Abstract
Sp3-rich compounds are underrepresented in libraries for probe- and drug-discovery, despite their promise of extending the range of accessible molecular shapes beyond planar geometries. With this in mind, a collection of single-enantiomer bicyclic, fused cyclopentenones underpinned by a complexity-generating Pauson–Khand cyclization was synthesized. A fingerprint of biological actions of these compounds was determined immediately after synthesis using real-time annotation−a process relying on multiplexed measurements of alterations in cell morphological features.
The composition of small-molecule screening libraries is critical for the success of early probe- and drug-discovery efforts, including those relying on fragment-based ligand discovery (FBLD) and high-throughput screening (HTS).1−4 Inclusion of diverse molecular shapes has been suggested as a key factor for library performance.5−9 However, in contrast to natural products, many synthetic libraries contain only a few sp3-rich scaffolds.8−11 Bridging this gap requires the development of efficient and scalable synthetic methods to access sp3-rich small molecules.
Piloting this approach, we recently reported several efficient, systematic routes to amino alcohol-derived low molecular weight compounds (or “fragments”) by way of 1,2-amino alcohols and bis-electrophiles.12 We now extend this approach by incorporating modern synthetic methods for low-to-medium molecular weight small-molecule library synthesis and to “de-risk” the involved synthetic pathways (e.g., explore amenability for expanding to additional structural variants and eventual optimization and scale-up). Here, we report the divergent synthesis of Pauson–Khand cyclization (PKC) derived tetrahydrocyclopenta[c]pyranone derivatives as novel low molecular weight small molecules for FBLD, HTS, and real-time biological annotation.
Since the rigidity of fused cyclic systems could be a desirable chemical feature for biological activity,13 we explored chiral building blocks amenable to cyclization reactions. In 2011, Fandrick and colleagues14 reported a general copper-catalyzed method for constructing historically difficult-to-access enantioenriched homopropargyl alcohols. Building upon this robust method, we aimed to construct a rigid, low-molecular weight bicyclic core. While many routes for cyclization to bicycles exist, we looked to synthesize a core skeleton allowing for molecule growth beyond what traditional sp2-enriched (hetero)aromatic libraries often provide, a reliance upon generally planar cores and appendage diversity arising from routine synthetic transformations (e.g., amide coupling or cross-coupling reactions).11 Having also considered the Astex Rule of Three,13,15 we proposed that a <300 Da, bicyclic, rigid core containing a functional handle (in this case, a β,β-disubstituted enone) would serve as an effective intermediate to generate a collection of sp3-carbon-enriched fragments and hit-to-lead-like small molecules.
In a previous study, our group compared the biological activity of skeletal rearrangements using a high-content imaging assay for “real-time biological annotation”.16 As a complementary approach, we explore here the biological annotation of derivatives originating from functional group interconversion reactions starting with a common core.
We anticipated that common enone intermediates 1 and 2 could be accessed by a Pauson–Khand cyclization of enyne 3. Building block 3 could be accessed by SN2′ allylation of chiral alcohol 5a, generated by deprotection17 of TMS-alkyne 4, the nonracemic product of a copper–BINAP-catalyzed homopropargylation reaction with acetophenone (Figure 1).14
Figure 1.
