NJ classification of the genetic analysis ABGD, BIN and GMYC (see Fig 1): FULL MATCH—pattern A, when all clustering methods generated the same partition (congruence between ABGD, GMYC and BIN with NJ), PARTIAL MATCH—pattern B, when two analyses generated the same cluster as NJ (GMYC and ABGD, for example), PARTIAL MATCH—pattern C, when only one method (ABGD or GMYC or BIN) generated the same cluster as NJ, or DISCORDANT—pattern D, when none of the methods are in agreement; in this case, the OTUs were delimited based on the 2% cutoff criterion of the NJ analysis as defined by the initial clustering.
Fig 1. Cluster delimitation method used in this study.
All clusters were first separated by 2% cutoff analysis and then tested by other delimitation methods, GMYC, BIN and ABGD. See text for further details.
Results
A total of 1675 barcoding sequences were obtained for Astyanax (including published records), covering the entire area of occurrence of the genus. These sequences included 64 nominal species, 12 species identified provisionally (A. aff. bimaculatus, A. aff. bockmanni Vari & Castro 2007, A. aff. intermedius Eigenmann 1908, A. aff. laticeps Cope 1894, A. cf. fasciatus, A. cf. pampa Casciotta, Almirón & Azpelicueta, 2005, A. cf. anterior Eigenmann, 1908, A. cf. asuncionensis, Géry 1972, A. cf. fasciatus, A. cf. jacuhiensis Cope 1894, A. cf. jequitinhonhae Steindachner 1877, and A. cf. scabripinnis), and 40 forms identified only as Astyanax sp. (S1 Table). The average COI sequence size was 633 bp, with no stop codons, deletions or insertions.
The dendrogram obtained by the NJ analysis (2% cutoff, data not shown) indicated the existence of four major (Clades 1–4) and one minor (clade 5) groups. Clade 1 includes mainly species belonging to the A. fasciatus and A. scabripinnis species complexes (Fig 2). Clade 2 encompasses the Central American species (Fig 3), and Clade 3 is formed primarily by species of the A. bimaculatus complex (Fig 4). Clades 4 (Fig 5) and 5 (Fig 6) correspond to the remaining species. This same separation was found in the bayesian analyses. Within these clades, 124 groups were identified based on NJ that correspond to potential species, with mean intragroup distances of 0.44% and intergroup distances of 18,8%, while 31 are singletons (represented by only one specimen). The final number of OTUs (congruence between methods) was 125.
Fig 2. Bayesian analyses and delimitation clusters of Astyanax clade 1.
The vertical red line indicates that the cluster A.sp./ A.bockmanni/ A.aff.bockmanni/ A.bifasciatus/ A. dissimlis/ A. cf. fasciatus/ A. fasciatus/ A. aff. intermedius/ A. minor/ A.paranae/ A.rivularis/ A.scabripinnis was found at 2% cutoff NJ analysis; although separating into 2 groups, they have only 1.28% of intra-cluster genetic distance. Nodes marked with an asterisk denote probabilities greater than 0.9. BINs marked with an asterisk: groups with the same letter share the same BIN (BOLD:AAC5910). ABGD marked with an asterisk: both groups clustered together in ABGD analysis. The left tree represents the full tree, Astyanax clade 1 is in red. The upper histogram indicates the total number of OTUs identified in the entire data, and the lower histogram indicates the clusters identified in Astyanax clade 1. For further details, see S1 Table.
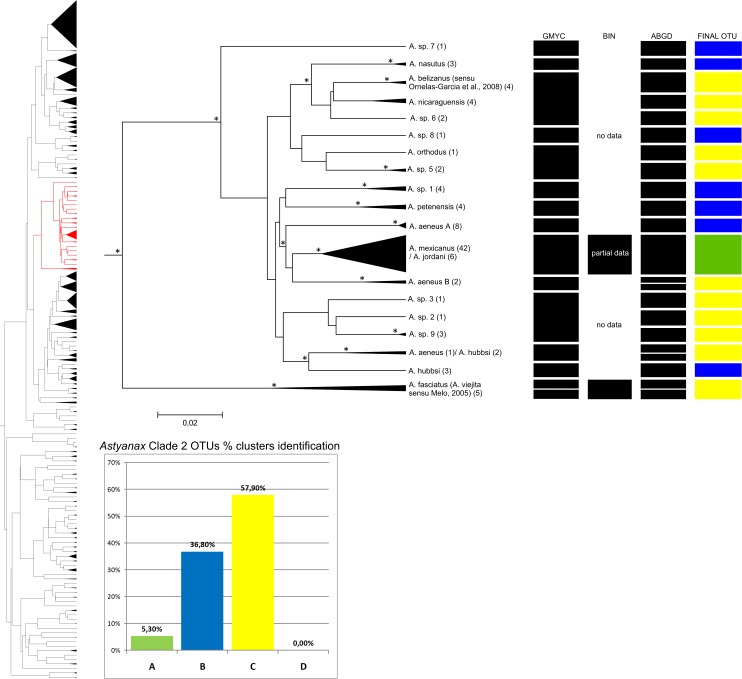
Fig 3. Bayesian analyses and delimitation clusters of Astyanax clade 2.
This clade is composed by trans-Andean species. BIN results are incomplete, once that these sequences are from public databases. In the left is represented the full tree, in red is represented the Astyanax clade 2. Spacers internal lines inside each cluster separate and indicate the number of subgroups found in a cluster, but does not represent the proportion of individuals found in each one. Histogram indicates the proportion of clusters identification in Astyanax clade 2. Nodes marked with an asterisk probabilities greater than 0.9. For further details, see S1 Table.
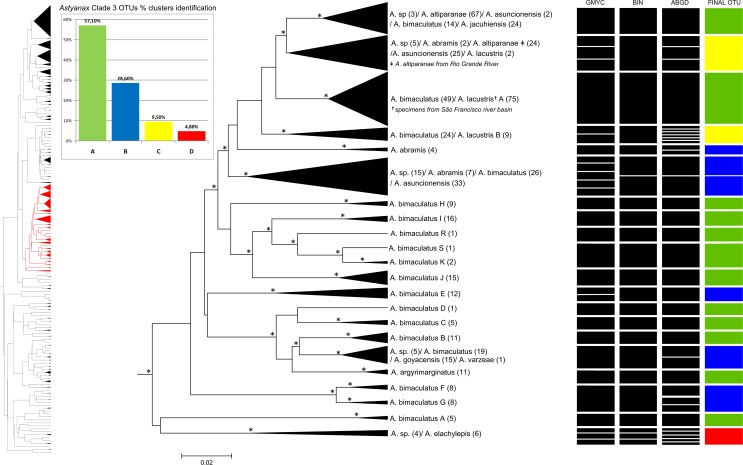
Fig 4. Bayesian analyses and delimitation clusters of Astyanax clade 3.
This clade is mainly composed by A. bimaculatus species complex. In the left is represented the full tree, in red is represented the Astyanax clade 3. Spacers internal lines inside each cluster separate and indicate the number of subgroups found in a cluster, but does not represent the proportion of individuals found in each one. Histogram indicates the proportion of clusters identification in Astyanax clade 3. Nodes marked with an asterisk probabilities greater than 0.9. For further details, see S1 Table.
