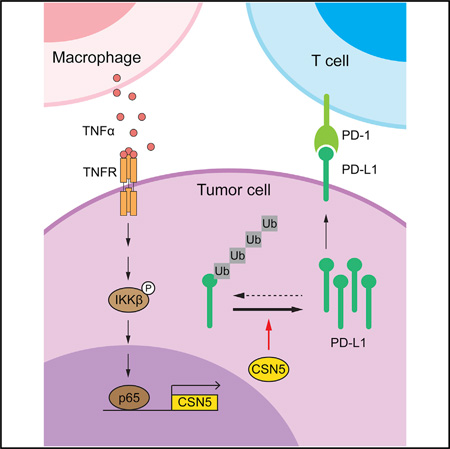
SUMMARY
Pro-inflammatory cytokines produced in the tumor microenvironment lead to eradication of anti-tumor immunity and enhanced tumor cell survival. In the current study, we identified tumor necrosis factor alpha (TNF-α) as a major factor triggering cancer cell immunosuppression against T cell surveillance via stabilization of programmed cell death-ligand 1 (PD-L1). We demonstrated that COP9 signalosome 5 (CSN5), induced by NF-κB p65, is required for TNF-α-mediated PD-L1 stabilization in cancer cells. CSN5 inhibits the ubiquitination and degradation of PD-L1. Inhibition of CSN5 by curcumin diminished cancer cell PD-L1 expression and sensitized cancer cells to anti-CTLA4 therapy.
Graphical Abstract
INTRODUCTION
Although the innate response of inflammation is critical for host immune defense, cancer cells often develop T cell resistance in the milieu of chronic inflammation, which is known as the seventh hallmark of cancer, facilitating angiogenesis, cancer cell progression, invasion, and metastasis (Colotta et al., 2009). Clinical studies have shown the risk of colorectal, esophageal, pancreatic, liver, and breast cancers is significantly increased in patients with inflammatory disease (Coussens and Werb, 2002). Thus, understanding the complex cancer cell and immune response during chronic inflammation could help us develop better treatments for cancers accompanied by inflammation.
The key upstream mediators linking inflammation to cancer include interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), nuclear factor κB (NF-κB), inducible nitric oxide synthase, cyclooxygenase 2, and hypoxia-inducible factor 1 alpha (HIF1α) (Lu et al., 2006). The pro-inflammatory cytokine TNF-α, which is mainly secreted by macrophages, promotes tumor growth by inducing cell survival (Wang et al., 2012), proliferation (Hu et al., 2004), angiogenesis (Lee et al., 2007), and epithelial-to-mesenchymal transition (Li et al., 2012) via NF-κB activation. TNF-α also activates the mammalian target of rapamycin (mTOR) (Lee et al., 2007), Hedgehog (Wang et al., 2012), AKT (Faurschou and Gniadecki, 2008), and extracellular signal-regulated kinases (ERK) (Yanagawa et al., 2002), providing diverse advantages for cell growth.
Bypassing immune surveillance by cancer cells is associated with the suppression of CD8+ T cell proliferation, cytokine release, or cytolytic activity (Krummel and Allison, 1995; Walunas et al., 1994). A major feature of immune evasion in cancer cells is the expression of multiple inhibitory ligands, notably the programmed cell death-ligand 1 (PD-L1), on the surface of cancer cells (Dong et al., 2002), dendritic cells (DCs) (Brown et al., 2003), and macrophages (Rodriguez-Garcia et al., 2011). Blocking co-inhibitory ligation using monoclonal antibodies reactivates tumor-infiltrating lymphocytes (TILs) (Brahmer et al., 2010; Sznol and Chen, 2013). Because programmed death-1 (PD-1) and PD-L1 blockade have yielded promising clinical effects, understanding the regulatory mechanism of PD-L1 may identify biomarkers and/or develop combinatorial strategies for clinical use (Pardoll, 2012). While transcriptional regulation of PD-L1 via STAT, NF-κB, or NFAT has been reported (Gowrishankar et al., 2015; Huang et al., 2013; Peng et al., 2015), it remains unclear whether and how PD-L1 is posttranscriptionally regulated.
COP9 signalosome 5 (CSN5) interacts with multiple signaling molecules, such as c-Jun, p27, migration inhibitory factor, HIF1α, Smad4, p53, and cullin1 (Bech-Otschir et al., 2001; Wan et al., 2002). CSN5 is the fifth component of the CSN complex and contains a conserved Jab1/Mpr1p and Pad1p N terminus (MPN) domain metalloenzyme (JAMM) motif. JAMM plays a critical role in CSN complex-mediated deneddylation and further regulates the activity of the Skp, Cullin, F box-containing complex (Cope et al., 2002). Ablation of CSN5 results in embryonic death, and CSN5−/− cells have an accumulation of p27, p53, and cyclin E, which results in impaired proliferation and accelerated apoptosis (Tomoda et al., 2004), suggesting that CSN5 acts as an oncogenic protein for cell survival. The COP9 signalosome also regulates the Carma1-Bcl10-Malt1 complex, a link between T cell receptor (TCR) signaling and the canonical IκB kinase/NF-κB pathway (Blonska and Lin, 2011). T cell activation triggers the interaction between CSN5 and the Carma1-Malt1 complex, and maintains Bcl10 protein stability through the COP9 signalosome, suggesting that CSN5 is a regulator of adaptive immunity activation in T cells (Welteke et al., 2009). Interestingly, CSN5 also possesses deubiquitination activity. For instance, deubiquitination of HSP70 and Snail by CSN5 were reported to modulate exosomal protein sorting (Liu et al., 2009) and to enhance cancer cell invasion and migration (Wu et al., 2009), respectively. Recently, phosphorylation of CSN6 by ERK was found to stabilize β-catenin for colon cancer cell proliferation (Fang et al., 2015). These results demonstrated that the deubiquitination activity of the CSN family is crucial for cancer progression.
In the current study, we investigate the effects of chronic inflammation on deubiquitinase CSN5 and inflammation-induced immunosuppression, with particular focus on PD-L1, and assess the therapeutic potential of CSN5 inhibition in basal-like breast cancer.
RESULTS
Macrophage-Secreted Inflammatory Cytokines Upregulate PD-L1 Protein Expression
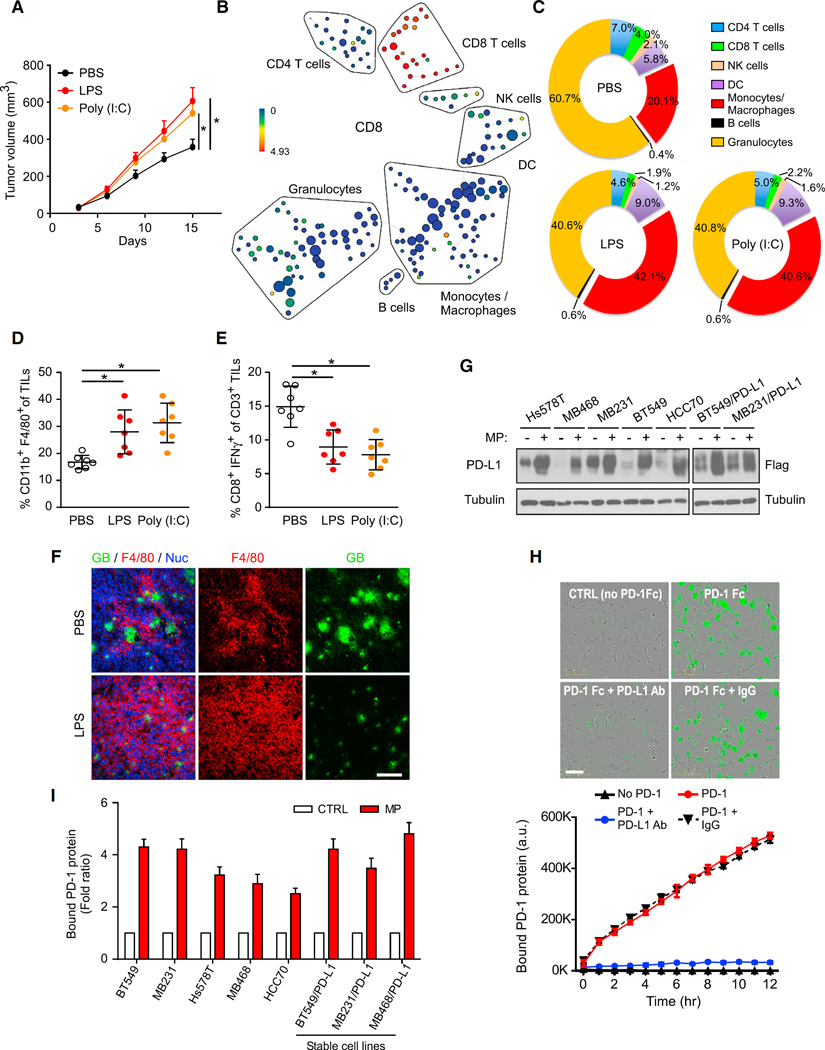
Cancer cells escape from immune surveillance by disrupting the balance between innate and adaptive immunity, both of which are influenced by factors that are produced from infiltrated macrophages in response to chronic inflammation in the vicinity of a cancerous lesion. We first established an inflammation-enhanced tumor model to study the mechanisms underlying inflammation-mediated anti-tumor immunity. Immunocompetent BALB/c mice carrying 4T1 cells were first challenged with lipopolysaccharide (LPS) or polyinosinic-polycytidylic acid (poly(I:C)) (Luo et al., 2004) to induce inflammation. As expected, LPS and poly(I:C) treatment enhanced 4T1 tumor growth in mice (Figure 1A), and the total number of TILs was increased (Figure S1A). When depleted of CD8+ T cells by either CD8 antibody or using Rag1 knockout mice (Mombaerts et al., 1992), both LPS and poly(I:C) failed to induce significant tumor growth (Figure S1B). To analyze the TIL population systematically, we used mass cytometry (CyTOF) to dissect the immunophenotype of TILs from 4T1 tumors by employing an 11-marker antibody panel (Supplemental Experimental Procedures). Figure 1B shows a CD8 marker expression in each subtype of TILs. CyTOF analysis showed a significant increase in monocytes and macrophages (CD11b+/Gr-1−) in mice treated with either LPS or poly(I:C) compared with mice treated with PBS (Figure 1C), implying that monocytes and macrophages contributed to the overall increase of the TIL population by LPS or poly(I:C) treatment. Although the number of TILs and macrophages (CD11b+/F4/80+) was increased by inflammatory stimuli (Figures 1D and S1A), the cytotoxic T cell activity in the tumors was decreased (Figure 1E). Results from granzyme B immunostaining, which indicates cytotoxic T cell activity, also supported the idea that macrophage (F4/80+) infiltration was increased but cytotoxic T cell activity was decreased after LPS treatment (Figure 1F), suggesting that cancer cells launch a defense mechanism against T cell surveillance in response to inflammation which enhances tumor development.
