Abstract
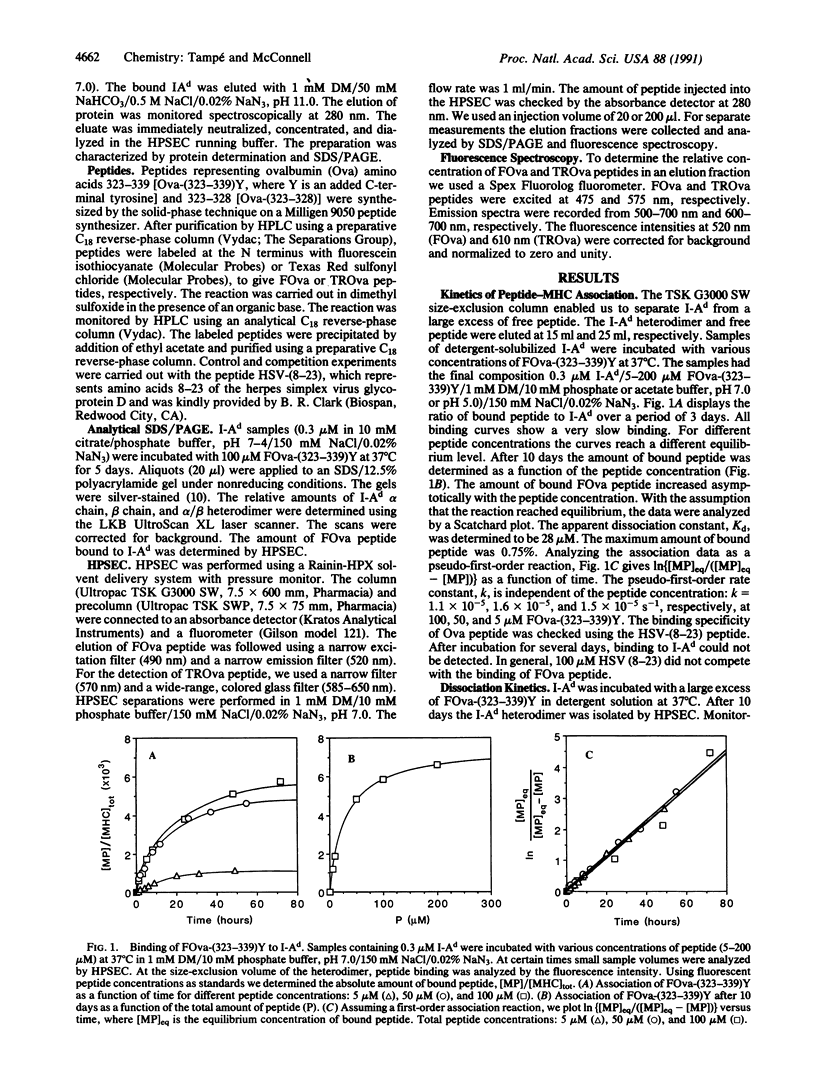
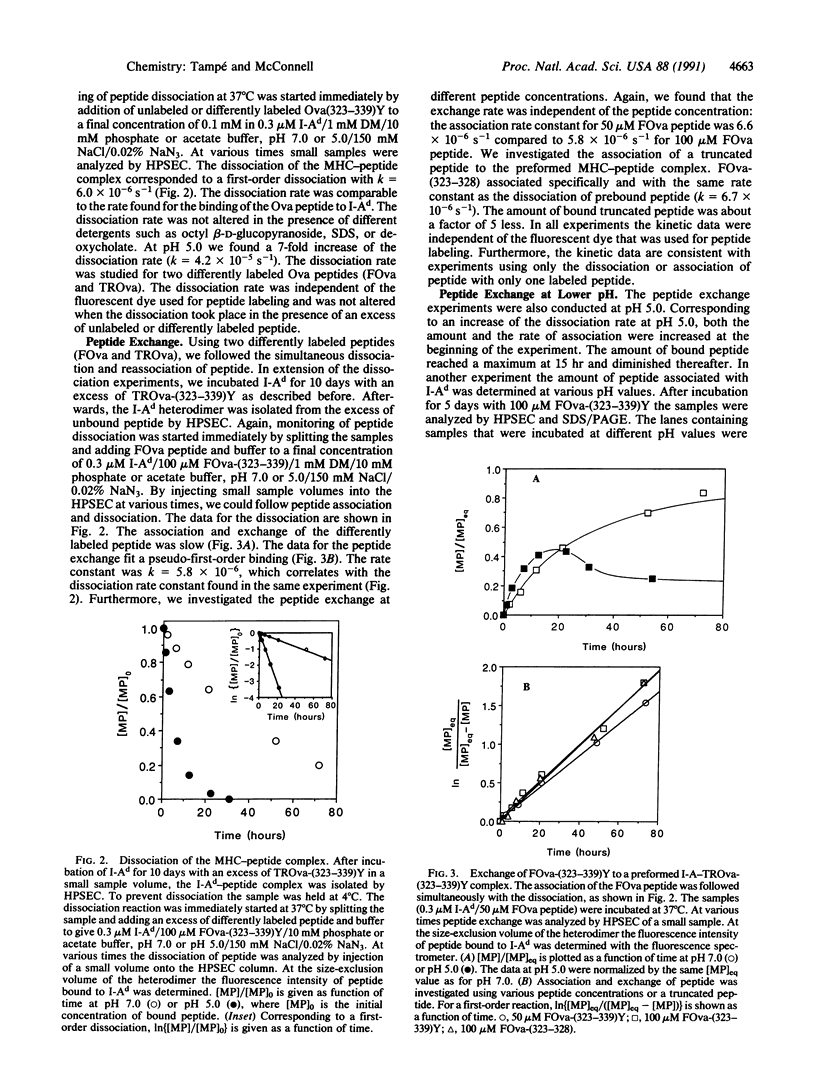
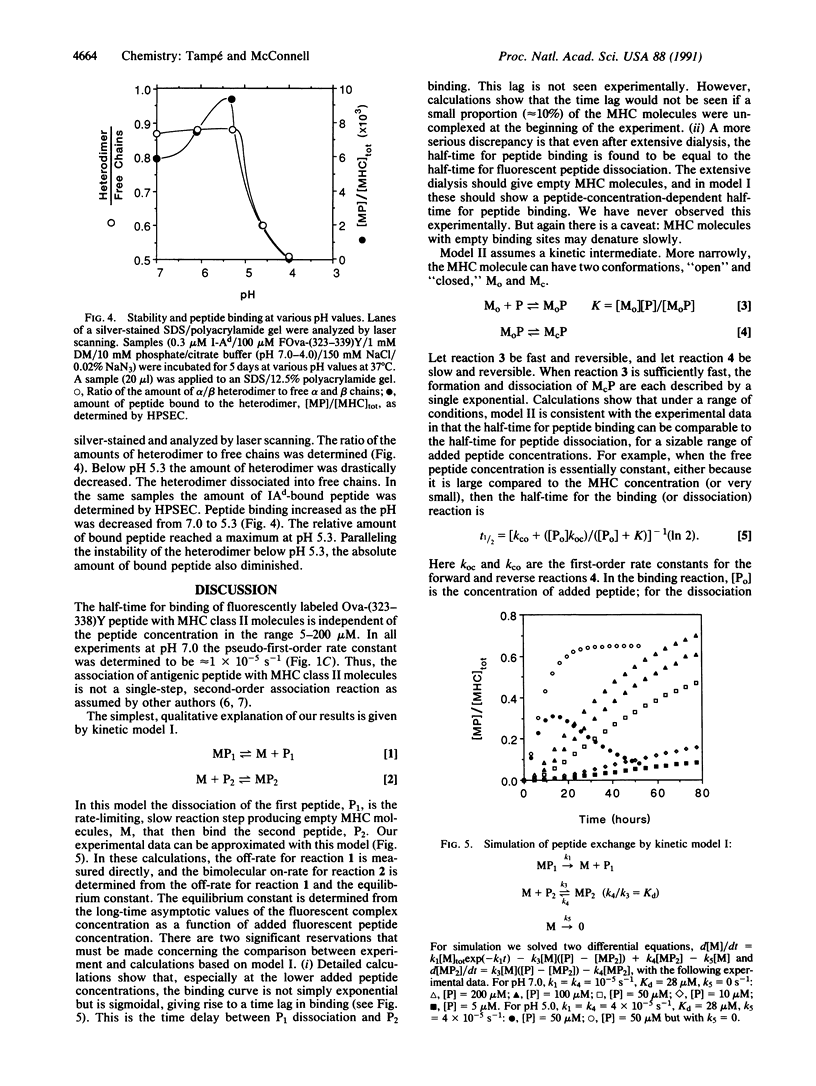
Using high-performance size-exclusion chromatography and fluorescence spectroscopy, we investigated the kinetics of fluorescent peptide reactions with detergent-solubilized I-Ad, a class II molecule of the mouse major histocompatibility complex. At pH 7.0 and 37 degrees C the half-time for the binding of a fluorescein-labeled synthetic peptide representing ovalbumin amino acids 323-339 [FOva-(323-339)Y] to I-Ad was 32 hr, independent of added fluorescent peptide concentration in the range 5-200 microM. Peptide exchange experiments were also carried out, where it was found that the half-time of FOva-(323-339)Y binding was equal to the half-time of dissociation of the Texas Red-labeled peptide TROva-(323-339)Y. These experiments show that slow peptide binding to class II major histocompatibility molecules may be limited by the slow dissociation of prebound peptides. Paradoxically, however, this kinetic behavior--a peptide concentration-insensitive on-reaction with a half-time for peptide binding approximately equal to the half-time for dissociation--can be modeled in