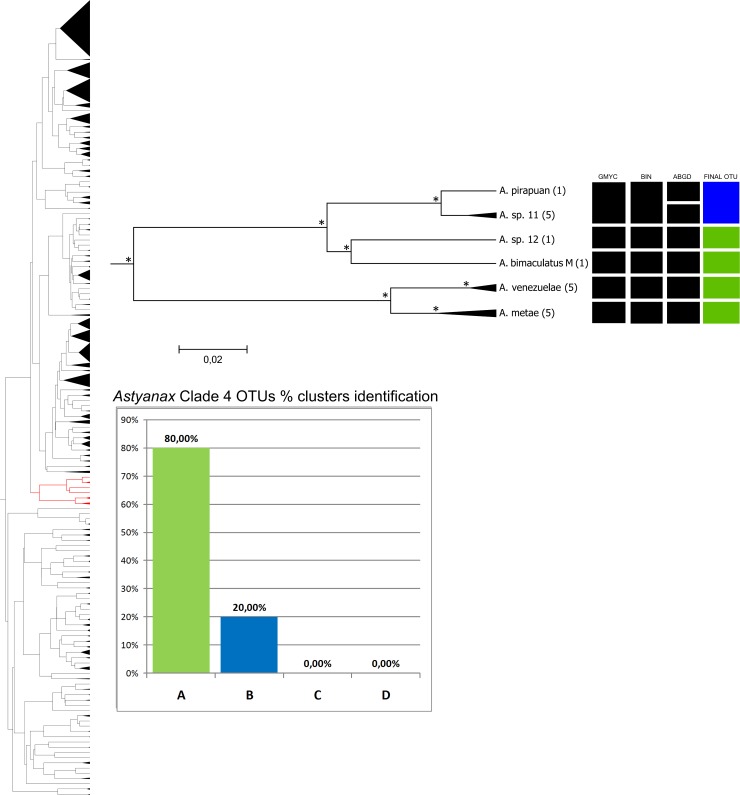
Fig 5. Bayesian analyses and delimitation clusters of Astyanax clade 4.
In the left is represented the full tree, in red is represented the Astyanax clade 4. Spacers internal lines inside each cluster separate and indicate the number of subgroups found in a cluster, but does not represent the proportion of individuals found in each one. Histogram indicates the proportion of clusters identification in Astyanax clade 4. Nodes marked with an asterisk probabilities greater than 0.9. For further details, see S1 Table.
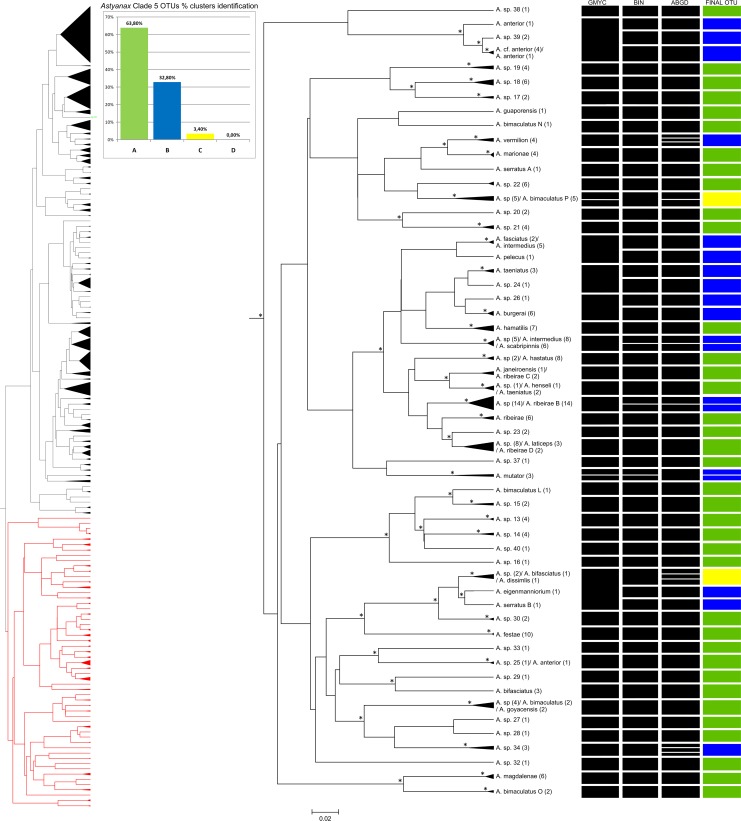
Fig 6. Bayesian analyses and delimitation clusters of Astyanax clade 5.
In the left is represented the full tree, in red is represented the Astyanax clade 5. Spacers internal lines inside each cluster separate and indicate the number of subgroups found in a cluster, but does not represent the proportion of individuals found in each one. Histogram indicates the proportion of clusters identification in Astyanax clade 5. Nodes marked with an asterisk probabilities greater than 0.9. For further details, see S1 Table.
The mean distance between the five major clades is 13.4%, while the mean distance within each clade ranged from 3.36% to 18.57% (Clade 2 and Clade 5, respectively; Table 1). The distances between groups of species ranged from 2% to 30.9% (S2 Table). The mean divergence within each cluster is shown in S3 Table.
Table 1. Mean distances within and between the five major clades.
| Clade 1 | Clade 2 | Clade 3 | Clade 4 | Clade 5 | |
|---|---|---|---|---|---|
| Clade 1 | 3.74* | 1.26 | 1.53 | 1.50 | 1.58 |
| Clade 2 | 14.42 | 3.36* | 1.35 | 1.48 | 1.53 |
| Clade 3 | 15.80 | 13.37 | 5.64* | 1.54 | 1.57 |
| Clade 4 | 15.61 | 15.73 | 16.44 | 7.14* | 1.45 |
| Clade 5 | 21.84 | 21.09 | 21.65 | 21.06 | 18.57* |
K2P distance within the four major clades identified (main diagonal, marked with asterisk) and average K2P divergence between these clades (below diagonal average values and above diagonal standard error).
The analysis of the ABGD dataset using the default parameters indicated the presence of 649 species groups, which is inconsistent with our other data. However, when the intraspecific minimum distance was set to 0.4% (which is equivalent to the mean estimated intraspecific cutoff of 2%) and the value of X to 0.1, the number of groups decreased to 156 (P = 0.0040), which is clearly more consistent with the data. The BIN results are only partial, given that the GenBank sequences were not in the BOLD system, but the results of its cluster analysis were similar to those of the ABGD and the NJ analysis. The GMYC analysis indicated the presence of 149 species (confidence interval: 137–161, threshold time: -0.02811409; Table 2). The NJ approach indicated 124 putative species.
