Abstract
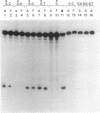
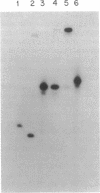
Substrate specificities of FPG protein (also known as formamidopyrimidine DNA glycosylase) and 8-hydroxyguanine endonuclease were compared by using defined duplex oligodeoxynucleotides containing single residues of 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG), 8-oxo-7,8-dihydro-2'-deoxyadenosine (8-oxodA), and 2,6-diamino-4-hydroxy-5-(N-methyl)formamidopyrimidine (Me-Fapy). Duplexes containing 8-oxodG positioned opposite dC, dG, or dT were cleaved, whereas single-stranded DNA and duplexes containing 8-oxodG.dA or 8-oxodA positioned opposite any of the four DNA bases were relatively resistant. Both enzymes cut duplexes containing 8-oxoG.dC 3' and 5' to the modified base but failed to cleave duplex DNA containing synthetic abasic sites, mismatches containing dG, or unmodified DNA. 8-Oxoguanine, identified by HPLC-electrochemical detection techniques, was released during the enzymatic reaction. Apparent Km values for FPG protein acting on duplex substrates containing a single Me-Fapy or 8-oxodG residue positioned opposite dC were 41 and 8 nM, respectively, and those for 8-hydroxyguanine endonuclease were 30 and 13 nM, respectively. Comparison of the properties of the two enzyme activities suggest that they are identical. In view of the widespread distribution of 8-oxodG in cellular DNA, the demonstrated miscoding and mutagenic properties of this lesion, and the existence of a bacterial gene coding for FPG protein, we propose that 8-oxodG DNA is the primary physiological substrate for a constituent glycosylase found in bacteria and mammalian cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailly V., Verly W. G. Importance of thiols in the repair mechanisms of DNA containing AP (apurinic or apyrimidinic) sites. Nucleic Acids Res. 1988 Oct 25;16(20):9489–9496. doi: 10.1093/nar/16.20.9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly V., Verly W. G., O'Connor T., Laval J. Mechanism of DNA strand nicking at apurinic/apyrimidinic sites by Escherichia coli [formamidopyrimidine]DNA glycosylase. Biochem J. 1989 Sep 1;262(2):581–589. doi: 10.1042/bj2620581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux S., Belleney J., Roques B. P., Laval J. Two rotameric forms of open ring 7-methylguanine are present in alkylated polynucleotides. Nucleic Acids Res. 1984 Jul 11;12(13):5429–5439. doi: 10.1093/nar/12.13.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux S., Laval J. Imidazole open ring 7-methylguanine: an inhibitor of DNA synthesis. Biochem Biophys Res Commun. 1983 Jan 27;110(2):552–558. doi: 10.1016/0006-291x(83)91185-3. [DOI] [PubMed] [Google Scholar]
- Boiteux S., O'Connor T. R., Laval J. Formamidopyrimidine-DNA glycosylase of Escherichia coli: cloning and sequencing of the fpg structural gene and overproduction of the protein. EMBO J. 1987 Oct;6(10):3177–3183. doi: 10.1002/j.1460-2075.1987.tb02629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux S., O'Connor T. R., Lederer F., Gouyette A., Laval J. Homogeneous Escherichia coli FPG protein. A DNA glycosylase which excises imidazole ring-opened purines and nicks DNA at apurinic/apyrimidinic sites. J Biol Chem. 1990 Mar 5;265(7):3916–3922. [PubMed] [Google Scholar]
- Breimer L. H. Enzymatic excision from gamma-irradiated polydeoxyribonucleotides of adenine residues whose imidazole rings have been ruptured. Nucleic Acids Res. 1984 Aug 24;12(16):6359–6367. doi: 10.1093/nar/12.16.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breimer L. H. Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: the role of DNA base damage. Mol Carcinog. 1990;3(4):188–197. doi: 10.1002/mc.2940030405. [DOI] [PubMed] [Google Scholar]
- Cabrera M., Nghiem Y., Miller J. H. mutM, a second mutator locus in Escherichia coli that generates G.C----T.A transversions. J Bacteriol. 1988 Nov;170(11):5405–5407. doi: 10.1128/jb.170.11.5405-5407.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetsanga C. J., Grigorian C. In situ enzymatic reclosure of opened imidazole rings of purines in DNA damaged by gamma-irradiation. Proc Natl Acad Sci U S A. 1985 Feb;82(3):633–637. doi: 10.1073/pnas.82.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetsanga C. J., Lindahl T. Release of 7-methylguanine residues whose imidazole rings have been opened from damaged DNA by a DNA glycosylase from Escherichia coli. Nucleic Acids Res. 1979 Aug 10;6(11):3673–3684. doi: 10.1093/nar/6.11.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetsanga C. J., Lozon M., Makaroff C., Savage L. Purification and characterization of Escherichia coli formamidopyrimidine-DNA glycosylase that excises damaged 7-methylguanine from deoxyribonucleic acid. Biochemistry. 1981 Sep 1;20(18):5201–5207. doi: 10.1021/bi00521a016. [DOI] [PubMed] [Google Scholar]