Figure 1. Macrophage-Secreted Inflammatory Cytokines Upregulate Expression of PD-L1 Protein.
(A) Tumor growth of 4T1 cells in BALB/c mice following treatment with lipopolysaccharide (LPS), polycytidylic acid (poly(I:C)), or PBS was measured at the indicated time points and dissected at the endpoint (n = 7 mice per group).
(B) SPADE tree derived from CyTOF (11-marker) analysis of tumor-infiltrating lymphocytes (TILs) from 4T1 tumors. Cell populations were identified as CD4 T cells (CD45+CD3+TCRβ+CD4+), CD8 T cells (CD45+CD3+TCRβ+CD8+), natural killer (NK) cells (CD45+NK1.1+), dendritic cells (DC; CD45+CD11c+), monocytes and macrophages (CD45+CD11b+Gr1−), B cells (CD45+B220+CD19+), and granulocytes (CD45+CD11b+Gr1+). The tree is colored by the intensity of CD8 marker shown to highlight CD8+ T cells.
(C) Percentages of immune cell populations within CD45+ TILs from 4T1 tumors, assessed with CyTOF (11-marker) and analyzed with SPADE (n = 3).
(D) Macrophage (CD11b+F4/80+) population in CD45+ TILs from 4T1 tumors (n = 7). Results are presented as mean ± SD from one representative experiment.
(E) Intracellular cytokine staining of IFN-γ+CD8+ in CD3+ T cell populations from 4T1 tumors (n = 7). Results are presented as mean ± SD from one representative experiment.
(F) Immunofluorescence staining of the protein expression pattern of F4/80 and granzyme B (GB) in 4T1 tumors (Nuc, nuclear). Scale bar, 100 µm.
(G) Western blot analysis of PD-L1 expression in various cancer cells treated with macrophage-conditioned medium (MP) for 12 hr.
(H) Time-lapse microscopy image showing the association of cancer cells with PD-1 at the 12-hr time point. Green fluorescent (green fluorescent-labeled PD-1/Fc protein) merged images of PD-L1-expressing cells are shown at the top, and a kinetic graph showing quantitative binding of PD-1/Fc protein on BT459 cells expressing PD-L1 at hourly time points is shown at the bottom. Scale bar, 100 µm.
(I) PD-1 binding affinity of various cancer cell lines examined using the PD-L1/PD-1 binding assay with MP or regular culture medium as a control.
*p < 0.05, Student’s t test. Error bars represent SD of three independent experiments.
See also Figure S1.
Because PD-L1 expression on cancer cells highjacks cytotoxic T cell activation via PD-1 engagement, we sought to determine whether inflammation-induced T cell resistance occurred via PD-L1 regulation. We measured the level of PD-L1 expression in breast cancer cell lines in response to LPS-treated pro-inflammatory cytokine-enriched macrophage-conditioned medium (MP). MP significantly increased the expression of PD-L1 protein but not mRNA expression in multiple breast cancer cell lines (Figures 1G and S1C). The protein expression of other immune inhibitory ligands, such as B7-H3 and poliovirus receptor (PVR), remained similar when cells were treated with MP (Figure S1D), indicating that MP selectively induced protein expression of PD-L1, but not other immune inhibitory ligands in cancer cells.
Next, to determine whether MP-treated cancer cells enhanced their ability to bind to PD-1, we developed a live cell imaging approach to re-create the dynamic interaction between PD-L1 and PD-1 in vitro. To avoid the influence from basal-level PD-L1, we first knocked down endogenous PD-L1 in BT549 cells using short hairpin RNA (shRNA) and then reconstituted it with a Flag-PD-L1, yielding BT549/PD-L1 cells (Figure S1E, vector design). Cells were then incubated with recombinant PD-l Fc protein together with green fluorescence (Alexa 488)-conjugated human Fc antibody (Figure S1F). The interaction of PD-L1 and PD-1 on the membrane of live cells was continuously increased during a 12-hr period by real-time quantitation of time-lapse images (Figure 1H). In the presence of PD-L1 or PD-1 neutralizing antibodies, the PD-L1/PD-1 interaction was disrupted (Figures 1H and S1G). A dose-dependent titration of neutralizing antibodies further confirmed the specificity of the assay (Figure S1G). Similar to the MP-induced PD-L1 protein expression (Figure 1G), MP also increased PD-1 binding (Figures 1I and S1H). MP treatment enhanced PD-L1 protein, but not PD-L1 mRNA expression (Figure S1C), suggesting it is not a transcriptional event. Consistently, stable expression of Flag-PD-L1 in BT549, MDA-MB231 (MB231), and MDA-MB468 (MB468) cells in which PD-L1 was driven by cytomegalovirus (CMV) promoter, but not endogenous promoter also increased the expression of exogenous Flag-PD-L1 (Figure 1G, right, data not shown) and the PD-1 binding ability of those cells (Figure 1I, right) in response to MP treatment. Taken together, these results suggested that macrophage-secreted inflammatory cytokines upregulate PD-L1 protein expression via a non-transcriptional regulatory mechanism such as posttranslational regulation.
TNF-α Induces Cancer Immunosuppression via PD-L1 Stabilization
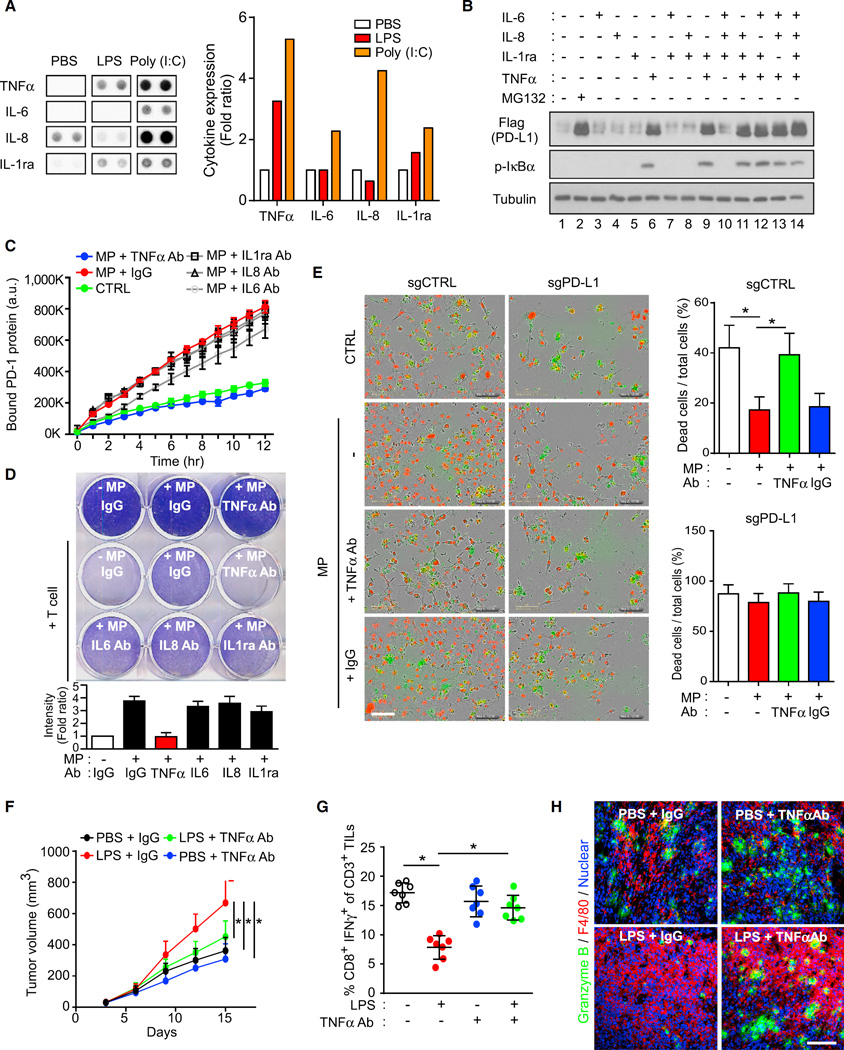
To ascertain which major macrophage-secreted inflammatory cytokines are involved in PD-L1 upregulation, we treated macrophages with LPS or poly(I:C) for 2 hr and then analyzed the cytokine expression in the MP. Both LPS and poly(I:C) increased TNF-α, IL-6, IL-8, or interleukin-1 receptor antagonist (IL-1ra) expression in the MP according to cytokine array analysis (Figures 2A and S2A). MG132 treatment significantly increased PD-L1 protein level (Figure 2B, lane 2). When BT549/PD-L1 cells were treated with TNF-α, IL-6, IL-8, and IL-1ra for 8 hr, only TNF-α was able to induce PD-L1 expression to a degree similar to that observed in cells treated with MG132 (Figure 2B, lanes 2 and 6). No cooperative effects were observed when combining TNF-α with other stimulation (Figure 2B, lanes 9 and 11–14). Meanwhile, PD-L1 mRNA expression did not change significantly (Figure S2B). In addition, levels of TNF-α, but not interferon gamma (IFN-γ), was significantly increased in 4T1 tumor tissues upon LPS or poly(I:C) treatment (Figure S2C). These data suggested that TNF-α is the major inflammatory cytokine in MP-induced PD-L1 stabilization.
