Abstract
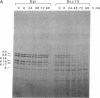
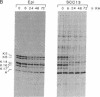
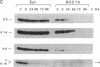
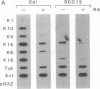
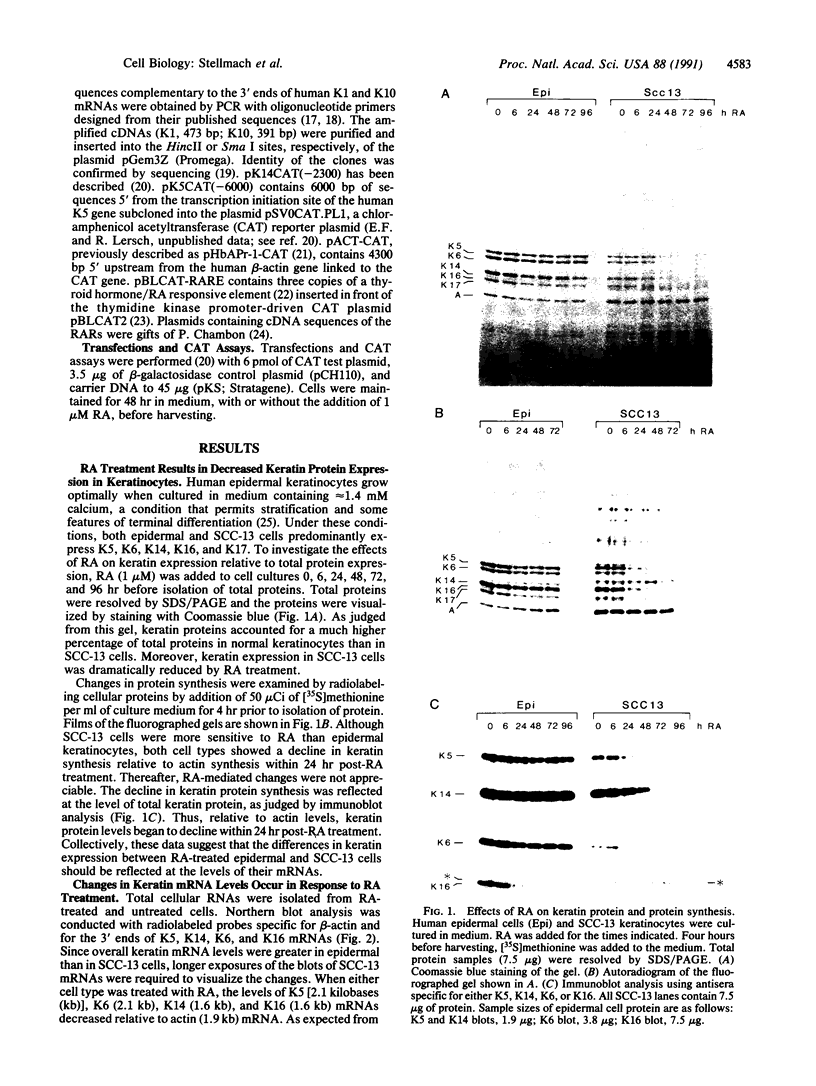
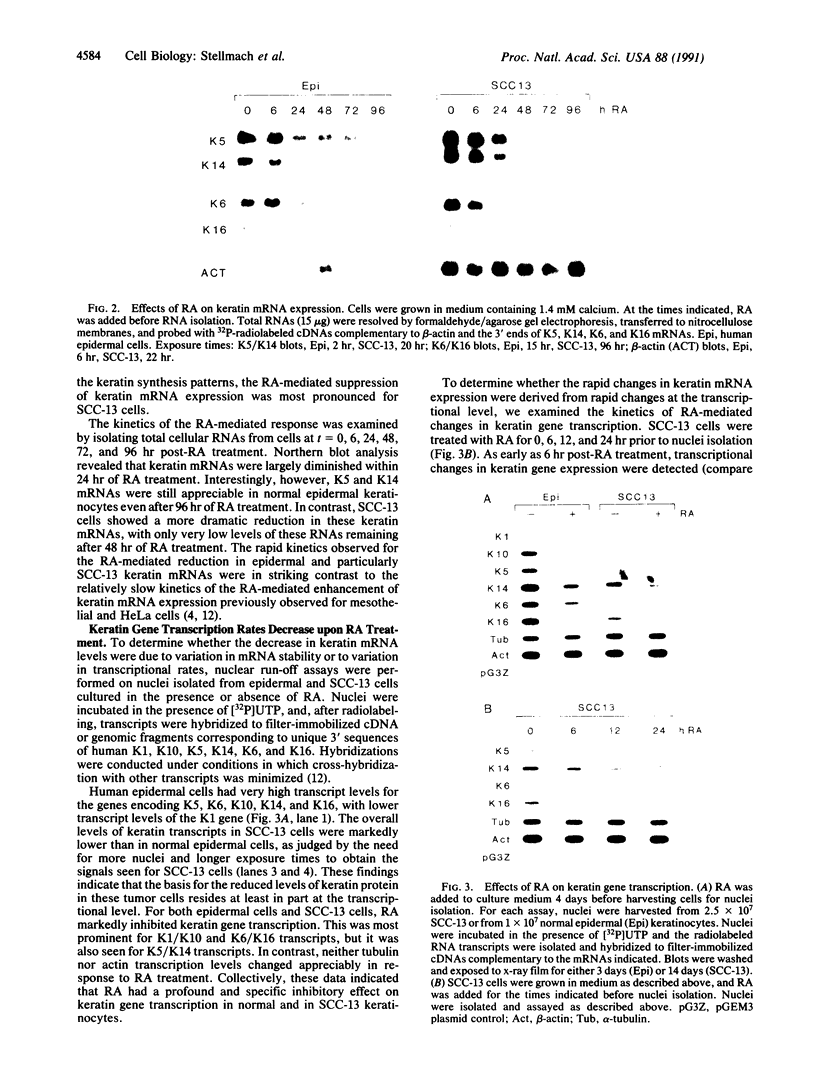
Vitamin A and other retinoids profoundly inhibit morphological and biochemical features of epidermal differentiation in vivo and in vitro. To elucidate the molecular mechanisms underlying the differential expression of epidermal keratins and their regulation by retinoids, we examined retinoid-mediated changes in total protein expression, protein synthesis, mRNA expression, and transcription in cultured human keratinocytes and in squamous cell carcinoma (SCC-13) cells of epidermal origin. Our studies revealed that the epidermal keratins, K5, K6, K14, and K16, their mRNAs, and their transcripts were diminished relative to actin as a consequence of retinoic acid (RA) treatment. The effects were most pronounced in SCC-13 and were detected as early as 6 hr post-RA treatment, with enhancement over an additional 24-48 hr. Repression was also observed when 5' upstream sequences of K14 or K5 genes were used to drive expression of a chloramphenicol acetyltransferase reporter gene in SCC-13 keratinocytes. Both cell types were found to express mRNAs for the RA receptors alpha and gamma, which may be involved in the RA-mediated transcriptional changes in these cells. The rapid transcriptional changes in epidermal keratin genes were in striking contrast to the previously reported slow transcriptional changes in simple epithelial keratin genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asselineau D., Bernard B. A., Bailly C., Darmon M. Retinoic acid improves epidermal morphogenesis. Dev Biol. 1989 Jun;133(2):322–335. doi: 10.1016/0012-1606(89)90037-7. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- FELL H. B., MELLANBY E. Metaplasia produced in cultures of chick ectoderm by high vitamin A. J Physiol. 1953 Mar;119(4):470–488. doi: 10.1113/jphysiol.1953.sp004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981 Sep;25(3):617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Gilfix B. M., Eckert R. L. Coordinate control by vitamin A of keratin gene expression in human keratinocytes. J Biol Chem. 1985 Nov 15;260(26):14026–14029. [PubMed] [Google Scholar]
- Glass C., Fuchs E. Isolation, sequence, and differential expression of a human K7 gene in simple epithelial cells. J Cell Biol. 1988 Oct;107(4):1337–1350. doi: 10.1083/jcb.107.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Watt F. M. Regulation by vitamin A of envelope cross-linking in cultured keratinocytes derived from different human epithelia. Mol Cell Biol. 1982 Sep;2(9):1115–1117. doi: 10.1128/mcb.2.9.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Gudas L. J. Cyclic AMP analogs and retinoic acid influence the expression of retinoic acid receptor alpha, beta, and gamma mRNAs in F9 teratocarcinoma cells. Mol Cell Biol. 1990 Jan;10(1):391–396. doi: 10.1128/mcb.10.