Abstract
Objectives
HIV infection is associated with increased prevalence of subclinical coronary plaque. The extent to which such plaque reflects effects of HIV infection or effects of long-term antiretroviral (ART) use remains unclear and was the goal of this analysis.
Design and Methods
We compared the prevalence and extent of coronary plaque and stenosis between users of specific ART drugs or drug classes using coronary computed tomography (CT) among HIV-infected men in the Multicenter AIDS Cohort Study. To account for time-dependent confounders, including cardiovascular disease (CVD) risk factors and time-varying reasons for using specific treatments, we conducted fully adjusted logistic and linear models with inverse probability of treatment weighting.
Results
There were 618 men who underwent non-contrast coronary CT; 450 also underwent coronary CT angiography. At the time of scanning 81% had undetectable plasma HIV RNA. In fully adjusted models, cumulative use of zidovudine, abacavir, darunavir and protease inhibitors as a drug class were inconsistently associated with specific forms of plaque presence or extent.
Conclusions
Among virally suppressed HIV-infected men with extensive ART exposure, no consistent associations between use of specific ART drugs and both subclinical coronary plaque presence and extent were apparent. Our findings support the hypothesis that, among virally suppressed persons, type of ART used is not in general a major determinant of subclinical coronary plaque risk.
Keywords: antiretroviral therapy, HIV, subclinical coronary plaque, atherosclerosis, coronary computed tomography (CT), coronary angiography
Introduction
In the era of highly active antiretroviral therapy (HAART), AIDS-related mortality has decreased significantly and age-related chronic non-opportunistic illnesses have become dominant causes of morbidity and mortality among aging HAART-treated HIV-infected persons, with cardiovascular disease (CVD) among these (1, 2). Compared to HIV-uninfected individuals, those who are HIV-infected have higher rates of acute coronary events (3) and subclinical coronary plaque (4, 5). Our group has reported on an increased prevalence of noncalcified coronary plaque among HIV-infected men compared with uninfected men (4). This association was recently confirmed in a meta-analysis that included 9 studies and more than 1200 HIV-infected individuals (6).
Chronic HIV infection is associated with increased levels of systemic immune activation and inflammation, both of which have been associated with increased risk of atherosclerosis in HIV-infected individuals (7-9). Several antiretroviral drugs and drug classes (including protease inhibitors, thymidine analog nucleotide reverse transcriptase inhibitors (TANRTIs) and abacavir) have been linked to metabolic abnormalities, such as insulin resistance and changes in body habitus, and increased risk of myocardial infarction(10-14). The extent to which HIV-associated subclinical coronary plaque reflects effects of long-term use of antiretroviral therapy (ART) versus effects of HIV infection itself remains unclear. Absence of association between specific ART drugs and subclinical plaque would suggest that the increased risk of or subclinical plaque is likely due to HIV infection and that ART type is a less important determinant of cardiovascular disease risk than achievement and maintenance of HIV virologic suppression, a factor known to be associated with avoidance of adverse cardiovascular clinical outcomes (15, 16). To address this gap we sought to characterize the associations between use of specific ART drugs or drug classes and presence and extent of subclinical coronary plaque among HIV-infected men.
Methods
Study population
The Multicenter AIDS Cohort Study (MACS) is an ongoing prospective cohort study of HIV infection among homosexual and bisexual men (17). Initial enrollment took place between 1984 and 1985 with subsequent enrollment periods between 1987 and 1991 and again between 2001 and 2003. Enrollment criteria into the ancillary cardiovascular study included active participation in MACS, age between 40 and 70 years, weight less than 136 kg, and no history of prior percutaneous coronary intervention or cardiac surgery. Participants underwent a non-contrast coronary computed tomography (CT) scan for coronary artery calcium (CAC) scoring. Men with glomerular filtration rate > 60 mL/min/1.73m2, no history of atrial fibrillation and no contrast allergy also underwent coronary CT angiography. The institutional review boards of all participating institutions approved this study. All participants provided informed consent.
