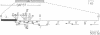Abstract
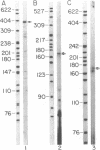
Decay-accelerating factor (DAF) expression modulates susceptibility of cells to autologous complement attack. To characterize the regulatory region controlling DAF gene transcription, genomic DNA extending from 815 base pairs (bp) upstream to approximately 4 kilobases downstream of DAF's AUG codon (designated +1) was cloned and sequenced. The 5' flanking sequence showed 59-76% G + C content (-355 to +1), at least one GC box(es) (-135 to -131), and variable length sequences (from -629 to -285) conforming to the motifs TCCTCC and TCn. Nuclease S1 digestions and primer extensions localized a major transcriptional start site to -82/-81, 38 bp downstream of a possible TATA variant, (A)TTTAA. In COS cell transfections, the sequence encompassing -815 to -67 functioned 2.5% as efficiently as the Rous sarcoma virus 3' long terminal repeat, but following deletion upstream of -355 its activity increased approximately 4-fold. Two octanucleotides exhibiting partial homology to phorbol 12-myristate 13-acetate (PMA) and cAMP responsive elements (PREs and CREs, respectively) were detected, and the respective modulators enhanced transcriptional efficiency 2- and approximately 10-fold, respectively. Thus, the DAF gene promoter (i) exhibits sequences resembling both conventional and unconventional transcriptional control elements, (ii) possesses a region with negative regulatory activity, and (iii) responds to PMA and cAMP induction presumably via PRE- and CRE-like enhancer elements.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Angel P., Baumann I., Stein B., Delius H., Rahmsdorf H. J., Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5'-flanking region. Mol Cell Biol. 1987 Jun;7(6):2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniahmad A., Muller M., Steiner C., Renkawitz R. Activity of two different silencer elements of the chicken lysozyme gene can be compensated by enhancer elements. EMBO J. 1987 Aug;6(8):2297–2303. doi: 10.1002/j.1460-2075.1987.tb02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bryant R. W., Granzow C. A., Siegel M. I., Egan R. W., Billah M. M. Phorbol esters increase synthesis of decay-accelerating factor, a phosphatidylinositol-anchored surface protein, in human endothelial cells. J Immunol. 1990 Jan 15;144(2):593–598. [PubMed] [Google Scholar]
- Caras I. W., Davitz M. A., Rhee L., Weddell G., Martin D. W., Jr, Nussenzweig V. Cloning of decay-accelerating factor suggests novel use of splicing to generate two proteins. Nature. 1987 Feb 5;325(6104):545–549. doi: 10.1038/325545a0. [DOI] [PubMed] [Google Scholar]
- Cheung N. K., Walter E. I., Smith-Mensah W. H., Ratnoff W. D., Tykocinski M. L., Medof M. E. Decay-accelerating factor protects human tumor cells from complement-mediated cytotoxicity in vitro. J Clin Invest. 1988 Apr;81(4):1122–1128. doi: 10.1172/JCI113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio F. G., Sedmak D. D., Mahan J. D., Nahman N. S., Jr Localization of decay accelerating factor in normal and diseased kidneys. Kidney Int. 1989 Jul;36(1):100–107. doi: 10.1038/ki.1989.167. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson R. K., Rottman F. M. Alternative processing of bovine growth hormone mRNA: nonsplicing of the final intron predicts a high molecular weight variant of bovine growth hormone. Proc Natl Acad Sci U S A. 1987 May;84(9):2673–2677. doi: 10.1073/pnas.84.9.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourcade D., Holers V. M., Atkinson J. P. The regulators of complement activation (RCA) gene cluster. Adv Immunol. 1989;45:381–416. doi: 10.1016/s0065-2776(08)60697-5. [DOI] [PubMed] [Google Scholar]
- Johnson A. C., Ishii S., Jinno Y., Pastan I., Merlino G. T. Epidermal growth factor receptor gene promoter. Deletion analysis and identification of nuclear protein binding sites. J Biol Chem. 1988 Apr 25;263(12):5693–5699. [PubMed] [Google Scholar]
- Klein J. L., Shows T. B., Dupont B., Trapani J. A. Genomic organization and chromosomal assignment for a serine protease gene (CSPB) expressed by human cytotoxic lymphocytes. Genomics. 1989 Jul;5(1):110–117. doi: 10.1016/0888-7543(89)90093-1. [DOI] [PubMed] [Google Scholar]