Table 2. Number of clusters identified on the different analysis.
| Number of clusters | Number of clusters | Number of clusters | Number of clusters | |
|---|---|---|---|---|
| Method | NJ (Cutoff value 2%) | ABGD (relative gap width-0.1) | BIN | GMYC |
| All dataset | 124 | 156 | 113* | 149 |
| Clade 1 | 22 | 27 | 23 | 49 |
| Clade 2 | 19 | 22 | 2* | 15 |
| Clade 3 | 22 | 36 | 23 | 30 |
| Clade 4 | 6 | 6 | 5 | 5 |
| Clade 5 | 55 | 65 | 58 | 50 |
Each column shows the different method including NJ, ABGD, BIN and GMYC. Also is presented the number of clusters identified in each clade.
*Incomplete sampling (see text for details)
Species with low values of genetic interspecific distance (<2%) are found mainly in clade 1, but can also be found in other clades. A number of these clusters appear to include at least two species, indicating the occurrence of geographical regionalization (e.g., the cluster composed by A. dissimilis Garavello & Sampaio 2010 / A. bifasciatus Garavello & Sampaio 2010) or even the existence of other groups with an large distribution (e.g., cluster composed of Astyanax sp. / A. abramis Garavello & Sampaio 2010 / A. altiparanae Garutti & Britski 2000 / A. asuncionensis Géry 1972 / A. lacustris Lütken 1875, which includes specimens from Argentina and Brazil). Furthermore, specimens identified as A. anterior, A. bifasciatus, A. bimaculatus, A. laticeps, A. scabripinnis and A. fasciatus are present in more than one species group, indicating that they represent more than one species, with a divergence greater than 2%.
Discussion
Following the scheme presented in Fig 1, the analyses showed that 50.4% of the OTUs correspond to pattern A, 32.8% to pattern B, 13.6% to pattern C and 3.2% to pattern D. Given the complexity of Astyanax, the relatively low rate of full matches of approximately 50% was not unexpected. These results in fact exceed those obtained for Rineloricaria (Siluriformes), another hyperdiverse fish genus, using a similar clustering approach, which gave only 41% full matches [78].
Both the NJ (data not shown) and Bayesian (GMYC) analyses revealed the presence of four major groups and one minor one. The genetic distance analyses (NJ—K2P model) confirmed the distinction of the identified clades (Table 1). Although in the NJ and GMYC we used different models (K2P and GTR+G+I, respectively) the five main clades were found in both analysis. However, the total number of species clusters identified in the NJ analyses was smaller than that observed under the other three analysis (BIN, GMYC, ABGD). In special, in the Astyanax clade 1 where there are specimens from two species complexes (A. scabripinnis and A. fasciatus complex) the number of clusters identified by the GMYC analyses was almost twice higher than NJ, suggesting a better capacity of this method in species separation. Some studies suggest that in complex groups the K2P model may underestimated the total number of clusters [79, 80]. Thus, we can suggest that herein GMYC, in general, was the better method for species separation. Four of these clades (1, 2, 3, and 4) were characterized by low internal genetic distances (3.74%, 3.36%, 5.64% and 7.14%, respectively), whereas clade 5 had very high levels of internal distance (18.57%). Mean intra-genus divergence in Neotropical freshwater fish is usually less than 10% (means of 8.37% in [18] and 6.8% in [19]). However, values of 10.2–12.5% were found in Tetragonopterus based on barcode sequences [67], although the values recorded in the present study are well beyond those found in Neotropical fish genera up to now.
On the other hand, previous studies of a small number of Astyanax samples found uncommonly low genetic distances between some nominal species [81]. These low divergence values were observed between closely-related species from a restricted area, such as the São Francisco basin, where values ranged from 0, between A. lacustris and A. bimaculatus, to 0.93%, between A. cf. fasciatus and A. rivularis Lütken 1875 [23]. In this case, the reduced distance between the members of the A. bimaculatus complex is consistent with their belonging to the same species (as found in the A. bimaculatus / A. lacustris A cluster in clade 3), although similar barcode values among members of the A. fasciatus and A. scabripinnis species complexes were also found [23]. Furthermore, DNA barcoding of specimens from Argentina indicated a reduced genetic distance (0.62%) between A. eigenmanniorum and A. cf. pampa Casciotta, Almirón & Azpelicuetta, 2005 [82].
In the present study, specimens identified as A. bifasciatus, A. bimaculatus, A. laticeps and A. scabripinnis were present in two different clades (Astyanax clades 1 and 5) and A. fasciatus (Astyanax clades 1, 2 and 5), in three clades. Astyanax bimaculatus, A. scabripinnis and A. fasciatus are recognized species complexes [11–14, 60]. The specimens assigned to A. laticeps are distributed over a wide area, and demand a careful review of the available evidence. Astyanax bifasciatus was described from the Iguaçu River basin in a review of the local Astyanax species [61]. In the present study, samples identified as A. bifasciatus from neighboring sites in the Iguaçu River were assigned to distinct groups, even though they cannot be distinguished from morphometric data, reinforcing the need for a systematic review of the evidence.
Astyanax clade 1
This clade includes 763 individuals, 25 species and five groups identified at the genus level (Astyanax sp.). The different analytical approaches (Table 2) identified between 23 (BIN) and 49 (GMYC) clusters, although the NJ analysis with a 2% cutoff returned 22 groups (Fig 2). In this clade, more than one nominal species was observed in 59% of the NJ clusters.
The species of this clade are found in Brazil and neighboring western and southern countries, associated with the Paraná River basin. The large number of clusters identified and the overall genetic distance of approximately 4% impede the reliable separation of the species by DNA barcoding. In this clade, between two and 11 species were observed in the 2% threshold groups, most of which belong to the A. scabripinnis and A. fasciatus complexes. In some cases, the low levels of divergence indicate the relatively recent separation of the species or a very close relationship between them, as in the case of A. paranae and A. rivularis from the Paraná and São Francisco basins, respectively, which belong to the A. scabripinnis species complex. It is known that these two basins share their fauna [83]. It has already been reported that species from different river basins (A. fasciatus, A. taeniatus Jenyns 1842, A. scabripinnis and A. intermedius), with no intermediate forms and overlapping characters, form a “labyrinth”, which might justify a reduction in the number of species identified [7].There may be a number of potential explanations for the cases of low divergence (<2%) found in clade 1. One is the phenotypic plasticity of the species, which may hamper the reliable identification of the specimens [20]. The COI gene may also evolve at distinct rates in different groups, affecting the arrangement of the clusters [81, 84], and different groups may have distinct evolutionary histories, with some radiating more recently than others [81]. In a study of Astyanax, Ornelas-Garcia et al. [85] using one nuclear (RAG1) and three mitochondrial (16S, Cytb and COI) markers have identified different groups in Mesoamerica, which was attributed to a recent colonization of the region followed by rapid expansion of local populations. The invasion of Central America by ancestral Astyanax appears to have occurred between 3.1 and 8.1 million years ago, confirming that the radiation was relatively recent [85, 86]. An alternative explanation here is that many local populations are being described as new species due only to their restricted geographical distribution or local adaptations and should be synonymized in the future. Any one of these scenarios would demand further investigation of the status of the respective OTUs.