Computed tomography scanning and analysis
These procedures have been described in detail elsewhere (18). Briefly, men received beta-blockers or calcium channel blockers as needed in preparation for cardiac imaging. Sublingual nitroglycerin was administered prior to intravenous contrast injection unless contraindicated. Imaging equipment consisted of either 64-slice or 320-row multi-detector CT. All CT images were transferred to a core CT reading center (Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center) where readers, blinded to participant characteristics assessed the presence, size, and composition of coronary plaque and the degree of luminal stenosis in all assessable coronary artery segments. Plaque size for each segment was graded as 0 for no plaque; 1 for a mild; 2 for a moderate; or 3 for a severe amount. Segment stenosis was defined as 0 (none), 1 (1%to 29% vascular luminal narrowing), 2 (30% to 49%), 3 (50% to 69%), or 4 (70%, stenosis). The overall extent of atherosclerosis (expressed as total plaque score) was calculated by summing the plaque size score for all assessable coronary segments that showed any plaque (calcified, noncalcified, or mixed), up to a maximum score of 45. Each coronary segment was classified as normal or containing calcified, noncalcified, or mixed (<50% of plaque area occupied by calcium) plaque. The calcified, noncalcified, and mixed plaque scores for each participant were calculated by separately summing the scores for calcified, noncalcified, and mixed plaque across all coronary segments. CAC score was computed using the Agatston method from non-contrast images (19).
Clinical variables
MACS participants are seen approximately every 6 months at research visits that include physical examination and cardiovascular disease risk factor assessment based on self-reported clinical history and blood tests. Race was based on self-report (Caucasian, African American, Hispanic/other). Standardized laboratory measurements performed at the time of visit included CD4 + T lymphocyte cell counts/mm3 (CD4); CD4/CD8 + T lymphocyte cell counts/mm3 ratio (CD4/CD8 ratio); plasma HIV RNA levels in copies/mm3; fasting serum glucose, total cholesterol and high-density lipoprotein (HDL) cholesterol levels; and hepatitis B (HBV) and hepatitis C (HCV) viremia. The estimation of cumulative pack-years of tobacco use, prescription of lipid lowering, diabetes and anti-hypertensive medications was based on self-report using a standardized questionnaire. HIV parameters including history of clinical AIDS and ART use were assessed at each study visit.
We evaluated cumulative use of classes of ART drugs and then of specific ART drugs in association with coronary plaque presence and extent. Specific ART drugs evaluated were chosen by the investigators on the basis of clinical data reported from our and other cohorts and other published data that have identified associations between specific drug use with risks for CVD, including plasma hyperlipidemia (protease inhibitors (PIs), including pharmaco-enhancing doses of ritonavir), insulin resistance (PIs, stavudine), body habitus changes (PIs, stavudine), risk of myocardial infarction (MI) (lopinavir/ritonavir, other PIs, abacavir) or other metabolic abnormalities (the TANRTIs zidovudine and stavudine) (10-14, 20-22). We defined cumulative use of classes of ART and specific ART drugs as continuous variables; individuals not exposed to the drug or drug class were considered to have a cumulative use of zero.
Statistical analysis
We analyzed the presence and extent of coronary plaque as separate outcomes. Each plaque outcome was analyzed independently including presence and extent of CAC; total, calcified, noncalcified and mixed plaque; and coronary stenosis. The presence of CAC or plaque was defined as a score greater than zero; presence of coronary stenosis was defined as stenosis > 50%. To assess associations between ART use and presence of plaque or coronary stenosis we performed logistic regression with the cumulative use of the drug of interest (per 1 year) as the predictor variable and adjusted for race and age at the time of scanning (minimally adjusted model). Since prior medical history (including CVD risk factors) may affect use of medications which then may affect these same risk factors, we used inverse probability of treatment weighting (IPTW) of the models that were fully adjusted by demographics, HIV parameters and CVD risk factors to account for this time-dependent confounding (23).