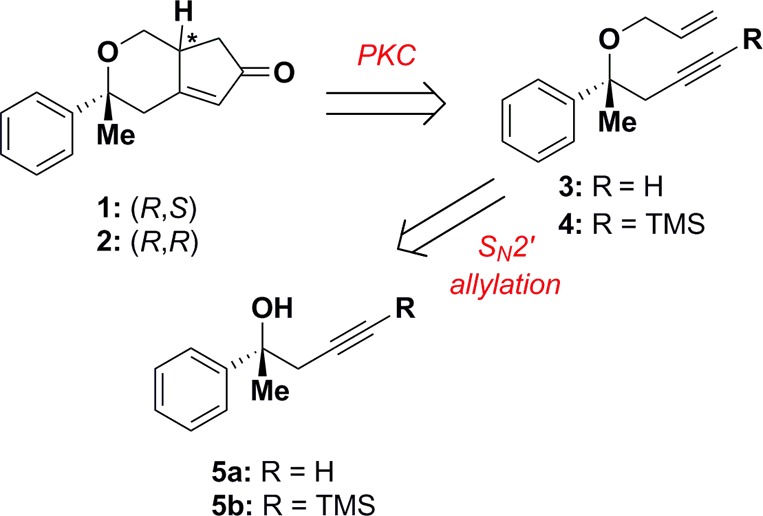
Retrosynthetic analysis of bicyclic enones 1 and 2.
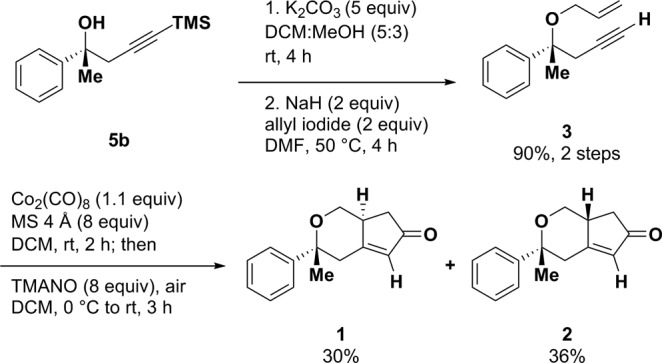
Allylation of tertiary alcohol 5a with dry sodium hydride and allyl iodide proceeded cleanly to afford 3 in high yield (95%). Direct allylation of TMS-protected alkyne 5b resulted in either no reaction or majority decomposition, presumably via an inter- or intramolecular Brook rearrangement (Table S2).18 Enyne 3 was subjected to a tertiary amine N-oxide promoted Pauson–Khand cyclization, which provided gram-scale quantities of enones 1 and 2 (Scheme 1).19,20 Cyclization of either 3 or 4 afforded a mixture of easily separable enone diastereomers.
Scheme 1. Synthetic Method to Access Gram-Scale Quantities of Enones 1 and 2.
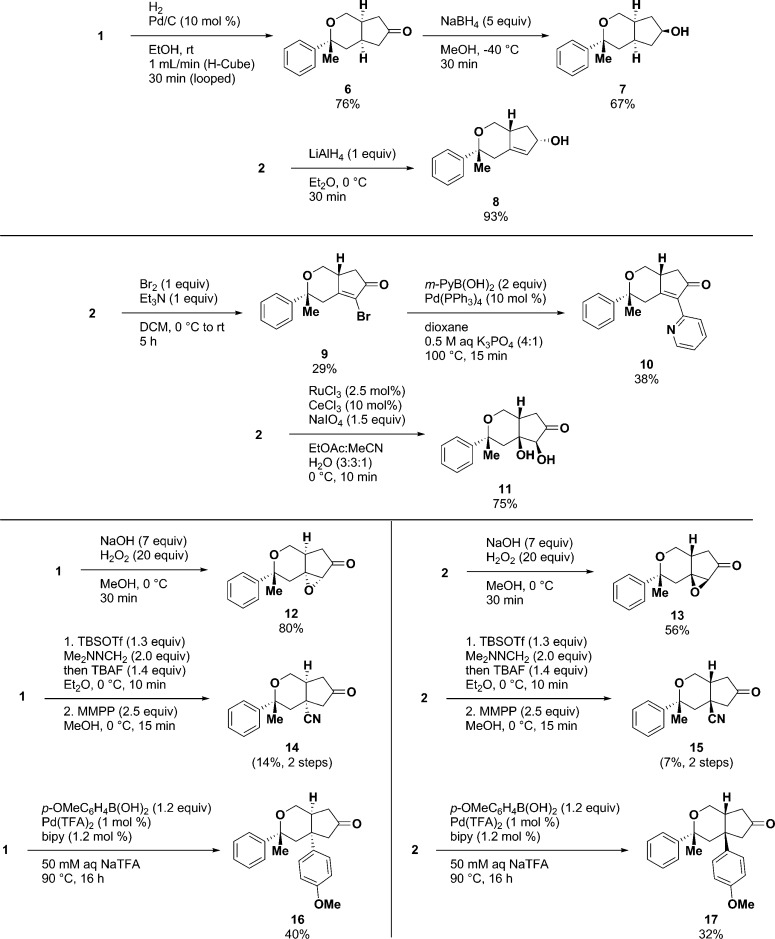
With sufficient quantities of 1 and 2 in hand, a range of conditions was explored to survey the reactivity of the key cyclic β,β-disubstituted enone functional handle (Scheme 2).