Figure 2. TNF-α Induces Cancer Immunosuppression via PD-L1 Stabilization.
(A) Analysis of cytokines secreted from LPS-, poly(I:C)-, or PBS-treated macrophages. Representative images are shown on the left and quantification of TNF-α, IL-6, IL-8, and IL-1ra is shown on the right.
(B) PD-L1 analyzed by Flag antibody in BT549/PD-L1 cells serum-starved overnight and then treated with the indicated cytokines (10 ng/mL for each cytokine) for 8 hr.
(C) PD-l binding assay in BT459/PD-L1 cells treated with MP and neutralization antibodies for the indicated cytokines.
(D) T cell-meditated tumor cell killing assay in BT459/PD-L1 cells treated with MP and neutralization antibodies for the indicated cytokines. Activated T cell and BT549/PD-L1 cells were co-cultured in 12-well plates for 4 days and then surviving tumor cells were visualized by crystal violet staining. Relative fold ratios of surviving cell intensities are shown.
(E) T cell-meditated tumor cell killing assay in PD-L1 knockout (PD-L1 KO) BT549 cells. Representative phase, red fluorescent (nuclear restricted RFP), and green fluorescent (NucView 488 caspase-3/7 substrate) merged images of activated T cell co-cultures in the presence of caspase-3/7 substrate at 96 hr are shown. T cells were activated with CD3 antibody (100 ng/mL) and IL-2 (10 ng/mL). Green fluorescent cells were counted as dead cells. The quantitative ratio of dead cells is shown in the bar graph on the right. Scale bar, 100 µm.
(F) Tumor growth of 4T1 cells in BALB/c mice following treatment with LPS or TNF-α antibody. Tumor growth was measured at the indicated time points and dissected at the endpoint (n = 7 mice per group).
(G) Intracellular cytokine staining of CD8+ IFN-γ+ in CD3+ T cell populations from isolated TILs. Results are presented as mean ± SD from one representative experiment.
(H) Immunofluorescence staining of the protein expression pattern of F4/80 and granzyme B in 4T1 tumor masses. Scale bar, 100 µm.
*p < 0.05, Student’s t test. Error bars represent SD of three independent experiments.
See also Figure S2.
To further validate the role of TNF-α in PD-L1-mediated immunosuppression, we used TNF-α antibody to neutralize TNF-α in MP. Only TNF-α antibody, but not other antibodies, e.g., IL-8 and IL-1ra, inhibited MP-induced PD-L1 stabilization (Figure S2D and data not shown), PD-1 binding (Figures 2C and S2E), and T cell-mediated cancer cell killing (Figure 2D). We also established two PD-L1 knockout cells by CRISPR/Cas9-sgRNA in BT549 and MB231. PD-L1 was depleted from these cells, whereas the expression of other tumor ligands, including B7-H3 and PVR, remained similar (Figure S2F). Of note, the T cell-mediated killing of BT549 cells was significantly suppressed by MP treatment (Figure 2E). The addition of TNF-α antibody, but not the control immunoglobulin G, reversed the effect of MP (Figure 2E). However, BT549 and MB231 cells with PD-L1 depletion did not respond to either MP or TNF-α antibody (Figures 2E and S2G), suggesting that PD-L1 expression is required for MP-secreted TNF-α-mediated suppression of T cell activity. We next examined the role of TNF-α in 4T1 and EMT6 syngeneic BALB/c mouse models. Both TNF-α antibody (Figures 2F, S2H, and S2I) and clodronate liposome (a liposome for macrophage depletion; CL in Figure S2J) but not control liposome (PL)-attenuated LPS-induced 4T1 and EMT6 tumor growth and the suppression of cytotoxic T cells as measured by fluorescence-activated cell sorting (FACS) (Figure 2G). In addition, IFN-γ antibody also inhibited LPS-induced 4T1 tumor growth, but TNF-α antibody more effectively inhibited tumor growth than did the IFN-γ antibody (Figure S2K). Thus, TNF-α is the major cytokine that mediated 4T1 immunosuppression in response to LPS. In the 4T1 tumor tissues sections, LPS increased macrophages (F4/80, red) and decreased T cell activity (granzyme B, green). In the presence of TNF-α antibody, LPS-induced T cell suppression was compromised (Figure 2H). Together, these results suggested that TNF-α secreted by macrophages in response to inflammation may stabilize PD-L1 and enhance tumor growth in immunocompetent mice.
p65 Activation Regulates TNF-α-Mediated PD-L1 Stabilization
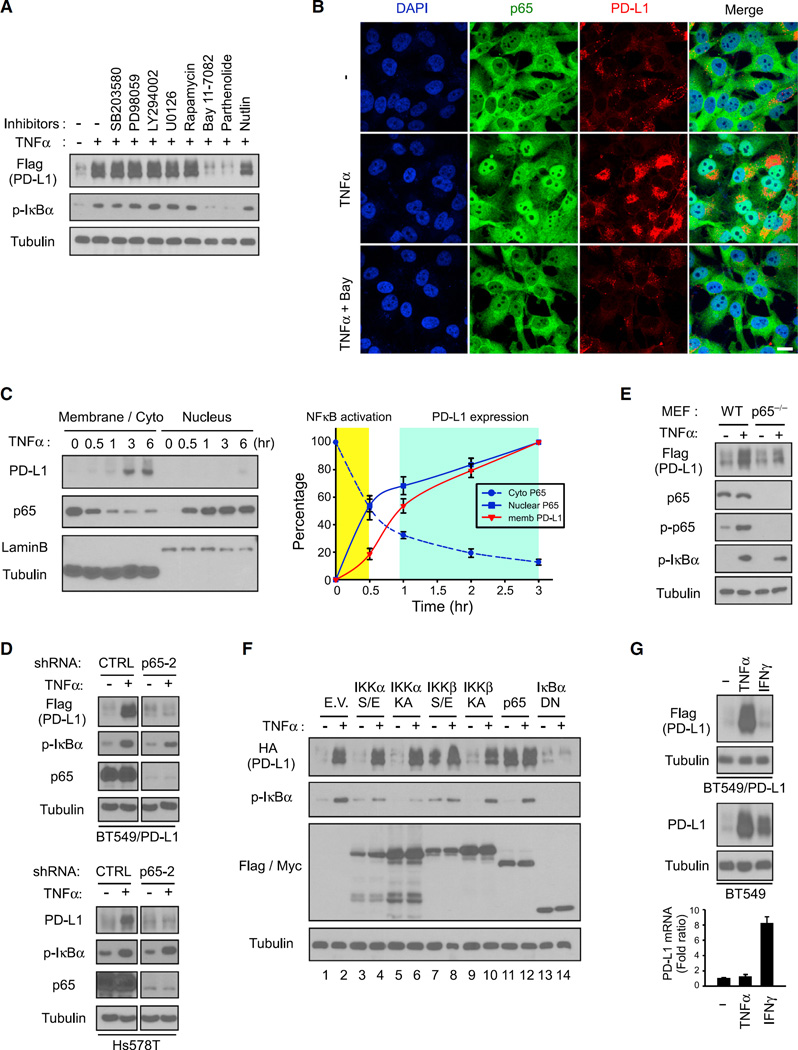
Because TNF-α activates several signaling pathways, including NF-κB, AKT, ERK, and mTOR/S6K1, it is of interest to pinpoint which signaling cascade is involved in TNF-α-mediated PD-L1 stabilization. To this end, we applied several inhibitors and found that only IκB kinase β (IKKβ) inhibitor BAY 11–7082 and NF-κB inhibitor parthenolide, but not other kinase inhibitors, e.g., SB203580, PD98059, LY294002, U0126, rapamycin, and nutlin, abolished TNF-α-induced PD-L1 stabilization (Figure 3A). Similarly, PD-L1 expression was significantly increased when reintroducing IKKβ by stable transfection in low IKKβ-expressing MCF7 and MDA-MB-453 cells (Lee et al., 2007) (Figure S3A). IKKβ gene knockout mouse embryonic fibroblasts (MEFs) no longer responded to the TNF-α-mediated PD-L1 stabilization (Figure S3B), suggesting IKKβ kinase activity and NF-κB may be involved in TNF-α-mediated PD-L1 stabilization (Figure 3A).
Figure 3. p65 Activation Regulates TNF-α-Mediated PD-L1 Stabilization.
(A) Exogenous PD-L1 expression determined by western blot analysis with the Flag antibody in BT549/PD-L1 cells pretreated with various inhibitors for 45 min, followed by treatment with TNF-α for 8 hr.
(B) Confocal microscopy image showing protein expression of p65 and PD-L1 after treatment with TNF-α in BT549 cells. Bay, Bay 11–7082. Scale bar, 20 µm.
(C) Nuclear translocation of p65 analyzed at the indicated time points using cell fractionation in BT549 cells treated with TNF-α. Protein expression over time is shown in the graph on the right. Error bars are expressed as mean ± SD of three independent experiments.