To estimate stabilized weights (SW), we ran two logistic models - a numerator model and a denominator model, to predict a specific ART drug use (yes or no) since last visit using longitudinal data from the visit when the ART drug was first used in the cohort up to the time of CT-scanning. The logistic model determining the numerator of the weights included time-fixed covariates (i.e., race and cohort entry status (enrolled after 2001 vs. pre-2001)), treatment history (i.e., cumulative years of any ART use before the drug was first used in the cohort, the same drug use at one visit before current visit, and the same drug use at two visits before current visit), and calendar year of the visit. The denominator model included all variables in the numerator model and also time-dependent covariates at the prior visit, which included: study center, age (years), body mass index (BMI, per 1 unit), cumulative pack-year smoking (per 1 year), prescription of anti-hypertensive medication (yes/no), systolic blood pressure (per 10mmHg), prescription of anti-diabetic medication (yes/no), fasting serum glucose (per 10 mg/dL), prescription of lipid-lowering medication (yes/no), HDL cholesterol (per 10 mg/dL), total cholesterol (per 10 mg/dL), chronic HBV infection (positive hepatitis B surface antigen), chronic HCV infection (detectable HCV RNA ), CD4/CD8 ratio, HIV RNA (per 1 log10) and history of clinical AIDS (yes/no). For each individual i at each visit j, we first calculate weights as the ratio (Rij) of the probability of treatment received from the numerator model to the probability of treatment received from the denominator model, and then multiply all weights (Ri1, Ri2,…, Ri(j-1), Rij) from the prior visits up to current visit to get SWij. For example, suppose an individual reported using abacavir at the first visit, but not at the second visit. Then his SWi2 at the second visit is the product of Ri1 (for the probability of receiving abacavir at the first visit) and Ri2 (for the probability of not receiving abacavir at the second visit). This was performed separately for each ART drug or class examined. The SW calculated at the last visit closest to CT-scanning were used in the fully-adjusted model, where we used logistic regression with generalized estimating equation including the cumulative use of the ART drug of interest (per 1 year increase) as a predictor variable with IPTW adjustment and further adjusted for age at the time of CT scanning, and all variables in the numerator model for weights. If the weights in fully-adjusted model were greater than ten, they were set to ten, which was >99th percentile of all weights (24). We used multiple imputation to generate missing values for those individuals who had missing covariates (25).
Five imputation data sets were created by using multivariate normal model including all variables in the denominator model for getting the SW. The final estimates of the association between plaque presence and cumulative ART drug use were obtained by averaging the weighted estimates from the five imputation data sets. Among men with coronary plaque present (i.e. plaque scores greater than zero), linear regression models were used to assess the association between ART use and extent of plaque (log-scale). The linear regression models were adjusted in the same manner as the logistic models. All analyses were done by using SAS 9.4 (SAS Institute, Cary, North Carolina, USA).
Results
A total of 618 HIV-infected men underwent non-contrast coronary CT scanning; 450 also underwent coronary CT angiography. The median age at the time of scanning was 53 years. Demographic and clinical characteristics prior to CT scanning are described in Table 1. At the time of scanning 81.4% of men had undetectable plasma HIV RNA levels (< 50 copies/mm3) and a median CD4 of 599. The median time since HAART initiation was 12 years and 56.3% of men had received mono- or dual- nucleotide reverse transcriptase inhibitors (NRTI) therapy before HAART initiation. Details about exposure to ART drug classes and specific ART drugs are described in Table 2.
Table 1. Demographic, laboratory and clinical characteristics at time of coronary CT scanning.
| Characteristic, median (IQR) or % | N = 618 |
|---|---|
| Age (years) | 53(48-58) |
| African American | 34% |
| Hispanic/Other | 13.40% |
| CD4 count (cells/mm3) | 599(422-756) |
| CD4/CD8 ratio | 0.74(0.5-1.04) |
| Detectable viral load* | 18.60% |
| Nadir CD4 count | 243(133-331) |
| History of AIDS | 14.20% |
| HAART use | 96% |
| HAART duration, years | 12(9-14) |
| ART use before HAART** | 56.30% |
| Duration of ART use before HAART, years | 8(3-11) |
| BMI, kg/m2 | 25(23-29) |
| Cumulative pack-years smoking | 6(0-24) |
| Hypertension medications | 36.10% |
| Systolic blood pressure (mm Hg) | 126(115-136) |
| Diabetes medications | 9.10% |
| Fasting glucose (mg/dL) | 98(90-107) |
| Lipid-lowering medications | 35.30% |
| Total Cholesterol (mg/dL) | 185(159-212) |
HIV RNA > 50 copies/mm3
mono- or dual- NRTI therapy before HAART initiation; IQR = interquartile range; NRTI = nucleotide reverse transcriptase inhibitors HAART = highly active antiretroviral therapy; BMI = body mass index; HDL = high density lipoprotein
Table 2. Cumulative antiretroviral exposure at the time of CT scanning.