Specimens identified as A. fasciatus were present in three of the five clades defined in the present study. Morphological studies of the A. fasciatus complex suggest that the A. fasciatus should only be applied to specimens from the São Francisco basin, as in the original description of Cuvier [87], while other specimens identified as A. fasciatus from the Paraná basin, eastern Brazil and Central America could be assigned to other species [13]. Alternative evidence, such as cytogenetics, has indicated the existence of many different forms of A. fasciatus [88, 89], reinforcing the need for a more thorough investigation of this species complex. In the present study, clade 1 best matches the morphology and distribution of the type specimens described originally by Cuvier (Fig 2). Interestingly, the genetic distances between the specimens of this form of A. fasciatus Cuvier and others from the A. scabripinnis complex (A. paranae, collected near the type locality) are less than 1.3% (based on the analysis of 238 specimens), reinforcing the idea of a recent radiation in these fish.
In clade 1, A. xavante Garruti & Venere 2009 and A. goyanensis (Miranda Ribeiro 1944) were the only two species that could be identified unequivocally on the basis of morphology and the DNA barcode. In some cases, such as Astyanax sp. / A. erytroptherus Holmberg 1891 and Astyanax sp. / A. fasciatus / A. schubarti Britiski 1964, a redefinition of species limits (based on morphology and geographic distribution) might help solve the taxonomic problems. In many other cases, new species should be described or existing ones redescribed, through analyses including additional, faster-evolving molecular markers.
Astyanax clade 2
This clade includes 100 individuals, 10 species and eight groups identified at the genus level (Astyanax sp.) (Fig 3). The different analytical approaches identified between 15 (GMYC) and 22 (ABGD) clusters (Table 2), while the NJ analysis with a 2% cutoff returned 19 groups and the BIN approach gave only two clusters due to a lack of data. More than one species was observed in 10.5% of the NJ clusters. This group includes all species from the west of the Andes, primarily those from Central America. The mean interspecific distance in this clade was 3.36%, with the largest distance of 10% being found between Astyanax sp. 8 and A. fasciatus (A. viejita sensu Melo [62]).
The cluster of A. mexicanus De Filippi 1853 specimens includes cave-dwelling fish identified as A. jordani Hubbs & Innes 1936 suggesting that they may be the same species (within-cluster distance of 0.73%). These data were derived from published records, in which were also describes marked similarities between Astyanax and Bramocharax (less than 1% divergence) and identify Astyanax as a polyphyletic genus [85]. Another study based on DNA barcoding also failed to separate species of the genera Bramocharax and Astyanax [17].
In this clade, a group of specimens from Lake Maracaibo in Venezuela identified as A. fasciatus represent the only sample from outside Central America, but can still be considered a species from the west of the Andes. Valenciennes described Tetragonopterus viejita from Lake Maracaibo [90], which was synonymized with A. fasciatus [9]. Considering the findings of the present study and those from Melo [62], it is possible to recognize A. viejita as a valid species.
In clade 2, A. orthodus Eigenmann 1907, A. belizanus Bocourt 1868 (sensu Ornelas-Garcia et al. [85]), A. nasutus Meek 1907, A. nicaraguensis Eigenmann & Ogle 1907, A. petenensis Günther 1864 and A. fasciatus (A. vieijita, sensu [62]) could all be identified unequivocally by the DNA barcoding.
Astyanax clade 3
This clade encompasses 565 individuals, 10 species and 13 groups with most specimens identified as A. bimaculatus (Fig 4). The different approaches permitted the identification of between 23 (BIN) and 36 clusters (ABGD) (Table 2) and the NJ analysis with a 2% cutoff found 22 groups. More than one species was observed in 27.3% of the NJ clusters.
Interestingly, this clade consisted basically of species of the A. bimaculatus complex, which encompasses the Astyanax species in which a horizontal oval humeral spot is found, with a spot on the caudal peduncle extending to the edge of the median caudal rays [11]. Only one cluster (Astyanax sp. / A. elachylepis Bertaco & Lucinda 2005) and one species (A. varzeae Abilhoa & Duboc 2007) do not correspond to the external body coloration of the A. bimaculatus species group. On the other hand, other species with the same color pattern as that of the A. bimaculatus species complex (A. bimaculatus L, A. bimaculatus M, A. bimaculatus N, A. bimaculatus O and A. bimaculatus P) were assigned to clade 4 of the present study.
One cluster had four species (A. altiparanae, A. jacuhiensis, A. asuncionensis and A. bimaculatus) together with one unidentified species (Astyanax sp.). The mean genetic distance between the specimens in this cluster was only 0.34%, and the minimum distance of any other cluster containing specimens identified as A. altiparanae was 2.8%. The distribution of this cluster includes coastal and continental basins in the Brazilian states of São Paulo, Goiás, Mato Grosso, Minas Gerais, Paraná and Rio Grande do Sul. The existence of two clusters with specimens identified as A. altiparanae reflects recent findings in cytogenetics [91] and DNA barcoding [19]. The sum of this evidence indicates the existence of two species, treated up to now as a single taxon (A. altiparanae). The type locality of A. altiparanae is the Grande River at Volta Grande Dam in Miguelópolis, São Paulo [63], and our nearest specimen is from Colômbia, São Paulo, where the specimens were grouped with those from the group composed of Astyanax sp. / A. abramis / A. altiparanae / A. asuncionensis / A. lacustris, indicating that the specimen identified as A. altiparanae and grouped with A. jacuhiensis, A. bimaculatus and Astyanax sp. actually belongs to a different species. It is interesting to note that A. altiparanae is cytogenetically diverse, with 22 cytotypes described [91].
Astyanax lacustris from the São Francisco basin was described as Tetragonopterus lacustris Lütken, 1875 and this name encompasses those Astyanax for the forms of the A. bimaculatus complex in this basin. We identified three clusters of specimens identified as A. lacustris, with genetic distances of 2.2–2.9% between clusters. Another study notes that A. lacustris is rarely compared to A. altiparanae given the fact that the two species are found in the São Francisco and Paraná basins, respectively, even though all the traits used to differentiate these species are variable and overlapping [83]. Our results thus reinforce the need for in-depth revision of A. altiparanae that includes samples of A. lacustris.
Indeed, a recent karyotypic analysis has suggested that A. altiparanae from the Grande and A. lacustris from the São Francisco basins correspond to a single biological unit [92]. Recently a review of the Astyanax bimaculatus 'caudal peduncle spot' subgroup of the La Plata and São Francisco basins suggested that two nominal species–A. lacustris and A. abramis–should be considered valid [64]. In this same paper, A. jacuhiensis, A. asuncionensis and A. altiparanae are recognized as junior synonyms of A. lacustris. As our data point to three genetically differentiated groups with specimens identified as A. lacustris, further studies are clearly necessary to elucidate the systematics of this group.
In fact, one consistent group of A. lacustris from Bahia state may belong to a different species, given that it groups with A. bimaculatus from local coastal rivers, and may in fact represent a cryptic species or even A. bahiensis, recently considered to be a valid species [64]. Further studies with additional samples from the putative type locality of A. bahiensis will be needed to confirm whether one of the groups identified in the present study can be assigned to A. bahiensis. In clade 3, only A. argyrimarginatus could be identified unequivocally by DNA barcoding.