Scheme 2. Diversification of Bicyclic Enone Core to Provide Tetrahydrocyclopenta[c]pyranone Derivatives.
Derivatization reactions have not been optimized for yield and were performed on a 0.1–0.2 mmol scale.
At the outset of our exploration, 1 and 2 were subjected to reductive conditions, for instance, hydrogenation to yield ketones, sodium borohydride treatment to yield aliphatic alcohols,21 and Luche (Ce3+) conditions to yield allylic alcohols.22 Interestingly, each diastereomer had a unique reaction profile across a number of transformations. Ketone 6 and aliphatic alcohol 7 were readily accessed from 1 (76% and 67%, respectively), yet catalytic hydrogenation of 2 resulted in scission of the benzyl C–O bond (an unexpected route toward generating nonracemic, benzyl-substituted cyclopentanones). Further, Luche reductions of both 1 and 2 led to mixtures of fully reduced aliphatic alcohols and, surprisingly, an epimerization of 2 to form 1. Allylic alcohol 8 was accessed as a single diastereomer by treatment of 2 with lithium aluminum hydride (93%), whereas reaction with 1 led to a mixture of diastereomers.
Next, α-halogenation was explored as a route toward α-aryl-substituted enones. We initially explored in situ generation of bromine, or bromine surrogate pyridinium tribromide, for this transformation; however, only treatment of 2 with molecular bromine (Br2) yielded the desired α-bromo enone 9.23,24 Reaction of 1 with bromine led to HBr-promoted decomposition or bromination at the bridgehead carbon. α-Bromo enone 9 was then subjected to straightforward Suzuki–Miyuara cross-coupling conditions with Pd(Ph3)4 and mild base to yield α-m-pyridyl enone 10 (11%, over two steps).
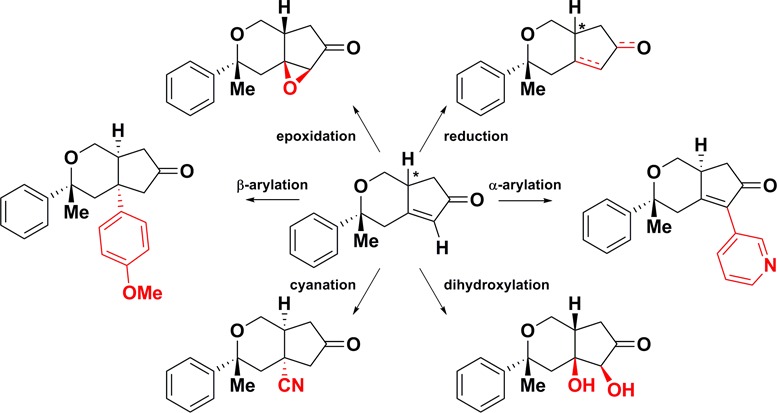
When considering oxidative transformations, we first explored dihydroxylation of the enone double bond. Again, 1 and 2 displayed differing reactivity profiles where treatment of 2 with catalytic Ru3+ and Ce3+ with sodium periodiate led to diol 11 (75%),25 yet identical conditions with 1 led to a recovery of starting material. In the case of base-promoted epoxidation, however, α,β-epoxy ketones 12 and 13 were isolated in 80% and 56% yields, respectively.26 Generating β-cyano ketones 14 and 15 required a two-step process where 1 and 2 were treated with tert-butyldimethylsilyl triflate (TBSOTf) and formaldehyde dimethylhydrazone and then oxidized to nitriles in low yields (7–14%, over two steps).27 Olefin hydroamination,28 reductive olefin coupling,29 and cuprate additions30,31 to 1 and 2 were explored, yet no desired products were isolated.