| ART drug type and drug | Number of men ever exposed, n (%) | Median duration of exposure in years (IQR) |
|---|---|---|
| NRTI | 594(96.1) | 11.9(8.2-14.7) |
| abacavir | 270(43.7) | 4.1(1.8-7.3) |
| didanosine | 254(41.1) | 2.2(0.8-4.6) |
| emtricitabine | 417(67.5) | 3.7(2.1-5.4) |
| lamivudine | 522(84.5) | 7(3.7-9.9) |
| stavudine | 340(55) | 3.5(1.5-5.7) |
| tenofovir disproxil fumarate | 498(80.6) | 4.9(3-7.1) |
| zidovidine | 437(70.7) | 5(2-8.2) |
| NNRTI | 499(80.7) | 5.2(2.4-9) |
| delavirdine | 16(2.6) | 1.1(0.2-3) |
| efavirenz | 389(62.9) | 4.1(1.3-7.8) |
| etravirine | 36(5.8) | 2.2(1.4-3.3) |
| nevirapine | 203(32.8) | 3.2(0.9-7.2) |
| rilpivirine | 5(0.8) | 0.9(0.3-1) |
| Protease inhibitors | 479(77.5) | 7.3(3.9-10.9) |
| atazanavir | 192(31.1) | 4(1.8-5.8) |
| darunavir | 90(14.6) | 1.6(0.7-3.1) |
| fosamprenavir | 48(7.8) | 3.5(1.4-5.2) |
| indinavir | 201(32.5) | 2.8(0.9-5.1) |
| lopinavir | 186(30.1) | 4.1(1.3-6.4) |
| nelfinavir | 202(32.7) | 2(0.6-4.2) |
| ritonavir | 369(59.7) | 5.8(2.7-8.1) |
| saquinavir | 142(23) | 2(0.6-4.2) |
| Fusion/attachment inhibitors | 27(4.4) | 2(0.8-3.9) |
| enfuviritide | 20(3.2) | 1.4(0.6-3.5) |
| maraviroc | 12(1.9) | 1.8(0.6-2.9) |
| Integrase inhibitors | 117(18.9) | 1.8(0.8-2.7) |
| elvitegravir | 2(0.3) | 0.3(0.2-0.5) |
| raltegravir | 117(18.9) | 1.8(0.8-2.7) |
ART = antiretroviral therapy; IQR = interquartile range; NRTI = nucleoside reverse transcriptase inhibitor; NNRTI = non-nucleoside reverse transcriptase inhibitor
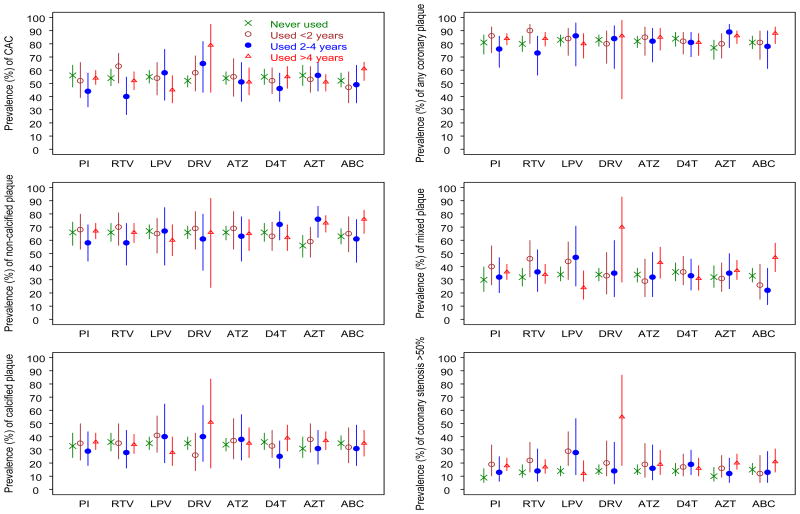
The overall prevalence of CAC was 53.1% with a median CAC score of 70. Among men who underwent coronary CT angiography (n=450) the prevalence of total plaque score > 0 (any coronary plaque) was 77.6% (table S1, Supplemental Digital Content 1). Noncalcified plaque was the most prevalent plaque type, identified in 63.3% of men overall. Figure 1 shows the age- and race-adjusted prevalence of specific types of plaque stratified by duration of exposure to each of the ART drugs and drug class selected for analysis. In these minimally adjusted models, the only consistent and significant (P=0.042) dose-effect response observed was CAC with increased use of darunavir. Long-term use (> 4 years) of darunavir use also was associated with stenosis.
