Abstract
Pancreatic ductal adenocarcinoma (PDAC) has long being viewed as a consequence of recurrent or unresolved inflammation, associated with fibrogenesis and extensive cross-communication between different cell types within the tumor microenvironment. A plethora of cytokines, chemokines and DAMPs such as TNF-α, IL-6, MCP-1, ATP and HMGB1, produced in response to pancreas damage from injury fuel inflammatory and fibrogenic responses. These and other factors contribute to the intercellular interplay within the complex milieu of the tumor microenvironment. However, precisely which factors represent appropriate or inappropriate signals, which cell type(s) produces the factors, and the detailed understanding of the sequence of events by which regeneration and malignant transformation are orchestrated have not yet been unraveled. Clearly, further understanding of cause-and effect relationships between the diverse cell populations and the factors they release into the microenvironment is urgently needed.
Pancreatic stellate cells modulate tumorigenesis and tumor progression
In the past 15 years, a major role for a unique cell type, the activated pancreatic stellate cell (PSC) has been defined in the production of dense stroma, or fibrotic plaques that characterize PDAC and chronic pancreatitis (CP).1,2 PSC, analogous to a closely related resident stellate cell population in the liver and other organs, are initially quiescent, but with appropriate stimuli, convert to an activated state with characteristics of myofibroblasts, a cell type conferring properties of a cancer-promoting niche. Activated PSC are highly proliferative, secrete abundant extracellular matrix proteins, and enter into cross-communication with tumor cells. A flood of studies showed that PSC support the growth of precancerous pancreatic lesions (PanINs) and cancer cells. More recently, a few prominent publications described studies in murine models that reached a different conclusion, that the presence of stroma rich in activated PSC greatly restrained tumor progression. 3,4 These apparently conflicting results have given rise to a new concept, i.e., that PSC can be reprogrammed into multiple identities, with abilities to promote versus restrain tumor progression and invasion. Thus, it now appears that PSC possess more divergent functional states than previously recognized: (1) a quiescent, lipid storing state, (2) an activated, ECM producing state, (3) reversion from activation back to a quiescent-like state, or possibly, (4) a metabolically stressed, post-activated phenotype characterized by exosome production. Stellate cells in culture can exhibit sluggish growth and hypersecretion, behaviors often ascribed to senescence. Still, such populations may yet proliferate or undergo apoptosis in response to stimuli. Overall, the possibility of reprogramming implies that not all the functional states of PSC are yet known.
Intercellular communication, a major driver of tumorigenesis
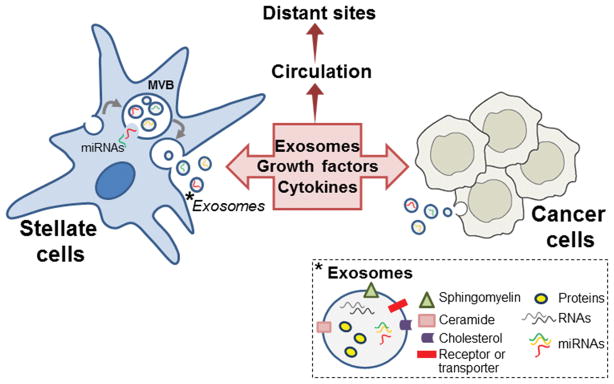
Many investigations have been aimed at understanding the role of substances produced locally or remotely to advance tumorigenesis, including by direct action on transformed cells. Previous studies demonstrated that conditioned medium (CM) from cultured PSC promotes proliferation and migration of pancreatic cancer cells and conversely, CM from PanIN cells or transformed cancer cells alters fibrogenic responses in activated PSC. These effects have been attributed to the presence of specific soluble growth factors, cytokines or chemokines in the CM.1,2 Chemokines expressed by cancer cells are widely hypothesized to recruit inflammatory cells and PSC by promoting migration towards tumor sites. The study from the laboratory of Shimosegawa in this issue of Pancreas 5 highlights the role of exosomes, 50–150-nm particles shed from mammalian cells, as potential factors contributing to these effects. As shown in Figure 1, exosomes are released from cells when subcellular double-membrane structures called multivesicular bodies (MVB) fuse with the plasma membrane (an alternative pathway for MVB disposal is fusion with lysosomes) and release their contents. 6,7,8 Recently, there has been a rapid growth of interest in whether exosomes and their incorporated protein, lipid and genetic material secreted from donor cells can be taken up and transferred into distinct recipient cells, and thereby transmit phenotype from a donor to a recipient cell. Recipient cells endocytose exosomes at some rate, and assimilate their content. Whereas approximately 300 proteins make up some of the cargo of exosomes, they also contain nucleic acids, including DNA, RNA and most notably, microRNA (miRNA). In this way, the protein and/or nucleic acid components of the exosome influence the recipient cell’s functional capacity and/or regulation.