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kim K. H., Schwartz F., Fuchs E. Differences in keratin synthesis between normal epithelial cells and squamous cell carcinomas are mediated by vitamin A. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4280–4284. doi: 10.1073/pnas.81.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. H., Stellmach V., Javors J., Fuchs E. Regulation of human mesothelial cell differentiation: opposing roles of retinoids and epidermal growth factor in the expression of intermediate filament proteins. J Cell Biol. 1987 Dec;105(6 Pt 2):3039–3051. doi: 10.1083/jcb.105.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R., Fuchs E. The use of retinoic acid to probe the relation between hyperproliferation-associated keratins and cell proliferation in normal and malignant epidermal cells. J Cell Biol. 1989 Jul;109(1):295–307. doi: 10.1083/jcb.109.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krust A., Kastner P., Petkovich M., Zelent A., Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A., Rosenberg M., Vassar R., Fuchs E. Regulation of a human epidermal keratin gene: sequences and nuclear factors involved in keratinocyte-specific transcription. Genes Dev. 1990 Nov;4(11):1985–1998. doi: 10.1101/gad.4.11.1985. [DOI] [PubMed] [Google Scholar]
- Liau G., Ong D. E., Chytil F. Interaction of the retinol/cellular retinol-binding protein complex with isolated nuclei and nuclear components. J Cell Biol. 1981 Oct;91(1):63–68. doi: 10.1083/jcb.91.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason I., Murphy D., Hogan B. L. Expression of c-fos in parietal endoderm, amnion and differentiating F9 teratocarcinoma cells. Differentiation. 1985;30(1):76–81. doi: 10.1111/j.1432-0436.1985.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Oshima R. G., Abrams L., Kulesh D. Activation of an intron enhancer within the keratin 18 gene by expression of c-fos and c-jun in undifferentiated F9 embryonal carcinoma cells. Genes Dev. 1990 May;4(5):835–848. doi: 10.1101/gad.4.5.835. [DOI] [PubMed] [Google Scholar]
- Oshima R. G., Trevor K., Shevinsky L. H., Ryder O. A., Ceceña G. Identification of the gene coding for the Endo B murine cytokeratin and its methylated, stable inactive state in mouse nonepithelial cells. Genes Dev. 1988 May;2(5):505–516. doi: 10.1101/gad.2.5.505. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Beckett M. A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981 May;41(5):1657–1663. [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Rubin A. L., Parenteau N. L., Rice R. H. Coordination of keratinocyte programming in human SCC-13 squamous carcinoma and normal epidermal cells. J Cell Physiol. 1989 Jan;138(1):208–214. doi: 10.1002/jcp.1041380128. [DOI] [PubMed] [Google Scholar]