(D) Western blot analysis of PD-L1 in p65 knockdown clones. Exogenous PD-L1 in BT549/PD-L1 cells was detected by Flag antibody.
(E) Western blot analysis of wild-type (WT) and p65−/− mouse embryonic fibroblasts serum-starved overnight prior to 8 hr of treatment with TNF-α.
(F) Expression of PD-L1, IKKα, IKKβ, IκBα, and p65 analyzed using western blot analysis. Flag-tagged IKKα/β, IκBα, or Myc-tagged p65 was expressed in HA-PD-L1-expressing BT549 cells. E.V., empty vector; S/E, constitutively active; KA, kinase-dead; DN, dominant-negative.
(G) Top, western blot analysis of PD-L1 in TNF-α- or IFN-γ-treated BT549 cells. Exogenous PD-L1 in BT549/PD-L1 cells was detected by the Flag antibody. Bottom, qRT-PCR analysis of PD-L1 mRNA expression. Error bars represent SD of three independent experiments.
See also Figure S3.
The nuclear translocation and downstream transactivation of p65 indicate NF-κB activation. Hence, we sought to determine whether p65 activation is required for TNF-α-mediated PD-L1 stabilization. As expected, TNF-α induced nuclear translocation of p65 and increased PD-L1 expression (Figures 3B and S3C). To further dissect these dynamics, we isolated nuclear and membrane or cytoplasmic fractions of BT549 cells at different time points during TNF-α treatment. TNF-α-induced nuclear translocation of p65 was first found at 30 min after the start of treatment, whereas stabilization of PD-L1 began after 3 hr of treatment with TNF-α (Figure 3C). To determine whether p65 is essential for PD-L1 stabilization, we knocked down p65 by shRNA in both BT549/PD-L1 and Hs578T cells and found that downregulation of p65 attenuated TNF-α-induced PD-L1 stabilization (Figure 3D). Similarly, TNF-α failed to induce PD-L1 stabilization in p65−/− MEFs (Figure 3E).
To better delineate NF-κB activation and PD-L1 expression, we expressed constitutively active or kinase-dead IKKα, IKKβ, and p65 or dominant-negative IκBα in BT549/PD-L1 cells and then treated the cells with TNF-α. Expression of both constitutively active IKKβ (Figure 3F, lane 7) and p65 (Figure 3F, lane 11) was sufficient to induce PD-L1 stabilization to a degree similar to that observed after treatment with TNF-α. In contrast, blocking NF-κB activation with dominant-negative IκBα attenuated PD-L1 expression (Figure 3F, lane 14). Similarly, p65-mediated PD-L1 membrane localization by TNF-α was attenuated by dominant-negative IκBα (Figure S3D). To establish clinical relevance to potentially target NF-κB-mediated PD-L1 stabilization, we pretreated BT549 cells with natural dietary anti-inflammatory supplements that are known to inhibit NF-κB prior to TNF-α stimulation. Emodin, curcumin, aspirin, and resveratrol reduced TNF-α-mediated PD-L1 stabilization (Figure S3E) and PD-1 binding (Figure S3F), resulting in enhanced T cell-mediated tumor cell killing (Figure S3G). These results suggested that p65 activation regulates TNF-α-mediated PD-L1 stabilization. Unlike IFN-γ-induced PD-L1 via transcription activation (Zou et al., 2016), the action of TNF-α-mediated PD-L1 induction was primarily at the posttranslational level as TNF-α did not influence PD-L1 mRNA expression (Figure 3G). Although both TNF-α and IFN-γ induced endogenous PD-L1 at a similar level (Figure 3G, middle panel), the exogenous PD-L1 (Flag-PD-L1, detected by Flag antibody), which was driven by a CMV promoter, was induced by TNF-α but not IFN-γ (Figure 3G, upper panel), further supporting the differential mechanism by which TNF-α and IFN-γ enhance PD-L1 expression.
TNF-α Upregulates CSN5 Expression to Stabilize PD-L1
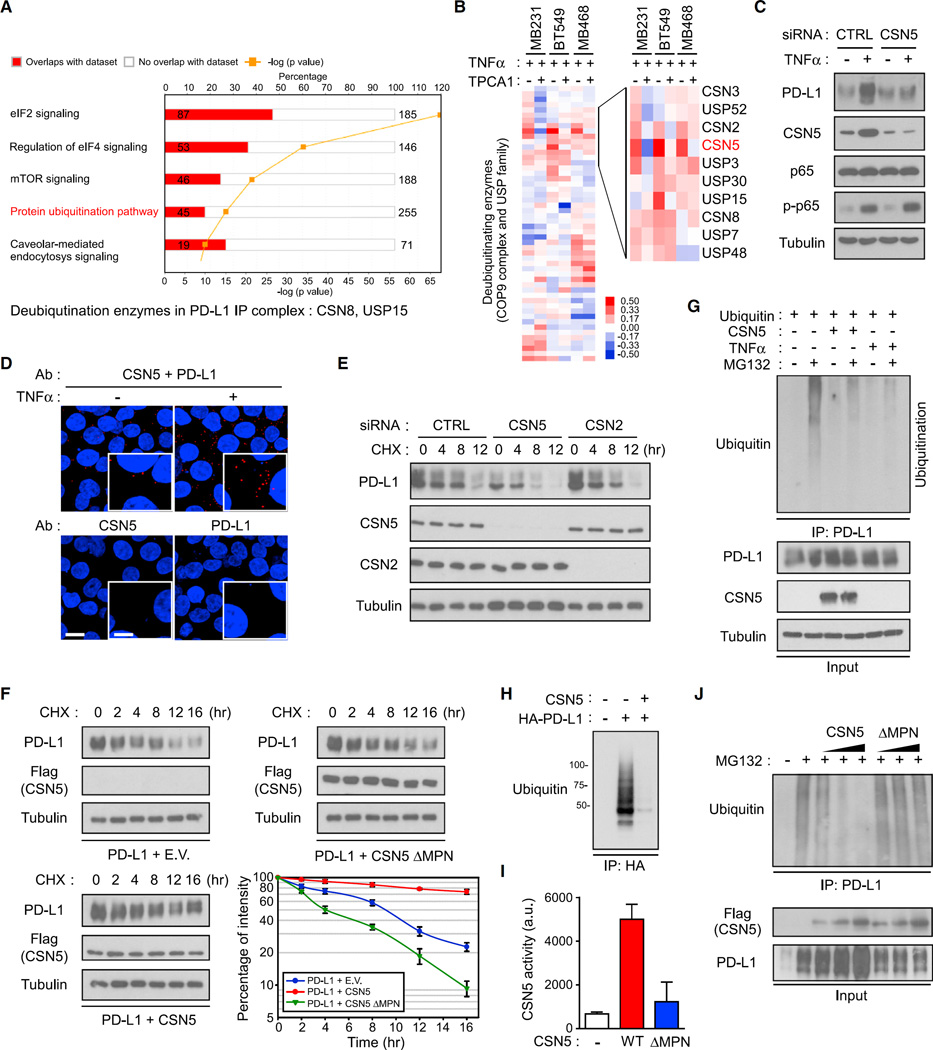
To identify the regulatory factor controlling TNF-α-mediated PD-L1 stabilization, we first analyzed the PD-L1 binding proteins using liquid chromatography-tandem mass spectrometry analysis and ingenuity pathway analysis (Figures 4A and S4A). Among the top five canonical pathways, only one was related to protein stability: the protein ubiquitination pathway (Figure 4A). Of the 930 binding proteins identified, two deubiquitinating enzymes, CSN8 (a subunit of COP9 signalosome) and ubiquitin-specific peptidase 15 (USP15), were found to associate with PD-L1 (Figure 4A). To determine whether TNF-α-induced PD-L1 stabilization may be regulated by deubiquitination, we searched for expression of deubiquitinating enzymes that responded to TNF-α by performing a PCR array analysis, focusing on CSN and the USP protein family. Among the 52 deubiquitinating enzymes identified, CSN5, which possesses enzyme activity in the COP9 signalosome, was robustly expressed in response to TNF-α treatment in three cell lines examined (Figure 4B). Downregulation of CSN5 reduced TNF-α-induced PD-L1 expression (Figure 4C), indicating that CSN5 is required for PD-L1 stabilization. Moreover, endogenous CSN5 and PD-L1 proteins formed a strong interaction as observed in Duolink II assay in vivo (Figure 4D) and co-immunoprecipitation assay in vitro (Figure S4B). In-depth analysis showed that PD-L1 selectively bound to CSN5 in the full-length and C-terminal regions (Figures S4C and S4D). Half-life analysis using cycloheximide pulse-chase analysis indicated that downregulation of CSN5, but not CSN2, which has been shown to destabilize Snail (Wu et al., 2009), reduced PD-L1 expression as well as its protein half-life (Figure 4E). Consistently, overexpression of wild-type CSN5 but not MPN domain deletion (ΔMPN) CSN5 stabilized PD-L1 expression (Figure 4F), suggesting that CSN5 enzyme activity is involved in PD-L1 stabilization.
Figure 4. CSN5 Induces PD-L1 Stabilization via PD-L1 Deubiquitination.
(A) PD-L1 binding proteins analyzed by liquid chromatography-tandem mass spectrometry and ingenuity pathway analysis. Flag-PD-L1 protein was immune-precipitated by Flag antibody-conjugated (M2) agarose resin and eluted by Flag peptide. The 930 identified PD-L1 binding proteins were analyzed by ingenuity pathway analysis.
(B) qRT-PCR analysis of deubiquitinating enzymes in the USP family and COP9 signalosome genes in MB231, BT549, and MB468 cells. The heatmap was generated using TreeView. TPCA1, IKKβ inhibitor.