Figure 1. Contribution of exosomes to reciprocal and multidirectional intercellular communication during tumorigenesis and metastasis.
In stellate cells and other parental cells including cancer cells, exosomes are formed within multivesicular bodies (MVB) budding from the plasma membrane and endosomes (not shown). MicroRNAs (miRNA) and other cargo are selectively incorporated into the exosomes and released into the extracellular space when MVB fuse with the plasma membrane. As indicated in the picture on the lower right corner, exosomes are composed of various bioactive molecules, including several plasma membrane components, intravesicular proteins, RNAs and DNAs, and miRNAs. Exosomes and soluble factors including growth factors, cytokines and chemokines secreted by stellate cells and cancer cells can regulate a diverse range of biological processes in recipient cells in the tumor microenvironment and in distant sites. Exosome components in distant sites such as blood, pancreatic juice and stool may be used as biomarkers for tumor progression and metastasis in PDAC.
Exosomes from PSC alter the behavior of pancreatic cancer cells
In their study, Takikawa et al 5 showed that PSC-derived exosomes were incorporated into, and promoted proliferation and migration in, two separate recipient pancreatic cancer cell lines, Panc-1 and SUIT-2. The exosomes differentially altered expression of a plethora of genes regulating various cellular processes including cell cycle, cellular assembly and organization, DNA replication, recombinant, and repair, cell death and survival, and cellular growth and proliferation in the recipient cancer cells. Among these genes, three chemokines, CCL20, CXCL1, CXCL2 were found upregulated in exosome-treated cancer cells. To further demonstrate the relevance of exosomes to PSC elicited responses on cancer cells, they treated PSC with GW4869, an inhibitor of neutral sphingomyelinases that prevents exosome formation. Indeed, the CM from GW 4869-treated PSC did elicit significantly less chemokine expression from cancer cells, supporting a role for exosomes in the observed effects. However, the secreted exosome profile was not directly examined in CM from GW 4869 treated PSC. Other studies have provided additional information by focusing on a small number of individual miRNAs identified in PSC-derived exosomes. 9,10
miRNA in exosomes: key messengers in PSC and cancer cell communications
Exosomes produced by PSC have previously been analyzed to elucidate their composition and assess their role in disease. 8 This recent study identified glypican-1, a glycoprotein found in PSC exosomes, as a valuable new biomarker to detect PDAC and as a tumor-promoter transferred between cells via exosomes. Besides proteins, exosomes also use their lipids and genetic material (DNA, mRNAs and miRNAs) (Figure 1) to regulate many cellular processes in recipient cells. Among these factors, miRNA have been recognized as central regulators of chromatin modification and gene regulation. Produced endogenously or taken up by cells, miRNA are small, non-coding RNAs (19–22 bases in length) that regulate protein-coding gene expression by promoting degradation of mRNAs in a sequence-specific manner, or by blocking translation. 11,12 Precursor miRNA are transcribed in the nucleus, and mature in the cytoplasm to a functional miRNA that exert their action by binding their “seed sequence” (an evolutionary-conserved region of 5–7 nt at the 5′-end of the miRNA) to a complementary sequence in the 3′ untranslated region of the targeted mRNA. 13 Mounting evidence indicates that miRNA may coordinately regulate a high percentage (perhaps 90%) of the genome in all organisms, affecting relevant cellular processes during normal homeostasis and disease states. Experimental tools and bioinformatic methods continue to be developed to catalogue and validate the increasing number of newly discovered miRNA in humans and other species, and to better predict and validate their molecular targets and functional relevance.