- Schweizer J., Fürstenberger G., Winter H. Selective suppression of two postnatally acquired 70 kD and 65 kD keratin proteins during continuous treatment of adult mouse tail epidermis with vitamin A. J Invest Dermatol. 1987 Aug;89(2):125–131. doi: 10.1111/1523-1747.ep12470544. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Role of retinoids in differentiation and carcinogenesis. Cancer Res. 1983 Jul;43(7):3034–3040. [PubMed] [Google Scholar]
- Steinert P. M., Parry D. A., Idler W. W., Johnson L. D., Steven A. C., Roop D. R. Amino acid sequences of mouse and human epidermal type II keratins of Mr 67,000 provide a systematic basis for the structural and functional diversity of the end domains of keratin intermediate filament subunits. J Biol Chem. 1985 Jun 10;260(11):7142–7149. [PubMed] [Google Scholar]
- Stellmach V. M., Fuchs E. Exploring the mechanisms underlying cell type-specific and retinoid-mediated expression of keratins. New Biol. 1989 Dec;1(3):305–317. [PubMed] [Google Scholar]
- Stoler A., Kopan R., Duvic M., Fuchs E. Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J Cell Biol. 1988 Aug;107(2):427–446. doi: 10.1083/jcb.107.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor J. M., Oshima R. G. Identification of mRNA species that code for extra-embryonic endodermal cytoskeletal proteins in differentiated derivatives of murine embryonal carcinoma cells. J Biol Chem. 1982 Aug 10;257(15):8771–8774. [PubMed] [Google Scholar]
- Tomic M., Jiang C. K., Epstein H. S., Freedberg I. M., Samuels H. H., Blumenberg M. Nuclear receptors for retinoic acid and thyroid hormone regulate transcription of keratin genes. Cell Regul. 1990 Nov;1(12):965–973. doi: 10.1091/mbc.1.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasios G. W., Gold J. D., Petkovich M., Chambon P., Gudas L. J. A retinoic acid-responsive element is present in the 5' flanking region of the laminin B1 gene. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9099–9103. doi: 10.1073/pnas.86.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Harris C. C. Altered differentiation of mouse epidermal cells treated with retinyl acetate in vitro. Exp Cell Res. 1974 May;86(1):95–105. doi: 10.1016/0014-4827(74)90653-3. [DOI] [PubMed] [Google Scholar]
- Zelent A., Krust A., Petkovich M., Kastner P., Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989 Jun 29;339(6227):714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- Zhou X. M., Idler W. W., Steven A. C., Roop D. R., Steinert P. M. The complete sequence of the human intermediate filament chain keratin 10. Subdomainal divisions and model for folding of end domain sequences. J Biol Chem. 1988 Oct 25;263(30):15584–15589. [PubMed] [Google Scholar]
- de The H., Marchio A., Tiollais P., Dejean A. Differential expression and ligand regulation of the retinoic acid receptor alpha and beta genes. EMBO J. 1989 Feb;8(2):429–433. doi: 10.1002/j.1460-2075.1989.tb03394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]