Astyanax clades 4 and 5
Neither of these clades encompass clearly-defined species complexes. Clade 4 includes 18 individuals (Fig 5) and clade 5 has 229 (Fig 6), with 28 species and 28 groups of specimens identified at the genus level in the two clades (Astyanax sp.). The different approaches permitted the identification of between five (BIN and GMYC) and six (ABGD) clusters in clade 4, and 50 (GMYC) to 65 (ABGD) clusters in clade 5 (Table 2). The NJ analysis with a 2% cutoff identified six groups in clade 4 and 55 in clade 5. In clade 5 more than one species was observed in only 15.7% of the NJ clusters, with 65% of final OTUs with the pattern A, being one of the best resolved clades, together with the clades 3 and 4 (65% and 80% OTUs in pattern A, respectively).
While a large number of unidentified species were found in clade 5, we also identified specimens belonging to the A. scabripinnis, A. bimaculatus and A. fasciatus species complexes. These species are widely distributed in Brazil, but some species are from Colombia, Guyana and Venezuela (e.g., A. metae Eigenmann 1914, A. magdalenae Eigenmann & Henn 1916, A. mutator Eigenmann 1909 and A. venezuelae Schultz 1944) and include the only species from the west of the Andes not found in clade 2, A. festae Boulenger 1898.
While the COI gene provides good resolution at the species level, the relationships among the different groups remain unclear because of the lack of a strong phylogenetic signal [93], although some insights can be gleaned from the analyses. In particular, the marked genetic distances (up to 30.9%) found between some of the clusters identified in clade 5 and other clades reinforce the conclusion that Astyanax is even more complex than previously thought.
In clade 4, A. pirapuan Tagliacollo, Britzke, Silva & Benine 2011, A. metae and A. venezuelae, were identified unequivocally by the DNA barcode, as were A. magdalenae, A. guaporensis Eigenmann 1911, A. festae, A. marionae Eigenmann 1911, A. vermilion Zanata & Camelier 2009, A. pelecus Bertaco & Lucena 2006, A. hamatilis Camelier & Zanata 2014, A. burgerai Zanata & Camelier 2009, and A. mutator in clade 5. Astyanax eigenmanniorum was also identified by barcoding, although the other specimens of this species were included in clade 1, a similar to that found in A. scabripinnis and A. fasciatus.
Conclusions
This study presents the most extensive investigation of Astyanax since the review of the last century [7]. The analysis of more than 1600 samples, including more than 70 nominal species (about half of the total number of valid species in the genus) and the other specimens identified only to the genus level, has further reinforced the complexity of the genus and the difficulty of identifying its species. We nevertheless identified five artificial clades separated by very high levels of genetic divergence (from 13.4% to 21.84%). One of the clades (clade 2) was formed by the Central American Astyanax forms, including A. mexicanus, the type species of the genus. We can thus speculate that this clade represents Astyanax strictu sensu, while the remaining groups may correspond to other genera, in line with other DNA barcoding studies of fish. This hypothesis will need to be tested with a molecular phylogeny of all the species currently included in the genus, although it was already recognized three different genera or subgenera in the Astyanax clade–Astyanax strictu sensu (which corresponds to A. mexicanus and clade 2), Poecilurichthys and Zygogaster [7].
The four major clades (1–4) presented very low intragroup genetic divergence (between 3.36% and 7.14%), whereas clade 5 presented high levels of intragroup divergence, indicating that speciation in the first four clades has been very rapid, further hampering species recognition.
Overall, only 21 morphological species (approximately 17% of the clusters in the 2% cutoff NJ analysis) could be identified unequivocally by DNA barcoding (A. xavante, A. goyanensis, A. orthodus, A. belizanus sensu Ornelas-Garcia et al., 2008, A. nasutus, A. nicaraguensis, A. petenensis, A. fasciatus, A. viejita sensu Melo, 2005, A. argyrimarginatus, A. pirapuan, A. metae, A. venezuelae, A. magdalenae, A. burgerai, A. guaporensis, A. festae, A. marionae, A. vermilion, A. pelecus, A. hamatilis and A. mutator). It is important to note, in addition, that a quarter of the diversity identified here was composed of unique specimens (31 singletons). This may be related to a combination of fast speciation, species with a broad geographic distribution, and the lack of descriptions of local morphotypes, as well as inadequate phylogenetic analyses. All these questions should be taken in account in future reviews of the genus.
Supporting Information
This list indicates the species, sampling details, vouchers numbers, clusters defined using the different approaches (NJ, GMYC, BIN and ABGD) and Genbank acession numbers.
(XLSX)
Pairwise distances between the clusters formed based on NJ-K2P.
(XLS)
Intra-cluster distances of the cluster formed based on NJ-K2P.
(XLS)
Acknowledgments
We thank all the individuals who assisted us in the collection and identification of the specimens analyzed in this study, as well as those who contributed through the donation of specimens and tissue samples. CO is a CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brasil) researcher (grant number 303854/2009-0). This investigation was supported by FAPESP (00264-2/2011 for BCR and 2010/17009-2 for CO), CONICET (D1009), CNPq and CAPES. The authors would like to thank the three anonymous reviewers for their helpful and constructive comments that greatly contributed to improving the final version of the paper.
Data Availability
Data are available in the Barcode of Life Database (BOLD): dx.doi.org/10.5883/DS-ABARCODE. The sequences are also available in GenBank, submission BankIt1972027: accessions KY266982—KY268290.
Funding Statement
This investigation was supported by Fundacao de Amparo a Pesquisa do Estado de Sao Paulo (www.fapesp.br; grant number 00264-2/2011 for BCR and 2010/17009-2 for CO), Consejo Nacional de Investigaciones Cientificas y Tecnicas (http://www.conicet.gov.ar/; grant number D1009 for JMDA), Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (http://cnpq.br/; grant number 303854/2009-0 for CO) and Coordenadoria de Aperfeicoamento do Ensino Superior (https://www.capes.gov.br/).
References
- 1.Albert JS, Reis RE. Historical biogeography of Neotropical Freshwater Fishes. Los Angeles: University of California Press, Berkeley;2011. [Google Scholar]
- 2.Reis RE, Kullander SO, Ferraris C. Check List of the Freshwater Fishes of South and Central America (CLOFFSCA). Porto Alegre (RS): EDIPUCRS; 2003. [Google Scholar]
- 3.Eschmeyer W, Fong D. Species by family/ subfamily. Catalog of Fishes. 2016; electronic version (accessed 8 February 2016).