(C) Western blot analysis of PD-L1 expression in CSN5-knockdown cells after treatment with TNF-α. CTRL, control small interfering RNA (siRNA).
(D) Interaction of endogenous CSN5 and PD-L1 proteins in BT549 cells. Cells were immunostained with CSN5 and PD-L1 antibodies and assessed using the Duolink II assay. Red foci indicate association between PD-L1 and CSN5 proteins. Scale bar, 20 µm. Inset scale bar, 10 µm.
(E) Protein stability of PD-L1 proteins in BT549/PD-L1 cells. Cells were treated with 20 µMcycloheximide (CHX) at indicated intervals and analyzed in western blot analysis.
(F) Protein stability of PD-L1 proteins in HEK293T cells. Cells were transfected with HA-PD-L1 and WT Flag-CSN5 or MPN deletion (ΔMPN) mutant and analyzed in western blot analysis. E.V., empty vector.
(G) Ubiquitination assay of PD-L1 in response to treatment with TNF-α. HEK293 cells were transiently transfected with the indicated constructs. Ubiquitinated PD-L1 was immunoprecipitated (IP) and subjected to western blot analysis with the ubiquitin antibody. Cells were treated with MG132 or TNF-α prior to ubiquitination analysis.
(H) In vitro PD-L1 deubiquitination assay. Purified PD-L1, CSN5, E1, E2, and ubiquitin were incubated for 2 hr prior to western blot analysis.
(I) CSN5 activity in an in vitro deubiquitination assay. The activity was measured by AMC released from the fluorogenic substrate, ubiquitin-AMC.
(J) HEK293 cells were transiently transfected with the indicated plasmids. PD-L1 ubiquitination was analyzed by western blot analysis. Error bars represent SD of three independent experiments.
See also Figure S4.
Next we asked whether CSN5 deubiquitinates PD-L1 to increase protein stabilization. Because protein degradation is accompanied by ubiquitin K48 chain ubiquitination, we analyzed PD-L1 ubiquitination in the presence of TNF-α or MG132 and found that MG132-induced PD-L1 ubiquitination (Figure S4E) was abolished by CSN5 overexpression or TNF-α treatment (Figure 4G), but not by the two other most well-known deubiquitinating enzymes in NF-κB signaling, CYLD and A20 (Figure S4F). Because CSN5 possesses deneddylation or deubiquitination activity (Echalier et al., 2013; Liu et al., 2009), we next tested whether CSN5 could serve as a deubiquitination enzyme for PD-L1. To assess this in vitro, we incubated purified HA-PD-L1 and Flag-CSN5 with E1, E2, ubiquitin, and ATP for 2 hr (Figure S4G). Ubiquitinated PD-L1 was then pulled down by an HA-tag and subjected to western blot analysis. Although PD-L1 had notable basal ubiquitination, CSN5 abolished ubiquitination of PD-L1 (Figure 4H). Purified CSN5, but not CSN5 ΔMPN, hydrolyzed ubiquitin from ubiquitin-7-amido-4-methylcoumarin (AMC), a fluorogenic substrate for ubiquitin hydrolases (Figure 4I), and ubiquitin-PD-L1 (Figure 4J) in a dose-dependent manner. The results suggested that TNF-α upregulates expression of CSN5, which interacts and deubiquitinates PD-L1 for protein stabilization.
Transcriptional Activation of CSN5 by p65 Is Required for TNF-α-Mediated PD-L1 Stabilization
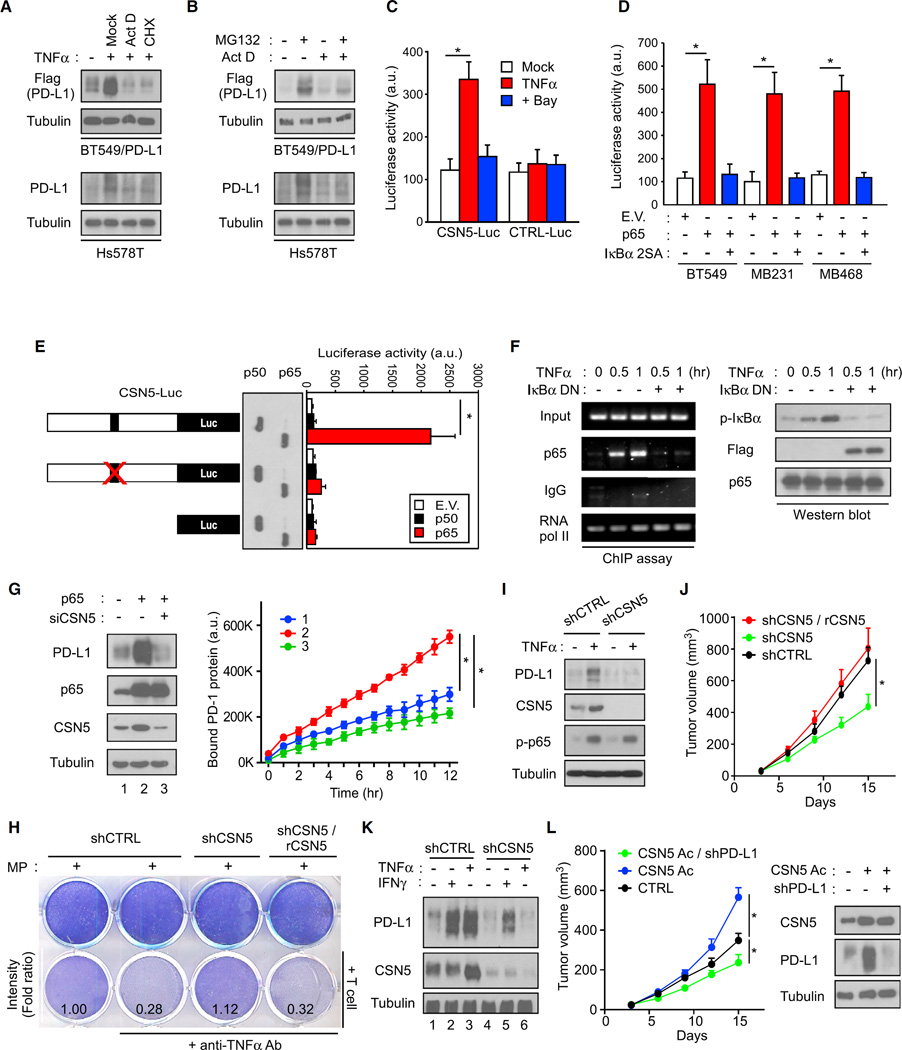
To determine how CSN5 contributes to TNF-α-mediated PD-L1 stabilization, we asked whether p65 activation transcriptionally regulates CSN5 expression. The protein synthesis inhibitor cycloheximide reasonably attenuated TNF-α-mediated PD-L1 expression (Figure 5A). Interestingly, the transcription inhibitor actinomycin D was also sufficient to block both TNF-α- and MG132-mediated PD-L1 expression (Figures 5A and 5B), suggesting that stabilization of PD-L1 by TNF-α may require p65-mediated transcriptional activation. In addition to PD-L1 in cancer cells, PD-L1 in myeloid DC and T cells was also found to be upregulated via posttranslational events (Figures S5A and S5B). Because activation of NF-κB allows p65 to associate with the NF-κB binding site for transcriptional activation, we sought to determine whether p65 regulates the COPS5 promoter (COPS5 encodes CSN5). We analyzed the ENCODE database of transcription factors using chromatin immunoprecipitation (ChIP) sequencing (such as TF ChIP-seq Uniform Peaks from ENCODE/USC/Analysis) and showed that p65 directly interacts with the COPS5 core promoter region after treatment with TNF-α (Figure S5C). In two shRNA p65 knockdown stable clones, CSN5 expression was reduced (Figure S5D). In a reporter assay, COPS5-promoter-luciferase (CSN5-Luc) responded to TNF-α stimulation. An IKKβ small-molecular inhibitor, Bay 11–7082 (Bay), reduced TNF-α-mediated CSN5 transactivation (Figure 5C). In addition, p65 stimulated CSN5 activation, whereas a dominant-negative IκBα mutant (IκBα 2SA) abolished p65-induced COPS5 promoter activation (Figure 5D).
Figure 5. Transcriptional Activation of CSN5 by p65 Is Required for TNF-α-Mediated PD-L1 Stabilization.
(A) PD-L1 expression detected by the Flag antibody in BT549/PD-L1 and Hs578T cells pretreated with actinomycin D (Act D) or cycloheximide (CHX) for 5 hr.
(B) PD-L1 expression detected by the Flag antibody in BT549/PD-L1 and Hs578T cells pretreated with Act D or MG132 for 5 hr.
(C) Luciferase activity measured and normalized according to Renilla luciferase activity in BT549 cells transiently transfected with the COPS5 luciferase promoter (CSN5-Luc).
(D) CSN5-Luc promoter analysis in BT549, MB231, and MB468 cells transfected with p65 or IκBα 2SA. E.V., empty vector.
(E) CSN5-Luc and p65 binding site-mutated CSN5-Luc activity in response to p65 or p50 expression.
(F) Chromatin immunoprecipitation assay analyzing the binding of p65 to the COPS5 promoter. Mouse immunoglobulin G (IgG) was used as a negative control. RNA polymerase II was used as a positive control. DN, dominant-negative.
(G) Western blot analysis of PD-L1 expression in p65-overexpressed and CSN5-knockdown cells (left). Results of the PD-1/PD-L1 binding assay (right).