As a final transformation involving bond formation at the β-carbon, Pd-catalyzed addition of p-methoxyboronic acid in aqueous sodium trifluoroacetamide led to β-aryl ketones 16 and 17 in 40% and 32% yields, respectively.32
In order to assess the ability of the resulting novel compounds to modulate the functions of cells, the collection was exposed to a high-dimensional, multiplex “cell-painting” assay.3,16,33,34 Using six readily available small-molecule dyes, we stained seven cellular components, from which hundreds of morphological features were extracted by automated imaging (Figure 2). Epoxy ketone diastereomers 12 and 13 displayed the most striking cellular response and induced consistent morphology changes across all doses. Notably, the two compounds had related yet distinguishable effects that were not seen in any other compound (Figures S1–S5). Encouraged by these results, we are continuing to explore this family of small molecules, especially by expanding the scope of the pathway and by comparing their morphological “signatures” to a growing database of signatures resulting from other small molecules.3
Figure 2.

Cell painting of U-2 OS cells reveals the biological activity of 13 (100 μM; right) through reduced cell counts and morphology changes compared to DMSO (negative control; left). False color shows mitochondrial, DNA, RNA, Golgi apparatus, endoplasmic reticulum, plasma membrane, and F-actin stains (for additional images, see Figure S9).
Acknowledgments
We thank Dr. Andrew J. Phillips (C4 Therapeutics) for helpful input and encouragement; Dr. Stephen Johnston (Broad Institute) for high-resolution mass spectrometry data; Kate Hartland (Broad Institute) for assistance with the cell-painting assay; the compound-management team at The Broad Institute for curating all synthesized compounds; and Dr. Peter Müller (Massachusetts Institute of Technology) for X-ray crystallographic structural analysis. The National Institutes of General Medical Sciences (GM038627 awarded to S.L.S.) supported this research. S.D.N. is funded in part by Harvard University’s Graduate Prize Fellowship and an Eli Lilly Graduate Fellowship in Chemistry. S.L.S. is an investigator with the Howard Hughes Medical Institute.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.6b03118.
The authors declare no competing financial interest.