- 4.Géry J. Characoids of the World. Neptune City: TFH Publications;1977. [Google Scholar]
- 5.Oliveira C, Avelino GS, Abe KT, Mariguela TC, Benine RC, Ortí G, et al. Phylogenetic relationships within the speciose family Characidae (Teleostei: Ostariophysi: Characiformes) based on multilocus analysis and extensive ingroup sampling. BMC Evol Biol. 2011;11:275 10.1186/1471-2148-11-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirande JM. Phylogeny of the family Characidae (Teleostei: Characiformes): from characters to taxonomy. Neotrop Ichthyol. 2010;8(3):385–568. [Google Scholar]
- 7.Eigenmann CH. The American Characidae. Mem Museum Comp Zool. 1921;43(3):209–310. [Google Scholar]
- 8.Eigenmann CH. The American Characidae . Mem Museum Comp Zool. 1927;43(4):311–428. [Google Scholar]
- 9.Lima FCT, Malabarba LR, Buckup PA, Silva JFP, Vari RP, Harold A, et al. Genera Incertae Sedis in Characidae In: Reis R. E., Kullander S. O., & Ferraris C. J. (Eds.). Check list of the freshwater fishes of South and Central America (CLOFFSCA). Porto Alegre: EDIPUCRS; 2003;106–169. [Google Scholar]
- 10.Pie MR, Baggio RA, Boeger WA, Patella LA, Ostrensky A, Vitule JRS, et al. Molecular data reveal a diverse Astyanax species complex in the upper Iguaçu River. J Fish Biol. 2009;75:2357–2362. 10.1111/j.1095-8649.2009.02438.x [DOI] [PubMed] [Google Scholar]
- 11.Garutti V. Revisão taxonômica dos Astyanax (Pisces, Characidae), com mancha umeral ovalada e mancha no pedúnculo caudal, estendendo-se à extremidade dos raios caudais medianos, das Bacias do Paraná, São Francisco e Amazônica [thesis]. São José do Rio Preto (SP):Universidade Estadual Paulista, Instituto de Biociências, Letras e Ciências Exatas;1995.
- 12.Garutti V, Langeani F. Redescription of Astyanax goyacensis Eigenmann, 1908 (Ostariophysi: Characiformes: Characidae). Neotrop Ichthyol. 2009;7(3):371–376. [Google Scholar]
- 13.Melo FAG, Buckup PA. Astyanax henseli, a new name for Tetragonopterus aeneus Hensel, 1870 from southern Brazil (Teleostei: Characiformes). Neotrop Ichthyol. 2006;4(1):45–52. [Google Scholar]
- 14.Moreira-Filho O, Bertollo LA. Astyanax scabripinnis (Pisces, Characidae): a species complex. Rev Bras Genet. 1991;14(2):331–357. [Google Scholar]
- 15.Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PD. DNA barcoding Australia´s fish species. Phil Trans R Soc B. 2005;360:1847–1857. 10.1098/rstb.2005.1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakra WS, Verma MS, Goswami M, Lal KK, Mohindra V, Punia P, et al. DNA barcoding Indian marine fishes. Mol Ecol Resour. 2011;11:60–71. 10.1111/j.1755-0998.2010.02894.x [DOI] [PubMed] [Google Scholar]
- 17.Váldez-Moreno M, Ivanova NV, Elías-Gutiérrez M, Contreras-Balderas S, Hebert PD. Probing diversity in freshwater fishes from Mexico and Guatemala with DNA barcodes. J Fish Biol. 2009;74:377–402. 10.1111/j.1095-8649.2008.02077.x [DOI] [PubMed] [Google Scholar]
- 18.Hubert N, Hanner R, Holm E, Mandrak NE, Taylor E, Burridge M, et al. Identifying Canadian freshwater fishes through DNA barcodes. Plos One. 2008;3:e2490 10.1371/journal.pone.0002490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereira LHG, Hanner R, Foresti F, Oliveira C. Can DNA barcoding accurately discriminate megadiverse Neotropical freshwater fish fauna?. BMC Genet. 2013;14:20 10.1186/1471-2156-14-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hebert PDN, Cywinska A, Ball SL, de Waard JR. Biological identification through DNA barcodes. Proc R Soc Lond B. 2003;270:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratnasingham S, Hebert PDN. BOLD: The barcode of Life Data System (www.barcodinglife.org). Mol Ecol Notes. 2007;7:355–364. 10.1111/j.1471-8286.2007.01678.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lara A, León JLP, Rodríguez R, Casane D, Côté G, Bernatchez L, et al. DNA barcoding of Cuban freshwater fishes: evidence for cryptic species and taxonomic conflicts. Mol Ecol Resour. 2010;10:421–430. 10.1111/j.1755-0998.2009.02785.x [DOI] [PubMed] [Google Scholar]
- 23.Carvalho DC, Oliveira DAA, Pompeu SA, Leal CG, Oliveira C, Hanner R. Deep barcode divergence in Brazilian freshwater fishes:the case of the São Francisco River basin. Mitochondrial DNA. 2011;22(S1):80–86. [DOI] [PubMed] [Google Scholar]
- 24.Mabragaña E, Astarloa JMD, Hanner R, Zhang J, Castro MG. DNA Barcoding Identifies Argentine Fishes from Marine and Brackish Waters. Plos One. 2011;6:e28655 10.1371/journal.pone.0028655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira LHG, Maia GMG, Hanner R, Foresti F, Oliveira C. DNA barcodes discriminate freshwater fishes from the Paraíba do Sul River Basin, São Paulo, Brazil. Mitochondrial DNA. 2011a;22(S1):71–79. [DOI] [PubMed] [Google Scholar]
- 26.Pereira LHG, Pazian MF, Hanner R, Foresti F, Oliveira C. DNA barcoding reveals hidden diversity in the Neotropical freshwater fish Piabina argentea (Characiformes: Characidae) from the Upper Paraná Basin of Brazil. Mitochondrial DNA. 2011;22(1):87–96. [DOI] [PubMed] [Google Scholar]
- 27.Hebert PDN. Penton EH, Burns JM, Janzen DH, Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astrapes fulgerator. Proc Natl Acad Sci. USA. 2004;101:14812–14817. 10.1073/pnas.0406166101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer CP, Paulay G. DNA barcoding: error rates based on comprehensive sampling. PLoS Biol. 2005;3:2229–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puillandre N, Lambert A, Brouillet S, Achaz G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol. 2012;21(8):1864–1877. 10.1111/j.1365-294X.2011.05239.x [DOI] [PubMed] [Google Scholar]
- 30.Puillandre N, Modica MV, Zhang Y, Sirovich L, Boisselier MC, Cruaud C, et al. Large-scale species delimitation method for hyperdiverse groups. Mol Ecol. 2012b;21(11):2671–2691. [DOI] [PubMed] [Google Scholar]
- 31.Ratnasingham S, Hebert PDN. A DNA-based registry for all animal species: The Barcode Index Number (BIN) System. Plos One. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pons J, Barraclough TG, Gomez-Zurita J, Cardoso A, Duran DP, Hazell S, et al. Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Syst Biol. 2006;55:595–609. [DOI] [PubMed] [Google Scholar]