(H) T cell-meditated tumor cell killing assay in macrophage-conditioned medium (MP-treated control (shCTRL), CSN5 knockdown (shCSN5), or CSN5 reconstituted (shCSN5/rCSN5) BT549 cells. Activated T cell and BT549 cells were co-cultured in 12-well plates for 4 days and the surviving tumor cells were visualized by crystal violet staining. Relative fold ratios of surviving cell intensity are shown.
(I) Western blot analysis of PD-L1 and CSN5 expression in TNFα-treated shCTRL or shCSN5 cells.
(J) Tumor growth of shCTRL, shCSN5, or shCSN5/rCSN5 4T1 cells in LPS-treated BALB/c mice. Tumor growth was measured at the indicated time points and dissected at the endpoint (n = 7 mice per group).
(K) Western blot analysis of PD-L1 and CSN5 expression in CSN5 knockdown, re-expression cells.
(L) Tumor growth of endogenous CSN5 overexpressing (CSN5 Ac; by CSN5 CRISPR activation) or CSN5 overexpressing and PD-L1 knockdown (CSN5 Ac/shPD-L1) 4T1 cells in BALB/c mice (left). CTRL, parental 4T1 cells. Tumor growth was measured at the indicated time points and dissected at the endpoint (n = 7 mice per group). Western blot analysis of PD-L1 and CSN5 expression in isolated 4T1 tumor cells (right).
*p < 0.05, Student’s t test. Error bars represent SD of three independent experiments.
See also Figure S5.
To pinpoint the exact p65 binding sites, we analyzed the COPS5 promoter sequence from −1,032 to +1 and found that this sequence contained one p65 binding site in both program predictions (Figure S5E). p65, but not its heterodimeric partner p50, stimulated CSN5 activation through the p65 binding site, and mutation of the binding site on the COPS5 promoter abrogated p65-mediated COPS5 promoter activity (Figure 5E). In terms of the binding dynamics, we found that p65 bound to the COPS5 promoter as early as after 30 min TNF-α treatment by ChIP assay (Figure 5F). CSN5 mRNA expression peaked at about 1 hr following TNF-α treatment (Figure S5F). These results suggested that p65 binds to the COPS5 gene promoter to transcriptionally upregulate its expression.
To validate a role of CSN5 in PD-L1 regulation, we performed a PD-L1/PD-1 interaction assay (Figure 5G) and T cell killing assay (Figure 5H) and found that CSN5 was functionally required for TNF-α- or p65-mediated PD-L1 regulation (Figure 5I). Downregulation of CSN5 also significantly attenuated LPS-induced tumor growth and PD-L1 expression (Figures 5J and 5K). Moreover, the tumor growth activity of CSN5 required PD-L1 because downregulation of PD-L1 attenuates CSN5-mediated tumorigenesis in BALB/c mice (Figure 5L). Together with the positive correlation between p65 and CSN5 found in the Cancer Cell Line Encyclopedia (Figure S5G), these results suggested that TNF-α-activated p65 transcriptionally upregulates CSN5 expression, which is required for TNF-α-mediated PD-L1 stabilization and related functions.
Since TNF-α and IFN-γ upregulated PD-L1 via separate mechanisms (Figure 3G), we asked whether CSN5 is also involved in IFN-γ-mediated PD-L1 expression. While mRNA level remains the same, the addition of TNF-α indeed enhanced more PD-L1 protein expression (Figure S5H). In addition, PD-L1 protein degraded faster than its corresponding mRNA upon IFN-γ withdrawal from the medium, suggesting that the basal-level CSN5 stabilizes IFN-γ-induced PD-L1 (Figure S5I). We next analyzed PD-L1 expression in CSN5 knockdown cells and found that TNF-α upregulated both CSN5 and PD-L1 protein levels and that CSN5 expression was required for the TNF-α-induced PD-L1 protein expression (Figures 5I and 5K). In contrast, IFN-γ did not induce CSN5 expression. Compared with CSN5 intact cells, IFN-γ-induced less PD-L1 protein expression in low CSN5-expressing cells (lanes 2 and 5, Figure 5K). These results suggested that although IFN-γ does not upregulate CSN5 to trigger PD-L1 stabilization, the basal-level CSN5 also stabilizes PD-L1 that has been induced by IFN-γ.
Correlations of p-p65, CSN5, PD-L1, and Granzyme B Expression in Human Tumor Tissues
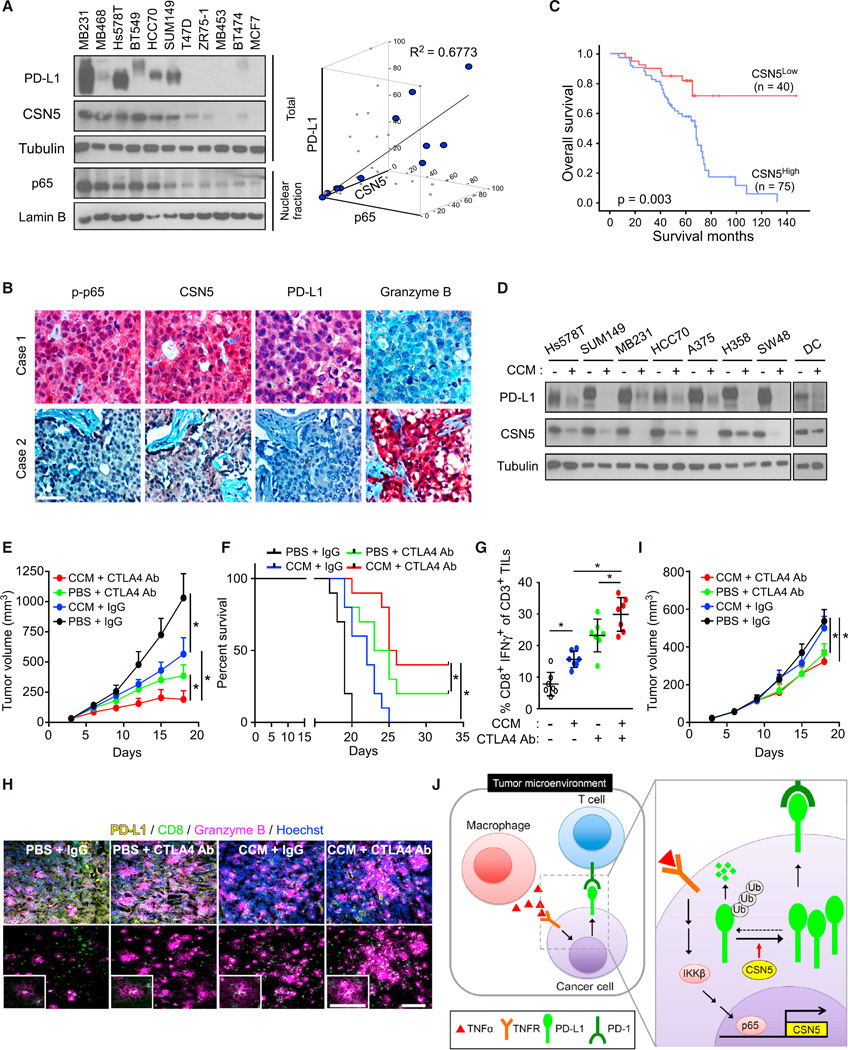
To further validate our findings in human cancer patient samples, we analyzed the correlations between nuclear p65, CSN5, and PD-L1 expression in breast cancer cell lines and human breast tumor specimens. We found that PD-L1 protein levels were highly correlated with nuclear p65 or CSN5 expression (Figures 6A and S6A). To further validate the pathologic relevance of the identified mechanism, we studied the expression of phospho-p65 (p-p65), CSN5, PD-L1, and granzyme B in 261 human breast tumor specimens using immunohistochemical staining. CSN5 was detected in 98 (60.1%) of the 163 specimens with high PD-L1 expression but in only 28 (32.9%) of the specimens with low PD-L1 expression, indicating that there is a positive correlation between CSN5 and PD-L1 expression (p = 0.0001). In addition, we found that CSN5 expression was positively correlated with p-p65 expression (p = 0.0001) but inversely correlated with granzyme B expression (p = 0.002; Figure 6B and Table S1). The overall survival durations in patients with high CSN5 expression were significantly shorter than in those with low or no CSN5 expression (Figure 6C). Similarly, high expression of p65 and CSN5 mRNA were associated with poor recurrence-free survival (Figure S6B) in 4,400 patients with breast cancer. These results supported the notion that the identified pathway may be pathologically relevant.
Figure 6. PD-L1 Is Required for Immunosuppression Mediated by Inflammation In Vivo.
(A) Correlation between protein expression of nuclear p65, total PD-L1, and CSN5 expression in 11 breast cancer cell lines.
(B) Two representative immunohistochemical staining results for p-p65, CSN5, PD-L1, and granzyme B in human breast cancer tissues. Scale bar, 50 µm.
(C) Overall survival of patients with breast cancer whose specimens were used in our analysis (n = 115).
(D) Western blot analysis of PD-L1 and CSN5 expression in cells treated with curcumin (CCM).
(E) Tumor growth of 4T1 cells in BALB/c mice treated with CCM or CTLA4 antibody after treatment with lipopolysaccharide. Tumors were measured at the indicated time points and dissected at the endpoint (n = 7 mice per group).
(F) Survival of mice bearing syngeneic 4T1 tumors following treatment with CCM or CTLA4 antibody. Significance was determined by the log rank test (n = 10 mice per group).
(G) Intracellular cytokine staining of CD8+ IFN-γ+ cells in the CD3+ T cell populations from isolated tumor-infiltrating lymphocytes. Results are presented as mean ± SD from one representative experiment.
(H) Immunofluorescence staining of the protein expression pattern of PD-L1, CD8, and granzyme B in 4T1 tumor masses from the experiments shown in (E). Scale bar, 100 µm. Inset scale bar, 50 um.
