Abstract
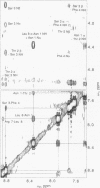
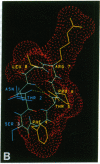
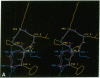
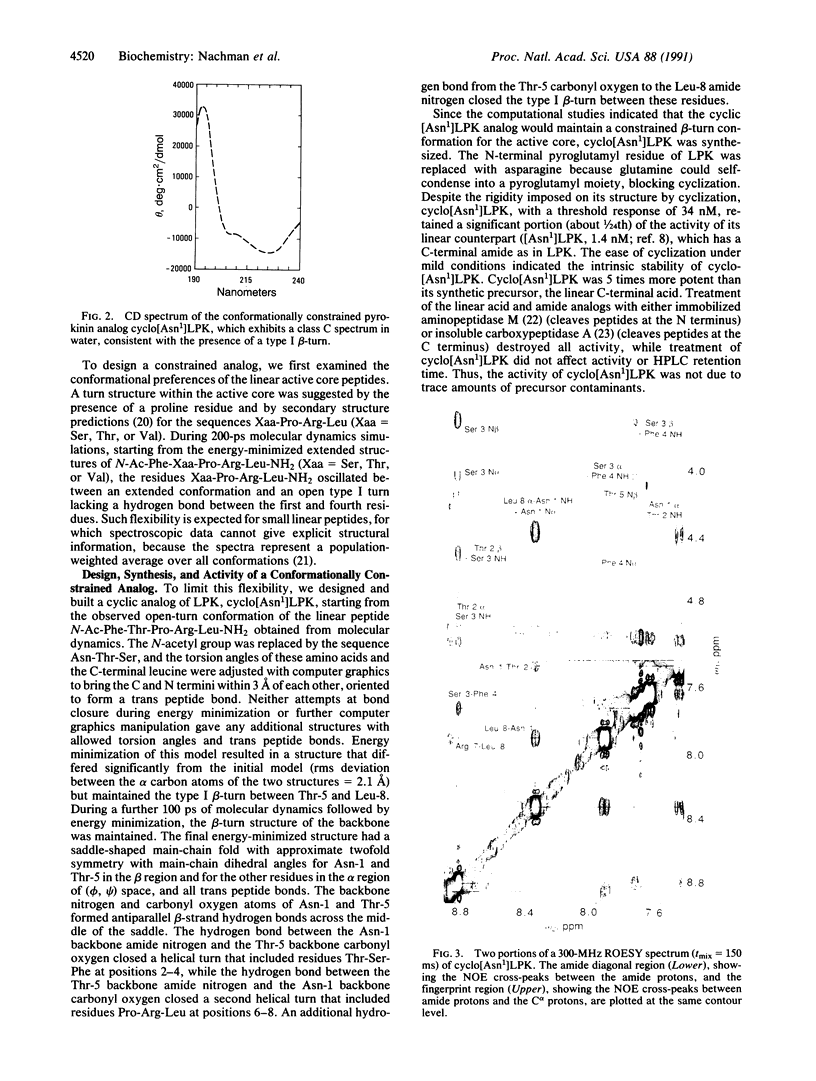
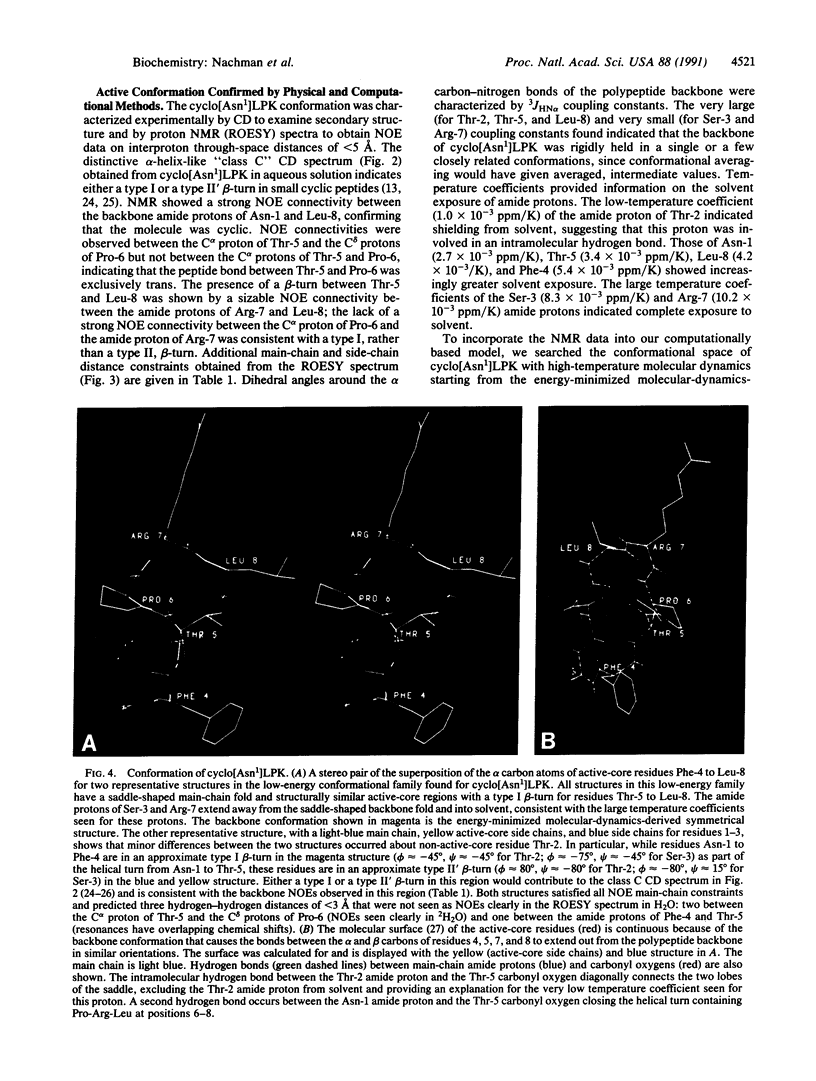
To understand the structural and chemical basis for insect neuropeptide activity, we have designed, synthesized, and determined the conformation of a biologically active cyclic analog of the pyrokinins, an insect neuropeptide family that mediates myotropic (visceral muscle contractile) activity. Members of this insect neuropeptide family share the common C-terminal pentapeptide sequence Phe-Xaa-Pro-Arg-Leu-NH2 (Xaa = Ser, Thr, or Val). Circular dichroic, nuclear magnetic resonance, and molecular dynamics analyses of the conformationally restricted cyclic pyrokinin analog cyclo(-Asn-Thr-Ser-Phe-Thr-Pro-Arg-Leu-) indicated the presence of a beta-turn in the active core region encompassing residues Thr-Pro-Arg-Leu. The rigid cyclic analog retains biological activity, suggesting that its C-terminal beta-turn is the active pyrokinin conformation recognized by the myotropic receptor. As individual pyrokinins and pyrokinin-like neuropeptides demonstrate both oviduct-contractile and pheromone-biosynthesis activities in various insects, the biologically active beta-turn structure reported here holds broad significance for many biological processes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COX D. J., BOVARD F. C., BARGETZI J. P., WALSH K. A., NEURATH H. PROCEDURES FOR THE ISOLATION OF CRYSTALLINE BOVINE PANCREATIC CARBOXYPEPTIDASE A. II. ISOLATION OF CARBOXYPEPTIDASE A-ALPHA FROM PROCARBOXYPEPTIDASE A. Biochemistry. 1964 Jan;3:44–47. doi: 10.1021/bi00889a008. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Beta-turns in proteins. J Mol Biol. 1977 Sep 15;115(2):135–175. doi: 10.1016/0022-2836(77)90094-8. [DOI] [PubMed] [Google Scholar]
- Collawn J. F., Wallace C. J., Proudfoot A. E., Paterson Y. Monoclonal antibodies as probes of conformational changes in protein-engineered cytochrome c. J Biol Chem. 1988 Jun 25;263(18):8625–8634. [PubMed] [Google Scholar]
- Connolly M. L. Solvent-accessible surfaces of proteins and nucleic acids. Science. 1983 Aug 19;221(4612):709–713. doi: 10.1126/science.6879170. [DOI] [PubMed] [Google Scholar]