- 33.Fujisawa T, Barraclough TG. Delimiting species using single locus data and the generalized mixed Yule coalescent approach. Syst Biol. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Kapli P, Pavlidis P, Stamatakis A. A General Species Delimitation Method with Applications to Phylogenetic Placements. Bioinformatics. 2013;29(22):2869–2876. 10.1093/bioinformatics/btt499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yule GU. A mathematical theory of evolution, based on the conclusions of Dr. J. C. Willis, F.R.S. Philos Trans R Soc Lon B. 1924;213:21–87. [Google Scholar]
- 36.Kingman JFC. On the genealogy of large populations. J Appl Probab. 1982;19:27–43. [Google Scholar]
- 37.Powell JR. Accounting for uncertainty in species delineation during the analysis of environmental DNA sequence data. Meth Ecol Evol. 2012;3:1–11. [Google Scholar]
- 38.Eigenmann CH. New characins in the collection of the Carnegie Museum. Ann Carnegie Mus. 1911;8(1):164–181. [Google Scholar]
- 39.Eigenmann CH, Henn AW, Wilson C. New fishes from western Colombia, Ecuador, and Peru. Contrib Zool Lab Ind Univ. Indiana University Studies.1914;133(19):1–15. [Google Scholar]
- 40.Eigenmann CH. The American Characidae. Mem Museum C Zool. Cambridge. 1917;23(1):1–102. [Google Scholar]
- 41.Schultz LP. The fishes of the family Characinidae from Venezuela, with descriptions of seventeen new forms. Proc U S Nat Mus. 1944;95(3181):235–367. [Google Scholar]
- 42.Garutti V, Britski HA. Descrição de uma nova espécie de Astyanax (Teleostei, Characidae), com mancha umeral ovalada horizontalmente, procedente da bacia do rio Guaporé, Amazônia. Pap Avulsos Zool. 1997; 40:217–229. [Google Scholar]
- 43.Garutti V. Descrição de Astyanax argyrimarginatus sp. n. (Characiformes, Characidae) procedente da bacia do Rio Araguaia, Brasil. Rev Bras Biol. 1999;59(4):585–591. [DOI] [PubMed] [Google Scholar]
- 44.Bertaco VA, Malabarba LR. Description of two new species of Astyanax (Teleostei: Characidae) from headwater streams of Southern Brazil, with comments on the “A. scabripinnis species complex”. Ichthyol Explor Fres. 2001;12(3):221–234. [Google Scholar]
- 45.Melo FAG. Revisão taxonômica das espécies do gênero Astyanax Baird & Girard, 1854 (Teleostei: Characiformes: Characidae) da região da serra dos Órgãos. Arch Mus Nac (Rio de J.). 2001;59:1–46. [Google Scholar]
- 46.Casciotta JR, Almirón AE, Azpelicueta MM. A new species of Astyanax from río Uruguay basin, Argentina (Characiformes: Characidae). Ichthyol Explor Fres. 2003;14(4):329–334. [Google Scholar]
- 47.Castro RM, Vari R. Astyanax biotae, a new species of stream fish from the Rio Paranapanema basin, upper Rio Paraná system, southeastern Brazil (Ostariophysi: Characiformes: Characidae). Proc Biol Soc Wash. 2004;117(3):330–338. [Google Scholar]
- 48.Casciotta JR, Almirón AE, Azpelicueta MM. Astyanax pampa (Characiformes, Characidae), a new species from the southernmost boundary of the Brazilian subregion, Argentina. Rev Suisse Zool. 2005;112(2):401–408. [Google Scholar]
- 49.Miquelarena AM, Menni RC. Astyanax tumbayaensis, a new species from northwestern Argentina highlands (Characiformes: Characidae) with a key to the Argentinean species of the genus and comments on their distribution. Rev Suisse Zool. 2005;112:661–676. [Google Scholar]
- 50.Vari RP, Castro RMC. New Species of Astyanax (Ostariophysi: Characiformes: Characidae) from the Upper Rio Parana System, Brazil. Copeia, 2007;1:150–162 [Google Scholar]
- 51.Garutti V, Venere PC. Astyanax xavante, a new species of characid from middle rio Araguaia in the Cerrado region, Central Brazil (Characiformes: Characidae). Neotrop Ichthyol. 2009;7(3):377–383. [Google Scholar]
- 52.Pavanelli CS, Oliveira CAM. A redescription of Astyanax gymnodontus (Eigenmann, 1911), new combination, a polymorphic characid fish from the rio Iguaçu basin, Brazil. Neotrop Ichthyol. 2009;7(4):569–578. [Google Scholar]
- 53.Zanata AM, Camelier P. Astyanax vermilion and Astyanax burgerai: new characid fishes (Ostariophysi: Characiformes) from Northeastern Bahia. Neotrop Ichthyol. 2009;7(2):175–184. [Google Scholar]
- 54.Bertaco VA, Lucena CAS. Redescription of Astyanax obscurus (Hensel, 1870) and A. laticeps (Cope, 1894) (Teleostei: Characidae): two valid freshwater species originally described from rivers of Southern Brazil. Neotrop Ichthyol. 2010;8(1):7–20. [Google Scholar]
- 55.Soneira P, Casciotta J, Almirón A, Ciotek L, Giorgis P. Redescription of Astyanax erythropterus (Holmberg, 1891) (Teleostei: Characiformes: Characidae) from La Plata basin in Argentina. Neotrop Ichthyol. 2010;8(4):779–785. [Google Scholar]
- 56.Tagliacollo VA, Britzke R, Silva GSC, Benine RC. Astyanax pirapuan: a new characid species from the upper Rio Paraguay system, Mato Grosso, central Brazil (Characiformes, Characidae). Zootaxa. 2011;2749:40–46. [Google Scholar]
- 57.Lucena C. A. S., Castro J. B. & Bertaco V. A.. Three new species of Astyanax from drainages of southern Brazil (Characiformes: Characidae). Neotrop Ichthyol. 2013;11(3): 537–552 [Google Scholar]
- 58.Marinho MMF, Ohara WM. Redescription of Astyanax guaporensis Eigenmann, 1911 (Characiformes: Characidae), a small characid from the rio Madeira basin. Zootaxa. 2013;3652:475–484. [DOI] [PubMed] [Google Scholar]
- 59.Camelier P, Zanata AM. A new species of Astyanax Baird & Girard (Characiformes: Characidae) from the Rio Paraguaçu basin, Chapada Diamantina, Bahia, Brazil, with comments on bony hooks on all fins. Journal of Fish Biology. 2014;84(2)475–490. 10.1111/jfb.12295 [DOI] [PubMed] [Google Scholar]
- 60.Bertaco VA, Lucena CAS. Two new species of Astyanax (Ostariophysi: Characiformes: Characidae) from eastern Brazil, with a synopsis of the Astyanax scabripinnis species complex. Neotrop Ichthyol. 2006;4(1):53–60. [Google Scholar]
- 61.Garavello JC, Sampaio FAA. Five new species of genus Astyanax Baird & Girard, 1854 from Rio Iguaçu, Paraná, Brazil (Ostariophysi, Characiformes, Characidae). Braz J Biol. 2010;70(3):847–865. [DOI] [PubMed] [Google Scholar]
- 62.Melo FAG. Revisão taxonômica do complexo de espécies Astyanax fasciatus (Cuvier, 1819) (Teleostei: Characiformes: Characidae) [Phd thesis]. Rio de Janeiro (RJ): UFRJ/MN; 2005.