Figure 1. Prevalence of plaque by antiretroviral drug use stratified by duration of exposure and adjusted for age and race.
The points represent the plaque prevalence and the bars represent the 95% confidence interval. CAC = coronary artery calcium, PI = protease inhibitor, RTV = ritonavir, LPV = lopinavir, DRV = darunavir, ATZ = atazanavir, D4T = stavudine, AZT = zidovudine, ABC = abacavir
To better characterize independent associations between cumulative exposure to specific ART drugs or to ART drug class with the presence and extent of coronary plaque we performed multivariable analysis as previously described (Table 3 and 4). Among the NRTIs evaluated, in fully adjusted models cumulative use of zidovudine was not associated with the presence of CAC, coronary plaque or stenosis. Among men in whom plaque was present, however, longer use of zidovudine was associated with increased extent of total plaque score and mixed plaque (3.3% increase per 1 year [standard error (SE)=0.012], P=0.005 and 2.5% increase per 1 year [SE=0.011], P=0.022). There was an association between zidovudine use and increased presence and extent of noncalcified plaque that was of borderline statistical significance (0.05<P<0.10). In fully adjusted models, cumulative use of abacavir was significantly associated with increased presence of noncalcified plaque (OR 1.1 [95% CI 1.01-1.19] per year, P=0.024) but not with extent of noncalcified plaque or with presence or extent of CAC, other forms of plaque or coronary stenosis. No significant associations with stavudine were observed.
Table 3. Association between antiretroviral therapy and the presence* of coronary plaque and coronary stenosis, OR per 1 year (95% CI).
| Outcome | abacavir | P | stavudine | P | zidovudine | P | PIs | P | atazanavir | P | darunavir | P | lopinavir | P | ritonavir | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAC | ||||||||||||||||
| minimally adjusted model | 1.06(1,1.12) | 0.042 | 0.99(0.93,1.05) | 0.674 | 0.99(0.96,1.03) | 0.715 | 1(0.97,1.04) | 0.903 | 1(0.92,1.08) | 0.942 | 1.26(1.01,1.56) | 0.038 | 0.95(0.88,1.02) | 0.130 | 0.99(0.95,1.04) | 0.688 |
| fully adjusted model | 1.05(0.99,1.12) | 0.129 | 0.95(0.86,1.05) | 0.321 | 0.99(0.94,1.06) | 0.871 | 0.97(0.9,1.05) | 0.437 | 1.15(0.95,1.38) | 0.144 | 1.15(0.67,1.99) | 0.613 | 0.96(0.83,1.1) | 0.540 | 1.04(0.91,1.17) | 0.581 |
| Any plaque | ||||||||||||||||
| minimally adjusted model | 1.07(0.98,1.17) | 0.109 | 0.96(0.87,1.05) | 0.340 | 1.04(0.98,1.1) | 0.166 | 1.01(0.96,1.07) | 0.580 | 1.06(0.94,1.19) | 0.372 | 1.04(0.77,1.41) | 0.805 | 0.98(0.9,1.07) | 0.670 | 1.03(0.97,1.1) | 0.329 |