more than one way. Models involving a kinetic intermediate are particularly attractive. The kinetics were significantly different at pH 5.0. The half-times for peptide binding and dissociation were approximately 7 times shorter than at pH 7.0. In addition the complex of the I-Ad alpha/beta heterodimer with FOva-(323-339)Y was unstable and dissociated into separate alpha and beta chains with a half-time of approximately 7 hr.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Buus S., Colon S., Smith C., Freed J. H., Miles C., Grey H. M. Interaction between a "processed" ovalbumin peptide and Ia molecules. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3968–3971. doi: 10.1073/pnas.83.11.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Jenis D. M., Grey H. M. Isolation and characterization of antigen-Ia complexes involved in T cell recognition. Cell. 1986 Dec 26;47(6):1071–1077. doi: 10.1016/0092-8674(86)90822-6. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Grey H. M. The interaction between protein-derived immunogenic peptides and Ia. Immunol Rev. 1987 Aug;98:115–141. doi: 10.1111/j.1600-065x.1987.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Unanue E. R. Antigen processing and intracellular Ia. Possible roles of endocytosis and protein synthesis in Ia function. J Immunol. 1989 Jan 1;142(1):12–19. [PubMed] [Google Scholar]
- Jensen P. E. Regulation of antigen presentation by acidic pH. J Exp Med. 1990 May 1;171(5):1779–1784. doi: 10.1084/jem.171.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. J., Kanellopoulos-Langevin C., Merwin R. M., Sachs D. H., Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979 Feb;122(2):549–554. [PubMed] [Google Scholar]
- Roche P. A., Cresswell P. High-affinity binding of an influenza hemagglutinin-derived peptide to purified HLA-DR. J Immunol. 1990 Mar 1;144(5):1849–1856. [PubMed] [Google Scholar]
- Rothenhäusler B., Dornmair K., McConnell H. M. Specific binding of antigenic peptides to separate alpha and beta chains of class II molecules of the major histocompatibility complex. Proc Natl Acad Sci U S A. 1990 Jan;87(1):352–354. doi: 10.1073/pnas.87.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadegh-Nasseri S., McConnell H. M. A kinetic intermediate in the reaction of an antigenic peptide and I-Ek. Nature. 1989 Jan 19;337(6204):274–276. doi: 10.1038/337274a0. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]