- 63.Garutti V, Britski HA. Descrição de uma espécie nova de Astyanax (Teleostei: Characidae) da bacia do Alto rio Paraná e considerações sobre as demais espécies do gênero na bacia. Comunicações do Museu de Ciências e Tecnologia da PUCRS, Série Zoologia. 2000;13:65–88. [Google Scholar]
- 64.Lucena CAS, Soares HG. Review of species of the Astyanax bimaculatus “caudal peduncle spot” subgroup sensu Garutti & Langeani (Characiformes, Characidae) from the rio La Plata and rio São Francisco drainages and coastal systems of southern Brazil and Uruguay. Zootaxa. 2016;4072(1):101–125. 10.11646/zootaxa.4072.1.5 [DOI] [PubMed] [Google Scholar]
- 65.Miya M, Nishida M. Use of mitogenomic information in teleostean molecular phylogenetics: a tree-based exploration under the maximum-parsimony optimality criterion. Mol Phylogenet Evol. 2000;17:437–455. 10.1006/mpev.2000.0839 [DOI] [PubMed] [Google Scholar]
- 66.Ivanova NV, Zemlak TS, Hanner RH, Hebert PDN. Universal primer cocktails for fish DNA barcoding. Mol Ecol Notes. 2007; 7:544–548. [Google Scholar]
- 67.Melo BF, Benine RC, Mariguela TC, Oliveira C. A new species of Tetragonopterus Cuvier, 1816 (Characiformes: Characidae: Tetragonopterinae) from the rio Jari, Amapá, northern Brazil. Neotrop Ichthyol. 2011;9(1):49–56. [Google Scholar]
- 68.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 69.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, and Kumar S. MEGA5:Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol. 2011;28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. [DOI] [PubMed] [Google Scholar]
- 72.Saitou M, Nei N. The Neighbor-joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol Biol Evol. 1987;4(4):406–425. [DOI] [PubMed] [Google Scholar]
- 73.Drummond AJ, Suchard MA, Xie D & Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012;29:1969–1973 10.1093/molbev/mss075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rambaut A, Suchard MA, Xie D, Drummond AJ. Tracer v1.6, 2014. Available from http://beast.bio.ed.ac.uk/Tracer (accessed December 2015).
- 75.Ezard T, Fujisawa T, Barraclough TG. SPLITS:SPecies' LImits by Threshold Statistics. R package version 1.0-18/r45;2009. Available from: http://R-Forge.R-project.org/projects/splits/ (accessed December 2015).
- 76.Monaghan MT, Wild R, Elliot M, Fujisawa T, Balke M, Inward DJG, et al. Accelerated species inventory on Madagascar using coalescent-based models of species delineation. Syst Biol. 2009;58:298–311. 10.1093/sysbio/syp027 [DOI] [PubMed] [Google Scholar]
- 77.Kekkonen M, Hebert PDN. DNA barcode-based delineation of putative species: efficient start for taxonomic workflows. Mol Ecol Resour. 2014;14(4):706–715. 10.1111/1755-0998.12233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Costa-Silva GJ, Rodriguez MS, Roxo FF, Foresti F, Oliveira C. Using Different Methods to Access the Difficult Task of Delimiting Species in a Complex Neotropical Hyperdiverse Group. Plos One, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barley AJ, Thomson RC. Assessing the performance of DNA barcoding using posterior predictive simulations. Mol Ecol. 2016;25(9):1944–1957. 10.1111/mec.13590 [DOI] [PubMed] [Google Scholar]
- 80.Zinger L, Philippe H. Coalescing molecular evolution and DNA barcoding. 2016; 25(9):1908–1910. 10.1111/mec.13639 [DOI] [PubMed] [Google Scholar]
- 81.Ward RD. DNA barcode divergence among species and genera of birds and fishes. Mol Ecol Resour. 2009;9:1077–1085. 10.1111/j.1755-0998.2009.02541.x [DOI] [PubMed] [Google Scholar]
- 82.Rosso JJ, Mabragaña E, Gonzalez Castro M, Díaz de Astarloa JM. DNA barcoding Neotropical fishes: recent advances from the Pampa Plain, Argentina. Mol Ecol Resour. 2012;12:999–1011. 10.1111/1755-0998.12010 [DOI] [PubMed] [Google Scholar]
- 83.Buckup PA. The Eastern Brazilian Shield In: Albert JS, Reis RE, editors. Historical Biogeography of Neotropical Freshwater Fishes. Berkeley and Los Angeles. University of California Press; 2011;203–210. [Google Scholar]
- 84.Frézal L.E, Leblois R. Four years of DNA barcoding: current advances and prospects. Infect Genet Evol. 2008;8(5):727–736. 10.1016/j.meegid.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 85.Ornelas-Garcia CP, Dominguez-Dominguez O, Doadrio I. Evolutionary history of the fish genus Astyanax Baird & Girard (1854) (Actynopterigii, Characidae) in Mesoamerica reveals multiple morphological homoplasies. BMC Evol Biol. 2008;8: 340 10.1186/1471-2148-8-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gross JB. The complex origin of Astyanax cavefish. BMC Evol Biol. 2012;12:105 10.1186/1471-2148-12-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cuvier LB. Memoirs du Museum D'Historie Naturelle. Paris. 1819; 5:352–353. [Google Scholar]
- 88.Pazza R, Kavalco SAF, Penteado PR, Kavalco KF, Almeida-Toledo LF . The species complex Astyanax fasciatus Cuvier (Teleostei, Characiformes)—a multidisciplinary approach. J Fish Biol. 2008;72:2002–2010. [Google Scholar]
- 89.Medrado AS, Figueiredo AVA, Waldschmidt AM, Affonso PRAM, Carneiro PLS. Cytogenetic and morphological diversity in populations of Astyanax fasciatus (Teleostei, Characidae) from Brazilian northeastern river basins. Genet Mol Biol. 2008;31:208–214. [Google Scholar]
- 90.Cuvier LB, Valenciennes A. Memoirs du Museum D'Historie Naturelle Des Poissons. Paris. 1848;22:1–149. [Google Scholar]
- 91.Fernandes CA, Martins-Santos IC. Cytogenetic studies in two populations of Astyanax altiparanae (Pisces, Characiformes). Hereditas. 2004;141:328–332. 10.1111/j.1601-5223.2004.01832.x [DOI] [PubMed] [Google Scholar]
- 92.Peres WAM, Bertollo LAC, Buckup PA, Blanco DR, Kantek DLZ, Moreira-Filho O. Invasion, dispersion and hybridization of fish associated to river transposition: Karyotypic evidence in Astyanax “bimaculatus group” (Characiformes: Characidae). Rev Fish Biol Fisher. 2012;22:519–526. [Google Scholar]
- 93.Hebert PDN, Gregory TR. The promise of DNA barcoding for taxonomy. Syst Biol. 2005;54:852–859. 10.1080/10635150500354886 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This list indicates the species, sampling details, vouchers numbers, clusters defined using the different approaches (NJ, GMYC, BIN and ABGD) and Genbank acession numbers.
(XLSX)
Pairwise distances between the clusters formed based on NJ-K2P.
(XLS)
Intra-cluster distances of the cluster formed based on NJ-K2P.
(XLS)
Data Availability Statement
Data are available in the Barcode of Life Database (BOLD): dx.doi.org/10.5883/DS-ABARCODE. The sequences are also available in GenBank, submission BankIt1972027: accessions KY266982—KY268290.