| fully adjusted model | 1.06(0.96,1.17) | 0.242 | 0.95(0.85,1.05) | 0.311 | 1.02(0.94,1.1) | 0.703 | 0.99(0.9,1.08) | 0.814 | 1.24(0.99,1.54) | 0.059 | 1.12(0.64,1.97) | 0.689 | 1.02(0.83,1.25) | 0.845 | 1.06(0.92,1.22) | 0.402 |
| Noncalcified plaque | ||||||||||||||||
| minimally adjusted model | 1.07(1,1.15) | 0.050 | 0.98(0.91,1.06) | 0.681 | 1.06(1.01,1.11) | 0.015 | 1.01(0.97,1.05) | 0.706 | 1(0.91,1.09) | 0.939 | 0.98(0.76,1.25) | 0.843 | 0.95(0.88,1.02) | 0.180 | 1(0.95,1.06) | 0.922 |
| fully adjusted model | 1.1(1.01,1.19) | 0.024 | 0.98(0.9,1.08) | 0.728 | 1.06(0.99,1.14) | 0.084 | 1.03(0.96,1.1) | 0.461 | 1.05(0.85,1.29) | 0.636 | 1.18(0.69,2.03) | 0.543 | 1.02(0.83,1.24) | 0.870 | 1.06(0.95,1.18) | 0.328 |
| Calcified plaque | ||||||||||||||||
| minimally adjusted model | 1.01(0.95,1.08) | 0.729 | 1.01(0.94,1.09) | 0.805 | 1.03(0.99,1.08) | 0.157 | 1.01(0.97,1.05) | 0.677 | 1.01(0.92,1.11) | 0.766 | 1.06(0.84,1.35) | 0.603 | 0.98(0.9,1.06) | 0.644 | 0.99(0.94,1.04) | 0.593 |
| fully adjusted model | 0.98(0.9,1.06) | 0.563 | 1(0.92,1.09) | 0.989 | 1.02(0.95,1.11) | 0.551 | 1.03(0.94,1.12) | 0.564 | 1.06(0.86,1.32) | 0.585 | 2.28(1.04,5.02) | 0.040 | 0.92(0.75,1.12) | 0.375 | 1.02(0.92,1.14) | 0.669 |
| Mixed plaque | ||||||||||||||||
| minimally adjusted model | 1.08(1.01,1.15) | 0.019 | 0.96(0.89,1.04) | 0.314 | 1.02(0.97,1.06) | 0.437 | 1.01(0.96,1.05) | 0.768 | 1.08(0.98,1.18) | 0.109 | 1.21(0.95,1.53) | 0.123 | 0.91(0.83,1) | 0.039 | 1(0.95,1.05) | 0.999 |
| fully adjusted model | 1.06(0.98,1.15) | 0.130 | 0.93(0.84,1.03) | 0.185 | 1.01(0.94,1.09) | 0.793 | 1.01(0.93,1.1) | 0.728 | 1.11(0.91,1.36) | 0.296 | 1.24(0.73,2.12) | 0.425 | 0.98(0.83,1.16) | 0.812 | 0.96(0.87,1.05) | 0.332 |
| Coronary stenosis | ||||||||||||||||
| minimally adjusted model | 1.06(0.99,1.14) | 0.108 | 0.99(0.9,1.09) | 0.861 | 1.06(1,1.11) | 0.040 | 1.06(1.01,1.12) | 0.030 | 1.06(0.95,1.18) | 0.313 | 1.25(0.96,1.64) | 0.100 | 0.94(0.84,1.06) | 0.309 | 1.04(0.98,1.11) | 0.221 |
| fully adjusted model | 1.04(0.95,1.13) | 0.387 | 0.98(0.87,1.09) | 0.666 | 1.07(0.96,1.19) | 0.227 | 1.05(0.96,1.15) | 0.321 | 1.04(0.8,1.33) | 0.788 | 2.54(0.94,6.9) | 0.067 | 1.12(0.93,1.35) | 0.246 | 1.07(0.94,1.23) | 0.311 |
Plaque score > 0; associations with P value < 0.05 for fully adjusted models are printed in bold. The minimally adjusted models adjusted for age and race; the fully adjusted models adjusted for inverse probability of treatment weighting (IPTW), see text for details. OR = odds ratio; CI = confidence interval; PIs = protease inhibitors; CAC = coronary artery calcium
Table 4. Association between antiretroviral therapy and extent of coronary plaque, estimate change in score* per 1 year (SE).