Dysregulation of multiple miRNA have been found in many type of cancers including pancreatic cancer, 14,15,16,17 and the functional relevance of these changes is the focus of active research. Assimilated miRNA exert a negative regulation of gene expression against distinct targets. In precancerous or malignantly transformed cells, for example, miRNA could be directed to switching off key tumor suppressor gene expression. Particular miRNA such as miR-21 are frequently upregulated and act as oncogenic miRNA with significant roles in the initiation, progression and metastasis of various types of cancers. Other miRNA including Let-7 are tumor suppressive and often found downregulated in cancer cells. The work of Takikawa et al 5 reinforces the notion that mesenchymal cells present in the pancreas, including activated PSC, produce exosomal miRNA that participate in the distinct phases of pancreatic inflammation and tumorigenesis. However, the coordinated regulation and functional effects on oncogenic pathways of many recently identified miRNA remain to be elucidated. We also need a better understanding of the mechanisms regulating miRNA expression, degradation and their selected sorting and incorporation in secreted exosomes and other vesicles.
Takikawa et al 5 also describe their microarray analysis of the miRNA signature in cultured human PSC, which is compared with that of the exosomes secreted by these cells. Several miRNA were identified as highly expressed in PSC-derived exosomes, whereas 344 miRNA were found to be differentially expressed in exosomes compared to the parental cells. Strikingly, most of these miRNA were enriched, and only a low number were depleted in the exosomes compared to the whole cells. This emphasis on specific miRNA becoming concentrated in exosomes is fascinating, as it implies the existence of machinery for sorting and selectively packaging certain miRNA for export. Whereas the functional effects or the molecular targets of individual miRNA were not enumerated, some of the listed miRNA are reported to regulate tumor progression in other studies. For example, upregulation of miR-21 (or miR-21-5p) is a common feature in several cancers and regulates the expression of target genes such as the tumor suppressors PTEN, MSH2, Cdc25A, SPRY2 and PDCD4. Aberrant expression of miR-451a, a miRNA highly represented in the PSC exosome profile, has been reported in several tumors and cancer cell lines, although both pro-tumorigenic 18 as well as tumor suppressive 19 roles have been described for this miRNA. miR-451 also regulates the drug-transporter protein P-glycoprotein, potentially promoting resistance to the chemotherapy drugs that utilize this transporter. 20
What remains to be learned about exosomes and miRNA?
Whereas pancreas exosome production is increased during tumorigenesis, whether they mostly come from PSC/TAFs or tumor cells is unclear. Transfer via exosomes appears to be multi-directional. Exchange between cancer cells and PSC and TAFs in the tumor microenvironment represents an axis of positive feedback that promotes tumor development. Since all cells produce exosomes, one possibility is that PSC in proximity to cancerous cells may transmit molecules that modulate the tumorigenic properties of the cancer cells. Reciprocally, cancer cell exosomes also modulate the phenotype of PSC. The term “reactive stroma” is used to describe the cancer cells’ ability to modulate the phenotype of stromal cells in the immediate environment. When glypican-1 enriched circulating exosomes were detected in PDAC, their cellular origin was not elucidated. Regardless of their cell of origin, exosomes entering the circulation have been hypothesized to influence metastasis. In the liver, for example, a recent study concluded that resident macrophages act as “intermediate” recipient cells, then producing local secretions that complete the conversion to a favorable niche, in part through the secondary fibrogenic actions of hepatic stellate cells. 21
In summary, the new study reported in this issue reinforces the emerging role of exosomes and their cargo including miRNA as factors influencing and modulating the processes whereby pancreatic inflammation progresses to cancer. Studies like that of Takikawa et al 5 represent important early steps toward understanding the extent to which exosomes modulate cellular responses, both in recipient tumor cells and distant metastatic sites. Nevertheless, further information is needed to clarify certain aspects of exosome function in tumorigenesis. The ability to isolate and test circulating exosomes for their potential to enable early pancreatic cancer diagnosis is highly advantageous, in that the presence of positive marker proteins (e.g., glypican-1) or perhaps, miRNA can reveal the presence of an incipient tumor much earlier than any imaging or endoscopic examinations currently in practice. However, miRNAs are diverse and identification of their targets remains incomplete. Target identifications retain ambiguity, and the efficacy of a particular miRNA on a target cell is linked to the expression of the target in that cellular context. These factors hinder easy comprehension of the effects of any particular ensemble of miRNAs. Further improving our understanding of noncoding (nc) RNAs is also necessary for continued progress in cancer research. Already sophisticated bioinformatics and next-generation sequencing have begun to be applied to identify and evaluate the functions of various ncRNAs. 22 Further research is urgently needed to clarify the interactions between cancer cells and other cell types in the tumor microenvironment such as pancreatic stellate cells, as well as the factors including exosomes, that mediate intercellular communication to promote and sustain malignant transformation and metastasis.