Supplementary Material
References
- Shelat A. A.; Guy R. K. Nat. Chem. Biol. 2007, 3 (8), 442–446. 10.1038/nchembio0807-442. [DOI] [PubMed] [Google Scholar]
- Petrone P. M.; Wassermann A. M.; Lounkine E.; Kutchukian P.; Simms B.; Jenkins J.; Selzer P.; Glick M. Drug Discovery Today 2013, 18 (13–14), 674–680. 10.1016/j.drudis.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Wawer M. J.; Li K.; Gustafsdottir S. M.; Ljosa V.; Bodycombe N. E.; Marton M. A.; Sokolnicki K. L.; Bray M.-A.; Kemp M. M.; Winchester E.; Taylor B.; Grant G. B.; Hon C. S.-Y.; Duvall J. R.; Wilson J. A.; Bittker J. A.; Dančík V.; Narayan R.; Subramanian A.; Winckler W.; Golub T. R.; Carpenter A. E.; Shamji A. F.; Schreiber S. L.; Clemons P. A. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (30), 10911–10916. 10.1073/pnas.1410933111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann A. M.; Lounkine E.; Hoepfner D.; Le Goff G.; King F. J.; Studer C.; Peltier J. M.; Grippo M. L.; Prindle V.; Tao J.; Schuffenhauer A.; Wallace I. M.; Chen S.; Krastel P.; Cobos-Correa A.; Parker C. N.; Davies J. W.; Glick M. Nat. Chem. Biol. 2015, 11 (12), 958–966. 10.1038/nchembio.1936. [DOI] [PubMed] [Google Scholar]
- Clemons P. A.; Bodycombe N. E.; Carrinski H. A.; Wilson J. A.; Shamji A. F.; Wagner B. K.; Koehler A. N.; Schreiber S. L. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (44), 18787–18792. 10.1073/pnas.1012741107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons P. A.; Wilson J. A.; Dančík V.; Muller S.; Carrinski H. A.; Wagner B. K.; Koehler A. N.; Schreiber S. L. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (17), 6817–6822. 10.1073/pnas.1015024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduk P. J.; Galloway W. R. J. D.; Spring D. R. Nature 2011, 470 (7332), 42–43. 10.1038/470042a. [DOI] [PubMed] [Google Scholar]
- Hung A. W.; Ramek A.; Wang Y.; Kaya T.; Wilson J. A.; Clemons P. A.; Young D. W. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (17), 6799–6804. 10.1073/pnas.1015271108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg D. G.; Kondo N.; Mitchell S. L.; Galloway W. R. J. D.; Sore H. F.; Madin A.; Spring D. R. Angew. Chem., Int. Ed. 2016, 55 (40), 12479–12483. 10.1002/anie.201606496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C. W.; Rees D. C. Angew. Chem., Int. Ed. 2016, 55 (2), 488–492. 10.1002/anie.201506783. [DOI] [PubMed] [Google Scholar]
- Brown D. G.; Boström J. J. Med. Chem. 2016, 59 (10), 4443–4458. 10.1021/acs.jmedchem.5b01409. [DOI] [PubMed] [Google Scholar]
- Haftchenary S.; Nelson S. D. Jr.; Furst L.; Dandapani S.; Ferrara S. J.; Bošković Ž. V.; Figueroa Lazú S.; Guerrero A. M.; Serrano J. C.; Crews D. K.; Brackeen C.; Mowat J.; Brumby T.; Bauser M.; Schreiber S. L.; Phillips A. J. ACS Comb. Sci. 2016, 18 (9), 569–574. 10.1021/acscombsci.6b00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congreve M.; Carr R.; Murray C.; Jhoti H. Drug Discovery Today 2003, 8 (19), 876–877. 10.1016/S1359-6446(03)02831-9. [DOI] [PubMed] [Google Scholar]
- Fandrick K. R.; Fandrick D. R.; Reeves J. T.; Gao J.; Ma S.; Li W.; Lee H.; Grinberg N.; Lu B.; Senanayake C. H. J. Am. Chem. Soc. 2011, 133 (27), 10332–10335. 10.1021/ja2028958. [DOI] [PubMed] [Google Scholar]
