Abstract
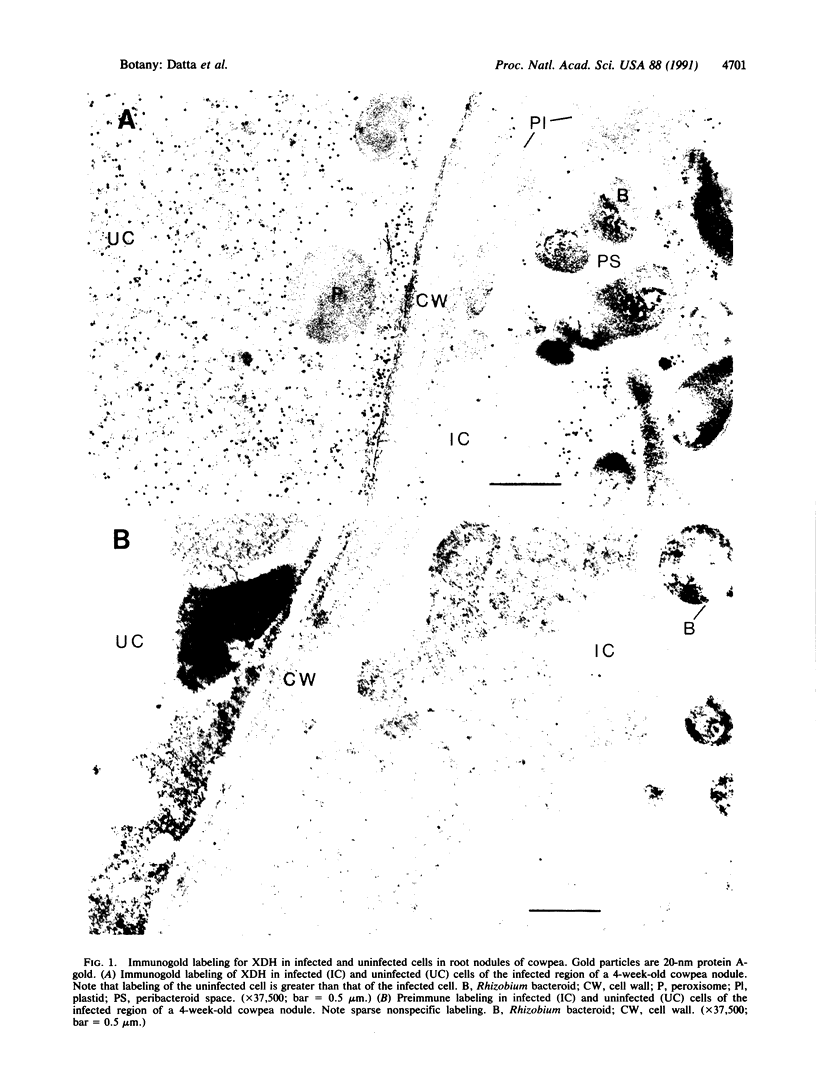
Immunocytochemistry was used to assess the location of xanthine dehydrogenase (EC 1.1.1.204) in the infected region of nodules of cowpea (Vigna unguiculata [L.] Walpers cv. Queen Anne Blackeye). Polyclonal antibodies raised against purified cowpea xanthine dehydrogenase were used to localize this enzyme at the electron microscopic level. Sparse nonspecific labeling was observed after treatment of nodule sections with preimmune serum. Although immune serum cross-reacted with the ground cytoplasm of both infected and uninfected cells, significantly more labeling was observed in the uninfected cells. No labeling above background was observed in peroxisomes, mitochondria, proplastids, endoplasmic reticulum, cytoplasmic or peribacteroid membranes, peribacteroid spaces, or bacteroids. The enzyme is soluble and not present in any organelle or membrane. The greater concentration of xanthine dehydrogenase in the uninfected cells suggests that xanthine or a precursor to xanthine, rather than uric acid, is the intermediate that moves from infected to uninfected cells during ureide biogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins C. A., Storer P. J., Pate J. S. Pathways of Nitrogen Assimilation in Cowpea Nodules Studied using N(2) and Allopurinol. Plant Physiol. 1988 Jan;86(1):204–207. doi: 10.1104/pp.86.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann H., Preddie E., Verma D. P. Nodulin-35: a subunit of specific uricase (uricase II) induced and localized in the uninfected cells of soybean nodules. EMBO J. 1983;2(12):2333–2339. doi: 10.1002/j.1460-2075.1983.tb01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland M. J., Schubert K. R. Biosynthesis of purines by a proplastid fraction from soybean nodules. Arch Biochem Biophys. 1983 Jan;220(1):179–187. doi: 10.1016/0003-9861(83)90398-3. [DOI] [PubMed] [Google Scholar]
- Hanks J. F., Schubert K., Tolbert N. E. Isolation and characterization of infected and uninfected cells from soybean nodules : role of uninfected cells in ureide synthesis. Plant Physiol. 1983 Apr;71(4):869–873. doi: 10.1104/pp.71.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks J. F., Tolbert N. E., Schubert K. R. Localization of enzymes of ureide biosynthesis in peroxisomes and microsomes of nodules. Plant Physiol. 1981 Jul;68(1):65–69. doi: 10.1104/pp.68.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T., Zelechowska M., Foster V., Bergmann H., Verma D. P. Primary structure of the soybean nodulin-35 gene encoding uricase II localized in the peroxisomes of uninfected cells of nodules. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5040–5044. doi: 10.1073/pnas.82.15.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelp B. J., Atkins C. A., Storer P. J., Canvin D. T. Cellular and subcellular organization of pathways of ammonia assimilation and ureide synthesis in nodules of cowpea (Vigna unguiculata L. Walp.). Arch Biochem Biophys. 1983 Jul 15;224(2):429–441. doi: 10.1016/0003-9861(83)90229-1. [DOI] [PubMed] [Google Scholar]
- Triplett E. W., Blevins D. G., Randall D. D. Allantoic Acid Synthesis in Soybean Root Nodule Cytosol via Xanthine Dehydrogenase. Plant Physiol. 1980 Jun;65(6):1203–1206. doi: 10.1104/pp.65.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W. Intercellular nodule localization and nodule specificity of xanthine dehydrogenase in soybean. Plant Physiol. 1985 Apr;77(4):1004–1009. doi: 10.1104/pp.77.4.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W., Lending C. R., Gumpf D. J., Ware C. F. Production, characterization, and applications of monoclonal antibodies reactive with soybean nodule xanthine dehydrogenase. Plant Physiol. 1986 Apr;80(4):965–971. doi: 10.1104/pp.80.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn K. C., Duke S. O., Duke S. H., Henson C. A. Ultrastructural localization of urate oxidase in nodules of Sesbania exaltata, Glycine max, and Medicago sativa. Histochemistry. 1982;74(3):309–318. doi: 10.1007/BF00493430. [DOI] [PubMed] [Google Scholar]