Acknowledgments
This work was supported by NIH Grants RO1 AA019954 (To A.L.), PO1 CA163200, and PO1 DK098108.
Footnotes
The authors declare no conflict of interest.
References
- 1.Apte MV, Wilson JS, Lugea A, et al. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210–1219. doi: 10.1053/j.gastro.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandol S, Gukovskaya A, Edderkaoui M, et al. Epidemiology, risk factors, and the promotion of pancreatic cancer: role of the stellate cell. J Gastroenterol Hepatol. 2012;27(Suppl 2):127–134. doi: 10.1111/j.1440-1746.2011.07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JJ, Perera RM, Wang H, et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc Natl Acad Sci U S A. 2014;111:E3091–3100. doi: 10.1073/pnas.1411679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takikawa T, Masamune A, Yoshida N, et al. Exosomes derived from pancreatic stellate cells: microRNA signature and effects on pancreatic cancer cells. Pancreas. 2016 doi: 10.1097/MPA.0000000000000722. [DOI] [PubMed] [Google Scholar]
- 6.Ohno S, Ishikawa A, Kuroda M. Roles of exosomes and microvesicles in disease pathogenesis. Adv Drug Deliv Rev. 2013;65:398–401. doi: 10.1016/j.addr.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Brinton LT, Sloane HS, Kester M, et al. Formation and role of exosomes in cancer. Cell Mol Life Sci. 2015;72:659–671. doi: 10.1007/s00018-014-1764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charrier A, Chen R, Chen L, et al. Connective tissue growth factor (CCN2) and microRNA-21 are components of a positive feedback loop in pancreatic stellate cells (PSC) during chronic pancreatitis and are exported in PSC-derived exosomes. J Cell Commun Signal. 2014;8:147–156. doi: 10.1007/s12079-014-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali S, Suresh R, Banerjee S, et al. Contribution of microRNAs in understanding the pancreatic tumor microenvironment involving cancer associated stellate and fibroblast cells. Am J Cancer Res. 2015;5:1251–1264. [PMC free article] [PubMed] [Google Scholar]
- 11.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 12.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman RC, Farh KK, Burge CB, et al. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iorio MV, Croce CM. MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol. 2009;27:5848–5856. doi: 10.1200/JCO.2009.24.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nana-Sinkam SP, Croce CM. MicroRNA regulation of tumorigenesis, cancer progression and interpatient heterogeneity: towards clinical use. Genome Biol. 2014;15:445. doi: 10.1186/s13059-014-0445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez YG, Lucas AL. MicroRNA in pancreatic ductal adenocarcinoma and its precursor lesions. World J Gastrointest Oncol. 2016;8:18–29. doi: 10.4251/wjgo.v8.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su Z, Ni L, Yu W, et al. MicroRNA-451a is associated with cell proliferation, migration and apoptosis in renal cell carcinoma. Mol Med Rep. 2015;11:2248–2254. doi: 10.3892/mmr.2014.2957. [DOI] [PubMed] [Google Scholar]
- 19.Fukumoto I, Kinoshita T, Hanazawa T, et al. Identification of tumour suppressive microRNA-451a in hypopharyngeal squamous cell carcinoma based on microRNA expression signature. Br J Cancer. 2014;111:386–394. doi: 10.1038/bjc.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu X, Li JY, Guo J, et al. Influence of MiR-451 on Drug Resistances of Paclitaxel-Resistant Breast Cancer Cell Line. Med Sci Monit. 2015;21:3291–3297. doi: 10.12659/MSM.894475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa-Silva B, Aiello NM, Ocean AJ, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller S, Raulefs S, Bruns P, et al. Next-generation sequencing reveals novel differentially regulated mRNAs, lncRNAs, miRNAs, sdRNAs and a piRNA in pancreatic cancer. Mol Cancer. 2015;14:94. doi: 10.1186/s12943-015-0358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]