- Dauber-Osguthorpe P., Roberts V. A., Osguthorpe D. J., Wolff J., Genest M., Hagler A. T. Structure and energetics of ligand binding to proteins: Escherichia coli dihydrofolate reductase-trimethoprim, a drug-receptor system. Proteins. 1988;4(1):31–47. doi: 10.1002/prot.340040106. [DOI] [PubMed] [Google Scholar]
- Donzel B., Rivier J., Goodman M. Synthesis of a cyclic analog of the luteinizing hormone releasing factor: [Glu4,D-Ala6,Orn7]LRF. Biopolymers. 1977 Nov;16(11):2587–2590. doi: 10.1002/bip.1977.360161121. [DOI] [PubMed] [Google Scholar]
- Dyson H. J., Rance M., Houghten R. A., Lerner R. A., Wright P. E. Folding of immunogenic peptide fragments of proteins in water solution. I. Sequence requirements for the formation of a reverse turn. J Mol Biol. 1988 May 5;201(1):161–200. doi: 10.1016/0022-2836(88)90446-9. [DOI] [PubMed] [Google Scholar]
- Dyson H. J., Wright P. E. Defining solution conformations of small linear peptides. Annu Rev Biophys Biophys Chem. 1991;20:519–538. doi: 10.1146/annurev.bb.20.060191.002511. [DOI] [PubMed] [Google Scholar]
- Gierasch L. M., Deber C. M., Madison V., Niu C. H., Blout E. R. Conformations of (X-L-Pro-Y)2 cyclic hexapeptides. Preferred beta-turn conformers and implications for beta turns in proteins. Biochemistry. 1981 Aug 4;20(16):4730–4738. doi: 10.1021/bi00519a032. [DOI] [PubMed] [Google Scholar]
- Holman G. M., Marks E. P. Synthesis, transport, and release of a neurohormone by cultured neuroendocrine glands from the cockroach, Leucophaea maderae. J Insect Physiol. 1974 Mar;20(3):479–484. doi: 10.1016/0022-1910(74)90156-5. [DOI] [PubMed] [Google Scholar]
- Holman G. M., Nachman R. J., Wright M. S. Insect neuropeptides. Annu Rev Entomol. 1990;35:201–217. doi: 10.1146/annurev.en.35.010190.001221. [DOI] [PubMed] [Google Scholar]
- Hruby V. J., al-Obeidi F., Kazmierski W. Emerging approaches in the molecular design of receptor-selective peptide ligands: conformational, topographical and dynamic considerations. Biochem J. 1990 Jun 1;268(2):249–262. doi: 10.1042/bj2680249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura A., Nagasawa H., Kataoka H., Inoue T., Matsumoto S., Ando T., Suzuki A. Amino acid sequence of pheromone-biosynthesis-activating neuropeptide (PBAN) of the silkworm, Bombyx mori. Biochem Biophys Res Commun. 1989 Aug 30;163(1):520–526. doi: 10.1016/0006-291x(89)92168-2. [DOI] [PubMed] [Google Scholar]