(I) Tumor growth of CSN5 knockout (CSN5 KO) 4T1 cells with PBS, curcumin (CCM), and/or CTLA4 antibody treatment in LPS-treated BALB/c mice. Tumor growth was measured at the indicated time points and dissected at the endpoint (n = 7 mice per group).
(J) Proposed model of TNF-α-mediated PD-L1 stabilization by CSN5 contributing to escape from T cell immune surveillance.
*p < 0.05, Student’s t test (or log rank test in F). Error bars represent SD of three independent experiments unless otherwise noted.
See also Figure S6 and Table S1.
Inhibition of CSN5 Destabilizes PD-L1 and Enhances the Therapeutic Efficacy of CTLA4 Blockade Therapy
Chemotherapy, radiation therapy, small-molecular inhibitors, and natural food compounds have been tested in clinical trials for potential synergy with immune checkpoint blockade (Pardoll, 2012). Because the protein stability of PD-L1 is stringently regulated by CSN5 (Figure 4), we sought to determine whether destabilization of PD-L1 by a CSN5 inhibitor would enhance anti-tumor immunity. Curcumin, which has been shown to inhibit CSN5-associated kinase activity (Uhle et al., 2003), inhibited not only CSN5 activity in a dose-dependent manner in vitro (Figure S6C) but also TNF-α-induced PD-L1 stabilization in cells from different types of cancers, including breast, colon, and lung cancer, and melanoma (Figures 6D and S6D).
The anti-PD-1 and anti-CTLA-4 combination has been recently reported to have a better therapeutic efficacy in several clinical trials (Callahan et al., 2014). Thus, we explored the possibility that decreasing PD-L1 expression by curcumin may sensitize inflammation-induced tumors to anti-CTLA4. To this end, tumor-bearing mice were treated with LPS followed by curcumin and/or anti-CTLA4 therapy (Figure S6E). Mouse mammary tumor growth and survival were monitored. Tumors resected from each mouse were subjected to TIL isolation followed by immune cell analysis by FACS. Curcumin reduced mouse tumor burden (Figure 6E) and improved survival rates (Figure 6F). The tumor-infiltrated activated CD8+ T cell population was also increased in curcumin-treated mice (Figures 6G, 6H, and S6G) with a concomitant PD-L1 reduction (Figure 6H). Similar to the enhanced efficacy observed for the anti-PD-1 and anti-CTLA4 combination therapy (Postow et al., 2015), the combination of curcumin and anti-CTLA4 therapy in 4T1 breast cancer, B16 melanoma, and CT26 colon cancer syngeneic mouse models did not induce any substantial changes in body weight or toxicity (Figures 6E, S6F, and S6H). Curcumin decreased PD-L1 expression (yellow) and enhanced the cytotoxic T cell activity (CD8 T cell, green; granzyme B, magenta) in the tumor mass when combined with anti-CTLA4 therapy (Figure 6H). Consistent with these findings, inhibition of PD-L1 by curcumin enhanced the efficacy of anti-CTLA4 blockade (Figures 6E–6I). Moreover, the combination of anti-CTLA4 and curcumin, which decreased PD-L1 stability, effectively suppressed tumor growth in multiple animal models (Figures 6E and S6H).
In addition, curcumin did not inhibit tumor growth of CSN5 knockout 4T1 cells in mice (Figure 6I), suggesting that curcumin-mediated tumor growth inhibition may be attributed primarily to CSN5-dependent regulation. It should be noted that while curcumin provided multiple benefits, inhibition of CSN5 enzyme activity was sufficient to induce the anti-tumor activity. When we knocked down endogenous mouse PD-L1 and re-expressed it under a CMV promoter, which lacks the p65 binding site, in 4T1 cells (4T1-mPD-L1), the addition of curcumin inhibited tumor growth in BALB/c mice inoculated with 4T1-mPD-L1 cells (Figures S6I and S6J). This result suggested that, by inhibiting CSN5 enzyme activity, curcumin is sufficient to enhance anti-tumor immunity in mice. In fact, curcumin was more effective in 4T1 cells (Figure 6E), suggesting that curcumin also inhibits PD-L1 expression through NF-kB-dependent transcriptional activation.
In our in vivo experimental models, we used LPS or poly(I:C) to mimic inflammatory conditions and also examine the role of PD-L1 under natural conditions, such as in non-LPS-treated tumor models. Without LPS treatment, PD-L1 knockout 4T1 cells already showed a significant reduction of tumor growth and more active cytotoxic T cell activities compared with 4T1 parental cells (Figures S6K and S6L). In addition, the combination treatment of curcumin and CTLA4 antibody also demonstrated better efficacy than a single treatment in BALB/c mice without LPS treatment (Figure S6M). These data implied that PD-L1 and CSN5 play important roles in evading immune surveillance and that the combination treatment improves efficacy in natural tumor condition.
Collectively, our results suggested that the pro-inflammatory cytokine TNF-α, secreted by macrophages, upregulates CSN5 to stabilize PD-L1, thereby enhancing its interaction with PD-1 to escape T cell immune surveillance (Figure 6J).
DISCUSSION
Our findings demonstrated that TNF-α induced PD-L1 stabilization through p65/CSN5 activation, namely TNF-α/p65/CSN5/PD-L1, and PD-L1 stabilization on cancer cells led to immune system evasion. Unlike CTLA-4 or PD-1, which are primarily expressed in immune cells (Brunet et al., 1987; Ishida et al., 1992), PD-L1 is expressed in cancer cells, macrophages, and DCs, and plays a major role in inhibiting immune surveillance (Dong et al., 2002). Aside from IFN-γ-mediated PD-L1 mRNA transcription through STAT3, we herein identified how T cells are suppressed through stabilization of PD-L1 by TNF-α. Since the regulatory mechanism was not limited to cancer cells, as PD-L1 in DCs and T cells also stabilized, our study revealed an underlying molecular mechanism of PD-L1 posttranslational modification. This mechanism represents a conceivable tumor-targeting strategy.
We identified the CSN complex as a PD-L1-interacting partner by mass spectrum analysis, confirming an association between PD-L1 and CSN5. Wu et al. (2009) reported that CSN2 is upregulated after TNF-α stimulation, which in turn inhibits Snail ubiquitination by blocking β-TrCP and Snail interaction, resulting in breast cancer cell invasion and migration. However, our results suggested that CSN5 directly deubiquitinates and stabilizes PD-L1 in cancer cells to escape from immune surveillance. Indeed, we found that TNF-α upregulated both CSN5 and CSN2 in breast cancer cells and further demonstrated that CSN5, but not CSN2, was involved in PD-L1 stabilization as downregulation of CSN2 did not affect PD-L1 protein expression and stability. Together, these findings suggested that non-redundant signaling regulation by the CSN complex may occur in different cancer cells.
Aside from inhibiting ubiquitination, CSN5 further deubiquitinates PD-L1 directly. Although the MPN domain of CSN5 is not responsible for PD-L1 interaction, disrupting the MPN domain affected CSN5-mediated PD-L1 stabilization and deubiquitination. As illustrated by deubiquitination assays, CSN5, purified from HEK293 cells, released the conjugation of ubiquitin-AMC and PD-L1 basal ubiquitination in vitro. These results indicated that TNF-α induces distinct deubiquitinating enzymes to exert independent actions for cancer cell survival, i.e.,CSN2 for epithelial-to-mesenchymal transition and CSN5 for immunosuppression.
The results from the mechanistic studies described here suggested that curcumin has the potential to enhance anti-cancer immunity for several reasons. First, curcumin is a natural dietary supplement that has been shown to inhibit NF-κB activation (Aggarwal et al., 2003). Indeed, it inhibited inflammation-mediated PD-L1 expression. Second, curcumin inhibits CSN5-associated kinase activity (Uhle et al., 2003) and, therefore, curcumin can inhibit pre-existing CSN5 activity in cancer cells as it can significantly reduce 4T1 tumor growth under natural conditions. Lastly, curcumin monotherapy has been evaluated for many diseases, including many types of cancer in human clinical trials (Aggarwal et al., 2003). The effects of curcumin as seen in 4T1 mPD-L1 cells (mPD-L1 lacking the endogenous PD-L1 promoter) did not seem as pronounced as those shown in 4T1 cells, suggesting that curcumin inhibits both transcriptional and posttranslational regulation of PD-L1. Our results from preclinical animal models, together with those from previous studies, suggested that curcumin attenuates immunosuppression.
In summary, we demonstrated a mechanism of immunosuppression in cancer cells through CSN5-mediated stabilization of PD-L1 by TNF-α. This regulatory event is critical for breast cancer cells to escape immune surveillance via PD-L1/PD-1 interaction. Importantly, inhibition of TNF-α-mediated PD-L1 stabilization in cancer cells promotes the tumor-infiltrating cytotoxic T cell immune response. Thus, targeting cancer cell PD-L1 stabilization through NF-κB/CSN5 inhibition represents a potential strategy to treat cancers that are associated with inflammatory diseases.
EXPERIMENTAL PROCEDURES
More detailed procedures can be found in the Supplemental Information.
Cell Culture, Stable Transfectants, and Transfection
All cell lines were obtained from the ATCC (Manassas, VA, USA) and were independently validated by short tandem repeat DNA fingerprinting at The University of Texas MD Anderson Cancer Center (Houston, TX, USA).
Animal Treatment Protocol
All procedures with BALB/c mice (6- to 8-week-old females; Jackson Laboratory) were conducted under guidelines approved by the IACUC at the MD Anderson Cancer Center. Mice were divided according to the mean tumor volume in each group. 4T1 or EMT6 cells (5 × 104 cells) in 50 µL of medium mixed with 50 µL of Matrigel (BD) were injected into the mammary fat fad (Li et al., 2016). The data on tumor growth show one experimental data with seven mice per group as indicated in the figure legends.