- Chetsanga C. J., Mavunga I. Chemical reclosure of opened imidazole ring of guanine. Chem Biol Interact. 1986 Apr;58(1):117–123. doi: 10.1016/s0009-2797(86)80091-6. [DOI] [PubMed] [Google Scholar]
- Cho B. P., Kadlubar F. F., Culp S. J., Evans F. E. 15N nuclear magnetic resonance studies on the tautomerism of 8-hydroxy-2'-deoxyguanosine, 8-hydroxyguanosine, and other C8-substituted guanine nucleosides. Chem Res Toxicol. 1990 Sep-Oct;3(5):445–452. doi: 10.1021/tx00017a010. [DOI] [PubMed] [Google Scholar]
- Chung M. H., Kasai H., Jones D. S., Inoue H., Ishikawa H., Ohtsuka E., Nishimura S. An endonuclease activity of Escherichia coli that specifically removes 8-hydroxyguanine residues from DNA. Mutat Res. 1991 Jan;254(1):1–12. doi: 10.1016/0921-8777(91)90035-n. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Wong P. K., Altmiller D. H., Rickard R. C. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun. 1986;1(3):163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- Fraga C. G., Shigenaga M. K., Park J. W., Degan P., Ames B. N. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski E., Rao G., Nackerdien Z., Dizdaroglu M. Modification of DNA bases in mammalian chromatin by radiation-generated free radicals. Biochemistry. 1990 Aug 28;29(34):7876–7882. doi: 10.1021/bi00486a014. [DOI] [PubMed] [Google Scholar]
- HEMS G. Effect of ionizing radiation on aqueous solutions of guanylic acid and guanosine. Nature. 1958 Jun 21;181(4625):1721–1722. doi: 10.1038/1811721a0. [DOI] [PubMed] [Google Scholar]
- Kadlubar F. F., Beranek D. T., Weis C. C., Evans F. E., Cox R., Irving C. C. Characterization of the purine ring-opened 7-methylguanine and its persistence in rat bladder epithelial DNA after treatment with the carcinogen N-methylnitrosourea. Carcinogenesis. 1984 May;5(5):587–592. doi: 10.1093/carcin/5.5.587. [DOI] [PubMed] [Google Scholar]
- Kasai H., Crain P. F., Kuchino Y., Nishimura S., Ootsuyama A., Tanooka H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986 Nov;7(11):1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- Kouchakdjian M., Bodepudi V., Shibutani S., Eisenberg M., Johnson F., Grollman A. P., Patel D. J. NMR structural studies of the ionizing radiation adduct 7-hydro-8-oxodeoxyguanosine (8-oxo-7H-dG) opposite deoxyadenosine in a DNA duplex. 8-Oxo-7H-dG(syn).dA(anti) alignment at lesion site. Biochemistry. 1991 Feb 5;30(5):1403–1412. doi: 10.1021/bi00219a034. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Mori F., Kasai H., Inoue H., Iwai S., Miura K., Ohtsuka E., Nishimura S. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987 May 7;327(6117):77–79. doi: 10.1038/327077a0. [DOI] [PubMed] [Google Scholar]
- Laval J., Boiteux S., O'Connor T. R. Physiological properties and repair of apurinic/apyrimidinic sites and imidazole ring-opened guanines in DNA. Mutat Res. 1990 Nov-Dec;233(1-2):73–79. doi: 10.1016/0027-5107(90)90152-t. [DOI] [PubMed] [Google Scholar]
- Margison G. P., Pegg A. E. Enzymatic release of 7-methylguanine from methylated DNA by rodent liver extracts. Proc Natl Acad Sci U S A. 1981 Feb;78(2):861–865. doi: 10.1073/pnas.78.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson B. J., Chang C. N., Grollman A. P., Henner W. D. Mechanism of DNA cleavage and substrate recognition by a bovine apurinic endonuclease. Biochemistry. 1989 May 2;28(9):3894–3901. doi: 10.1021/bi00435a040. [DOI] [PubMed] [Google Scholar]
- Shibutani S., Takeshita M., Grollman A. P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991 Jan 31;349(6308):431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- Shigenaga M. K., Gimeno C. J., Ames B. N. Urinary 8-hydroxy-2'-deoxyguanosine as a biological marker of in vivo oxidative DNA damage. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9697–9701. doi: 10.1073/pnas.86.24.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillwell W. G., Xu H. X., Adkins J. A., Wishnok J. S., Tannenbaum S. R. Analysis of methylated and oxidized purines in urine by capillary gas chromatography-mass spectrometry. Chem Res Toxicol. 1989 Mar-Apr;2(2):94–99. doi: 10.1021/tx00008a004. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Chang C. N., Johnson F., Will S., Grollman A. P. Oligodeoxynucleotides containing synthetic abasic sites. Model substrates for DNA polymerases and apurinic/apyrimidinic endonucleases. J Biol Chem. 1987 Jul 25;262(21):10171–10179. [PubMed] [Google Scholar]
- Wood M. L., Dizdaroglu M., Gajewski E., Essigmann J. M. Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry. 1990 Jul 31;29(30):7024–7032. doi: 10.1021/bi00482a011. [DOI] [PubMed] [Google Scholar]