- McGillis J. P., Mitsuhashi M., Payan D. G. Immunomodulation by tachykinin neuropeptides. Ann N Y Acad Sci. 1990;594:85–94. doi: 10.1111/j.1749-6632.1990.tb40470.x. [DOI] [PubMed] [Google Scholar]
- Nachman R. J., Holman G. M., Cook B. J. Active fragments and analogs of the insect neuropeptide leucopyrokinin: structure-function studies. Biochem Biophys Res Commun. 1986 Jun 30;137(3):936–942. doi: 10.1016/0006-291x(86)90315-3. [DOI] [PubMed] [Google Scholar]
- Nachman R. J., Holman G. M., Haddon W. F., Ling N. Leucosulfakinin, a sulfated insect neuropeptide with homology to gastrin and cholecystokinin. Science. 1986 Oct 3;234(4772):71–73. doi: 10.1126/science.3749893. [DOI] [PubMed] [Google Scholar]
- Raina A. K., Jaffe H., Kempe T. G., Keim P., Blacher R. W., Fales H. M., Riley C. T., Klun J. A., Ridgway R. L., Hayes D. K. Identification of a neuropeptide hormone that regulates sex pheromone production in female moths. Science. 1989 May 19;244(4906):796–798. doi: 10.1126/science.244.4906.796. [DOI] [PubMed] [Google Scholar]
- Rance M., Sørensen O. W., Bodenhausen G., Wagner G., Ernst R. R., Wüthrich K. Improved spectral resolution in cosy 1H NMR spectra of proteins via double quantum filtering. Biochem Biophys Res Commun. 1983 Dec 16;117(2):479–485. doi: 10.1016/0006-291x(83)91225-1. [DOI] [PubMed] [Google Scholar]
- Royer G. P., Andrews J. P. Immobilized derivatives of leucine aminopeptidase and aminopeptidase M. Applications in protein chemistry. J Biol Chem. 1973 Mar 10;248(5):1807–1812. [PubMed] [Google Scholar]
- Schoofs L., Holman G. M., Hayes T. K., Kochansky J. P., Nachman R. J., De Loof A. Locustatachykinin III and IV: two additional insect neuropeptides with homology to peptides of the vertebrate tachykinin family. Regul Pept. 1990 Dec 10;31(3):199–212. doi: 10.1016/0167-0115(90)90006-i. [DOI] [PubMed] [Google Scholar]
- Schoofs L., Holman G. M., Hayes T. K., Tips A., Nachman R. J., Vandesande F., De Loof A. Isolation, identification and synthesis of locustamyotropin (Lom-MT), a novel biologically active insect peptide. Peptides. 1990 May-Jun;11(3):427–433. doi: 10.1016/0196-9781(90)90038-7. [DOI] [PubMed] [Google Scholar]
- Starratt A. N., Brown B. E. Structure of the pentapeptide proctolin, a proposed neurotransmitter in insects. Life Sci. 1975 Oct 15;17(8):1253–1256. doi: 10.1016/0024-3205(75)90134-4. [DOI] [PubMed] [Google Scholar]