| Outcome | abacavir | P | stavudine | P | zidovudine | P | PIs | P | atazanavir | P | darunavir | P | lopinavir | P | ritonavir | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAC score | ||||||||||||||||
| minimally adjusted model | 0.029(0.026) | 0.272 | 0.023(0.031) | 0.457 | 0.013(0.02) | 0.512 | -0.005(0.019) | 0.770 | -0.002(0.04) | 0.959 | 0.047(0.09) | 0.607 | -0.063(0.039) | 0.106 | -0.024(0.023) | 0.280 |
| fully adjusted model | 0.03(0.033) | 0.365 | 0.056(0.04) | 0.168 | 0.001(0.024) | 0.965 | 0.032(0.028) | 0.253 | 0.024(0.071) | 0.732 | 0.38(0.134) | 0.005 | -0.051(0.086) | 0.559 | 0.051(0.051) | 0.318 |
| Total plaque score | ||||||||||||||||
| minimally adjusted model | 0.009(0.013) | 0.505 | 0.012(0.017) | 0.456 | 0.027(0.009) | 0.004 | 0.012(0.009) | 0.187 | 0.016(0.019) | 0.406 | 0.082(0.051) | 0.107 | -0.031(0.017) | 0.070 | -0.003(0.011) | 0.790 |
| fully adjusted model | 0.002(0.017) | 0.928 | 0.013(0.019) | 0.478 | 0.033(0.012) | 0.005 | 0.027(0.013) | 0.042 | 0.008(0.039) | 0.841 | 0.26(0.11) | 0.018 | -0.005(0.035) | 0.890 | 0.021(0.023) | 0.366 |
| Noncalcified plaque score | ||||||||||||||||
| minimally adjusted model | -0.005(0.012) | 0.670 | 0.007(0.016) | 0.653 | 0.012(0.009) | 0.187 | 0.019(0.009) | 0.029 | 0.015(0.019) | 0.429 | 0.055(0.051) | 0.286 | 0.012(0.018) | 0.509 | 0.016(0.011) | 0.127 |
| fully adjusted model | -0.009(0.014) | 0.510 | 0.002(0.022) | 0.930 | 0.022(0.012) | 0.078 | 0.028(0.015) | 0.061 | 0.009(0.038) | 0.810 | 0.17(0.137) | 0.217 | 0.012(0.026) | 0.643 | 0.01(0.018) | 0.579 |
| Calcified plaque score | ||||||||||||||||
| minimally adjusted model | -0.001(0.018) | 0.949 | 0.014(0.021) | 0.516 | 0.006(0.012) | 0.653 | 0.002(0.013) | 0.891 | 0.027(0.026) | 0.313 | -0.017(0.064) | 0.797 | -0.057(0.024) | 0.019 | -0.002(0.016) | 0.915 |
| fully adjusted model | 0.003(0.021) | 0.874 | 0.032(0.024) | 0.175 | 0.009(0.01) | 0.372 | 0.004(0.029) | 0.894 | -0.009(0.046) | 0.842 | 0.041(0.123) | 0.739 | -0.046(0.047) | 0.330 | 0.015(0.023) | 0.501 |
| Mixed plaque score | ||||||||||||||||
| minimally adjusted model | 0.007(0.016) | 0.683 | 0.004(0.021) | 0.856 | 0.023(0.012) | 0.053 | 0.002(0.013) | 0.884 | -0.036(0.024) | 0.128 | 0.104(0.058) | 0.076 | 0.02(0.031) | 0.514 | -0.01(0.015) | 0.511 |
| fully adjusted model | 0.008(0.016) | 0.631 | 0.005(0.027) | 0.862 | 0.025(0.011) | 0.022 | -0.01(0.015) | 0.492 | -0.058(0.05) | 0.250 | 0.186(0.111) | 0.095 | 0.034(0.057) | 0.553 | 0.059(0.04) | 0.142 |
Log-transformed; associations with P value < 0.05 for fully adjusted models are printed in bold. The minimally adjusted models adjusted for age and race; the fully adjusted models adjusted for inverse probability of treatment weighting (IPTW), see text for details. SE = standard error; PIs = protease inhibitors; CAC= coronary artery calcium
Although in the minimally adjusted model cumulative use of PIs was associated with the presence of stenosis, it was not associated with the presence of CAC, coronary plaque or coronary stenosis in fully adjusted models. However, among men for whom coronary plaque was present, longer duration of PI use was associated with increased extent of total plaque score in fully adjusted models (2.7% increase [SE=0.013] per year, P=0.042). Analysis by specific protease inhibitor type revealed that longer darunavir use was associated with increased presence of calcified plaque (OR 2.28 [95% CI 1.04-5.02] per year, P=0.04) but not with extent of calcified plaque in fully adjusted models. Cumulative use of darunavir was associated with increased extent of CAC and total plaque score (38 % increase [SE=0.134] per year, P=0.005 and 26% increase [SE=0.11] per year, P=0.018, respectively). Its associations with increased presence of coronary stenosis and extent of mixed plaque were of borderline statistical significance (0.05<P<0.10). No other associations with specific protease inhibitors were observed in the fully adjusted models.