- Jhoti H.; Williams G.; Rees D. C.; Murray C. W. Nat. Rev. Drug Discovery 2013, 12 (8), 644–645. 10.1038/nrd3926-c1. [DOI] [PubMed] [Google Scholar]
- Gerry C. J.; Hua B. K.; Wawer M. J.; Knowles J. P.; Nelson S. D. Jr.; Verho O.; Dandapani S.; Wagner B. K.; Clemons P. A.; Booker-Milburn K. I.; Boskovic Z. V.; Schreiber S. L. J. Am. Chem. Soc. 2016, 138 (28), 8920–8927. 10.1021/jacs.6b04614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirk S. M.; Tour J. M. Tetrahedron 2003, 59 (3), 287–293. 10.1016/S0040-4020(02)01527-2. [DOI] [Google Scholar]
- Brook A. G. Acc. Chem. Res. 1974, 7 (3), 77–84. 10.1021/ar50075a003. [DOI] [Google Scholar]
- Shambayati S.; Crowe W. E.; Schreiber S. L. Tetrahedron Lett. 1990, 31 (37), 5289–5292. 10.1016/S0040-4039(00)98052-3. [DOI] [Google Scholar]
- Velcicky J.; Lanver A.; Lex J.; Prokop A.; Wieder T.; Schmalz H. G. Chem. - Eur. J. 2004, 10 (20), 5087–5110. 10.1002/chem.200400079. [DOI] [PubMed] [Google Scholar]
- Aristoff P. A.; Johnson P. D.; Harrison A. W. J. Am. Chem. Soc. 1985, 107 (26), 7967–7974. 10.1021/ja00312a028. [DOI] [Google Scholar]
- Luche J.-L. J. Am. Chem. Soc. 1978, 100 (7), 2226–2227. 10.1021/ja00475a040. [DOI] [Google Scholar]
- Thede K.; Diedrichs N.; Ragot J. P. Org. Lett. 2004, 6 (24), 4595–4597. 10.1021/ol0479904. [DOI] [PubMed] [Google Scholar]
- Paquette L. A.; Liu Z.; Ramsey C.; Gallucci J. C. J. Org. Chem. 2005, 70 (20), 8154–8161. 10.1021/jo0513919. [DOI] [PubMed] [Google Scholar]
- Plietker B.; Niggemann M. J. Org. Chem. 2005, 70 (6), 2402–2405. 10.1021/jo048020x. [DOI] [PubMed] [Google Scholar]
- Boto A.; Hernández R.; Suárez E.; Betancor C.; Rodríguez M. S. J. Org. Chem. 1995, 60 (25), 8209–8217. 10.1021/jo00130a020. [DOI] [Google Scholar]
- Díez E.; Fernández R.; Gasch C.; Lassaletta J. M.; Llera J. M.; Martín-Zamora E.; Vázquez J. J. Org. Chem. 1997, 62 (15), 5144–5155. 10.1021/jo970481d. [DOI] [Google Scholar]
- Gui J.; Pan C.-M.; Jin Y.; Qin T.; Lo J. C.; Lee B. J.; Spergel S. H.; Mertzman M. E.; Pitts W. J.; La Cruz T. E.; Schmidt M. A.; Darvatkar N.; Natarajan S. R.; Baran P. S. Science 2015, 348 (6237), 886–891. 10.1126/science.aab0245. [DOI] [PubMed] [Google Scholar]
- Lo J. C.; Yabe Y.; Baran P. S. J. Am. Chem. Soc. 2014, 136 (4), 1304–1307. 10.1021/ja4117632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman H.; Jones R. G.; Woods L. A. J. Org. Chem. 1952, 17 (12), 1630–1634. 10.1021/jo50012a009. [DOI] [Google Scholar]
- Normant J. F. Synthesis 1972, 1972 (2), 63–80. 10.1055/s-1972-21833. [DOI] [Google Scholar]
- Van Zeeland R.; Stanley L. M. ACS Catal. 2015, 5 (9), 5203–5206. 10.1021/acscatal.5b01272. [DOI] [Google Scholar]
- Gustafsdottir S. M.; Ljosa V.; Sokolnicki K. L.; Wilson J. A.; Walpita D.; Kemp M. M.; Seiler K. P.; Carrel H. A.; Golub T. R.; Schreiber S. L.; Clemons P. A.; Carpenter A. E.; Shamji A. F. PLoS One 2013, 8 (12), e80999. 10.1371/journal.pone.0080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M.-A.; Singh S.; Han H.; Davis C. T.; Borgeson B.; Hartland C.; Kost-Alimova M.; Gustafsdottir S. M.; Gibson C. C.; Carpenter A. E. Nat. Protoc. 2016, 11 (9), 1757–1774. 10.1038/nprot.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.