Statistical Analysis
Data in bar graphs represent mean ± SD fold change, relative to untreated or control groups, of three independent experiments. Statistical analyses were performed using SPSS (V20; SPSS). For immunohistochemistry analysis, Fisher’s exact test and Spearman’s rank correlation coefficient were used. Student’s t test was used to compare experimental data. A p value <0.05 was considered statistically significant.
Supplementary Material
Highlights.
TNF-α stabilizes cancer cell PD-L1 in response to chronic inflammation
Activation of NF-κB by TNF-α induces CSN5 expression leading to PD-L1 stabilization
CSN5 enzyme activity controls T cell suppression via PD-L1 deubiquitination
Destabilization of PD-L1 by CSN5 inhibitor curcumin benefits anti-CTLA4 therapy
In Brief.
Lim et al. show that inflammation increases PD-L1 expression in tumors through TNF-α-mediated activation of NF-κB, leading to transactivation of CSN5. CSN5 reduces PD-L1 ubiquitination and stabilizes it. Inhibition of CSN5 cooperates with anti-CTLA4 to enhance anti-tumor T cell function and reduce tumor growth.
Significance.
Chronic inflammation in cancer is often associated with disease aggressiveness. In the current study of inflammation-mediated immune response in cancer, we report the TNF-α/p65/CSN5/PD-L1 signaling axis as a deubiquitination mechanism stabilizing cancer cell PD-L1 expression for immunosuppression. The role of CSN5 in modulating PD-L1 stabilization prompted us to use the CSN5 inhibitor, curcumin, to show that it could destabilize PD-L1. Curcumin reduced CSN5 activity to attenuate TNF-α-mediated PD-L1 stabilization and enhanced anti-tumor immunity. Preclinical data demonstrated inhibition of CSN5-sensitized cancer cells to anti-CTLA4 therapy, suggesting that the CSN5 inhibitor may be a useful adjuvant to enhance immune-based therapies.
Acknowledgments
This work was partially supported by the NIH(CA109311, CA099031, and CCSG CA016672); Cancer Prevention Research Institute of Texas (RP160710); the Patel Memorial Breast Cancer Endowment Fund; the National Breast Cancer Foundation; a Breast Cancer Research Foundation grant (to M.-C.H. and G.N.H.); The University of Texas MD Anderson Cancer Center-China Medical University and Hospital Sister Institution Fund (to M.-C.H.); the Ministry of Science and Technology, International Research-intensive Centers of Excellence in Taiwan (I-RiCE; MOST 105-2911-I-002-302); the Ministry of Health and Welfare, China Medical University Hospital Cancer Research Center of Excellence (MOHW105-TDU-B-212-134003); the Center for Biological Pathways; Susan G. Komen for the Cure Postdoctoral Fellowship (PDF12231298 to S.-O.L.); the Basic Science Research Program through the National Research Foundation of Korea funded by the Korean government (MSIP; NRF-2011-357-C00140 to S.-O.L.); the National Research Foundation of Korea grant for the Global Core Research Center funded by the Korean government (MSIP; 2011-0030001 to J.-H.C.). M.-C.H. received a sponsored research agreement from STCube Pharmaceuticals, Inc., through the MD Anderson Cancer Center. S.-O.L., C.-W.L., and M.-C.H. are inventors on a patent applications under review.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, six figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.ccell.2016.10.010.
AUTHOR CONTRIBUTIONS
S.-O.L. and C.-W.L. designed and performed the experiments, analyzed data, and wrote the manuscript; W.X., J.-H.C., L.-C.C., S.-S.C., W.-C.L., J.-M.H., Y.-S.H., T.K., W.-C.C., Q.D., Y.W., Y.Y., and C.-H.C. performed experiments and analyzed data; Y. Wu provided patient tissue samples; J.L.H. and H.Y. provided scientific input and wrote the manuscript; A.A.S., D.Y., and G.N.H. provided scientific and clinical input; and M.-C.H. supervised the entire project, designed the experiments, analyzed data, and wrote the manuscript.
REFERENCES
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- Bech-Otschir D, Kraft R, Huang X, Henklein P, Kapelari B, Pollmann C, Dubiel W. COP9 signalosome-specific phosphorylation targets p53 to degradation by the ubiquitin system. EMBO J. 2001;20:1630–1639. doi: 10.1093/emboj/20.7.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonska M, Lin X. NF-kappaB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res. 2011;21:55–70. doi: 10.1038/cr.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin superfamily–CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- Callahan MK, Postow MA, Wolchok JD. CTLA-4 and PD-1 pathway blockade: combinations in the clinic. Front Oncol. 2014;4:385. doi: 10.3389/fonc.2014.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- Cope GA, Suh GS, Aravind L, Schwarz SE, Zipursky SL, Koonin EV, Deshaies RJ. Role of predicted metalloprotease motif of Jab1/Csn5 in cleavage of Nedd8 from Cul1. Science. 2002;298:608–611. doi: 10.1126/science.1075901. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- Echalier A, Pan Y, Birol M, Tavernier N, Pintard L, Hoh F, Ebel C, Galophe N, Claret FX, Dumas C. Insights into the regulation of the human COP9 signalosome catalytic subunit, CSN5/Jab1. Proc. Natl. Acad. Sci. USA. 2013;110:1273–1278. doi: 10.1073/pnas.1209345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Lu W, Choi HH, Yeung SC, Tung JY, Hsiao CD, Fuentes-Mattei E, Menter D, Chen C, Wang L, et al. ERK2-Dependent phosphorylation of CSN6 is critical in colorectal cancer development. Cancer Cell. 2015;28:183–197. doi: 10.1016/j.ccell.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faurschou A, Gniadecki R. TNF-alpha stimulates Akt by a distinct aPKC-dependent pathway in premalignant keratinocytes. Exp. Dermatol. 2008;17:992–997. doi: 10.1111/j.1600-0625.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- Gowrishankar K, Gunatilake D, Gallagher SJ, Tiffen J, Rizos H, Hersey P. Inducible but not constitutive expression of PD-L1 in human melanoma cells is dependent on activation of NF-kappaB. PLoS One. 2015;10:e0123410. doi: 10.1371/journal.pone.0123410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- Huang G, Wen Q, Zhao Y, Gao Q, Bai Y. NF-kappaB plays a key role in inducing CD274 expression in human monocytes after lipopolysaccharide treatment. PLoS One. 2013;8:e61602. doi: 10.1371/journal.pone.0061602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL, Chao CH, Yamaguchi H, Yang NK, Ding Q, et al. Epithelial-mesenchymal transition induced by TNF-alpha requires NF-kappaB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72:1290–1300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CW, Lim SO, Xia W, Lee HH, Li CC, Kuo CW, Khoo KH, Chang SS, Cha JH, Kim T, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shah SV, Xiang X, Wang J, Deng ZB, Liu C, Zhang L, Wu J, Edmonds T, Jambor C, et al. COP9-associated CSN5 regulates exosomal protein deubiquitination and sorting. Am. J. Pathol. 2009;174:1415–1425. doi: 10.2353/ajpath.2009.080861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Hamanishi J, Matsumura N, Abiko K, Murat K, Baba T, Yamaguchi K, Horikawa N, Hosoe Y, Murphy SK, et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-kappaB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75:5034–5045. doi: 10.1158/0008-5472.CAN-14-3098. [DOI] [PubMed] [Google Scholar]
- Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Garcia M, Porichis F, de Jong OG, Levi K, Diefenbach TJ, Lifson JD, Freeman GJ, Walker BD, Kaufmann DE, Kavanagh DG. Expression of PD-L1 and PD-L2 on human macrophages is upregulated by HIV-1 and differentially modulated by IL-10. J. Leukoc. Biol. 2011;89:507–515. doi: 10.1189/jlb.0610327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin. Cancer Res. 2013;19:1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda K, Yoneda-Kato N, Fukumoto A, Yamanaka S, Kato JY. Multiple functions of Jab1 are required for early embryonic development and growth potential in mice. J. Biol. Chem. 2004;279:43013–43018. doi: 10.1074/jbc.M406559200. [DOI] [PubMed] [Google Scholar]
- Uhle S, Medalia O, Waldron R, Dumdey R, Henklein P, Bech-Otschir D, Huang X, Berse M, Sperling J, Schade R, Dubiel W. Protein kinase CK2 and protein kinase D are associated with the COP9 signalosome. EMBO J. 2003;22:1302–1312. doi: 10.1093/emboj/cdg127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Wan M, Cao X, Wu Y, Bai S, Wu L, Shi X, Wang N. Jab1 antagonizes TGF-beta signaling by inducing Smad4 degradation. EMBO Rep. 2002;3:171–176. doi: 10.1093/embo-reports/kvf024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ding Q, Yen CJ, Xia W, Izzo JG, Lang JY, Li CW, Hsu JL, Miller SA, Wang X, et al. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer Cell. 2012;21:374–387. doi: 10.1016/j.ccr.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welteke V, Eitelhuber A, Duwel M, Schweitzer K, Naumann M, Krappmann D. COP9 signalosome controls the Carma1-Bcl10-Malt1 complex upon T-cell stimulation. EMBO Rep. 2009;10:642–648. doi: 10.1038/embor.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa Y, Iijima N, Iwabuchi K, Onoe K. Activation of extracellular signal-related kinase by TNF-alpha controls the maturation and function of murine dendritic cells. J. Leukoc. Biol. 2002;71:125–132. [PubMed] [Google Scholar]
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016;8:328rv324. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.