- Laimins L., Holmgren-König M., Khoury G. Transcriptional "silencer" element in rat repetitive sequences associated with the rat insulin 1 gene locus. Proc Natl Acad Sci U S A. 1986 May;83(10):3151–3155. doi: 10.1073/pnas.83.10.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R., Harney J. W., Moore D. D. Repression mediates cell-type-specific expression of the rat growth hormone gene. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8283–8287. doi: 10.1073/pnas.83.21.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass J. H., Walter E. I., Burris T. E., Grossniklaus H. E., Roat M. I., Skelnik D. L., Needham L., Singer M., Medof M. E. Expression of two molecular forms of the complement decay-accelerating factor in the eye and lacrimal gland. Invest Ophthalmol Vis Sci. 1990 Jun;31(6):1136–1148. [PubMed] [Google Scholar]
- Law S. K. The covalent binding reaction of C3 and C4. Ann N Y Acad Sci. 1983;421:246–258. doi: 10.1111/j.1749-6632.1983.tb18113.x. [DOI] [PubMed] [Google Scholar]
- Lederman M. M., Purvis S. F., Walter E. I., Carey J. T., Medof M. E. Heightened complement sensitivity of acquired immunodeficiency syndrome lymphocytes related to diminished expression of decay-accelerating factor. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4205–4209. doi: 10.1073/pnas.86.11.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lublin D. M., Lemons R. S., Le Beau M. M., Holers V. M., Tykocinski M. L., Medof M. E., Atkinson J. P. The gene encoding decay-accelerating factor (DAF) is located in the complement-regulatory locus on the long arm of chromosome 1. J Exp Med. 1987 Jun 1;165(6):1731–1736. doi: 10.1084/jem.165.6.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Leroy P., Ruhlmann C., Gesnel M. C., Breathnach R. Isolation of the oncogene and epidermal growth factor-induced transin gene: complex control in rat fibroblasts. Mol Cell Biol. 1986 May;6(5):1679–1686. doi: 10.1128/mcb.6.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Lublin D. M., Holers V. M., Ayers D. J., Getty R. R., Leykam J. F., Atkinson J. P., Tykocinski M. L. Cloning and characterization of cDNAs encoding the complete sequence of decay-accelerating factor of human complement. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2007–2011. doi: 10.1073/pnas.84.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Roberts W. L., Haas R., Rosenberry T. L. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry. 1986 Nov 4;25(22):6740–6747. doi: 10.1021/bi00370a003. [DOI] [PubMed] [Google Scholar]
- Post T. W., Arce M. A., Liszewski M. K., Thompson E. S., Atkinson J. P., Lublin D. M. Structure of the gene for human complement protein decay accelerating factor. J Immunol. 1990 Jan 15;144(2):740–744. [PubMed] [Google Scholar]
- Quigg R. J., Nicholson-Weller A., Cybulsky A. V., Badalamenti J., Salant D. J. Decay accelerating factor regulates complement activation on glomerular epithelial cells. J Immunol. 1989 Feb 1;142(3):877–882. [PubMed] [Google Scholar]
- Reynolds G. A., Basu S. K., Osborne T. F., Chin D. J., Gil G., Brown M. S., Goldstein J. L., Luskey K. L. HMG CoA reductase: a negatively regulated gene with unusual promoter and 5' untranslated regions. Cell. 1984 Aug;38(1):275–285. doi: 10.1016/0092-8674(84)90549-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert P. S., Hansson G. K. Decay-accelerating factor is expressed on vascular smooth muscle cells in human atherosclerotic lesions. J Clin Invest. 1989 Aug;84(2):597–604. doi: 10.1172/JCI114204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver B. J., Bokar J. A., Virgin J. B., Vallen E. A., Milsted A., Nilson J. H. Cyclic AMP regulation of the human glycoprotein hormone alpha-subunit gene is mediated by an 18-base-pair element. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2198–2202. doi: 10.1073/pnas.84.8.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford H. A., Tykocinski M. L., Lublin D. M., Holers V. M., Rosse W. F., Atkinson J. P., Medof M. E. Normal polymorphic variations and transcription of the decay accelerating factor gene in paroxysmal nocturnal hemoglobinuria cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):880–884. doi: 10.1073/pnas.85.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildeman A. G., Zenke M., Schatz C., Wintzerith M., Grundström T., Matthes H., Takahashi K., Chambon P. Specific protein binding to the simian virus 40 enhancer in vitro. Mol Cell Biol. 1986 Jun;6(6):2098–2105. doi: 10.1128/mcb.6.6.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]