To further explore characteristics of men exposed to zidovudine and darunavir (the two antiretroviral drugs whose use demonstrated the most significant associations with coronary plaque) we examined differences in HIV characteristics between men who were exposed compared to men who were naïve to each of these drugs. We found that men who received zidovudine and men who received darunavir had longer median durations of HIV infection, lower median CD4 counts at the time of HAART initiation and a higher percentage of visits with detectable HIV RNA (>= 50 copies/mm3) compared to men who had not received these medications (table S2, Supplemental Digital Content 2).
Discussion
In this very-well characterized, longitudinally followed group of aging HIV-infected men, the presence and extent of subclinical coronary plaque was inconsistently linked to the use of antiretroviral therapy, either specific drugs or drug classes. When associations between cumulative use of ART and plaque were found, they in general involved modest effect sizes and marginal statistical significance for either plaque presence or plaque extent but not both. The HIV-infected participants were, in general, a group that was durably virologically suppressed while receiving combination ART. While we did not have available for comparison a sizable group of HIV-infected persons who were not receiving ART, the implications of these findings may include that the types of ART used do not comprise a distinct substantial risk for coronary plaque presence or extent. Furthermore, these findings support the hypothesis that ART type is a less important determinant of cardiovascular disease risk than achievement and maintenance of HIV virologic suppression, a factor known to be associated with avoidance of adverse cardiovascular clinical outcomes (15, 16). In this context, our findings support the assertion that virologic suppression achieved through ART use may outweigh or even offset any potential negative impact of individual or combination ART on coronary atherogenesis or its predisposing conditions.
We found that cumulative use of the TANRTI zidovudine was significantly associated with increased extent of total plaque score and mixed plaque; also with presence and extent of noncalcified plaque with borderline statistical significance. While positive associations between use of TANRTIs and increases in traditional CVD risk factors have been reported extensively in the literature, we found no significant associations between stavudine, the other commonly used TANRTI, and coronary plaque, raising the question of a possibly unique risk conferred by zidovudine use that might be distinct from the class effects of TANRTIs. Alternatively, these associations might be a surrogate marker for longer duration of HIV infection, including more prolonged exposure to unsuppressed HIV viral replication among persons exposed to zidovudine.
We found a limited positive association between abacavir use and presence, but not extent, of noncalcified plaque. The existing literature on the association between abacavir and cardiovascular disease is extensive, conflicting and not yet conclusive(26). Moreover, reported associations between abacavir use and risk for MI suggest that MI risk is associated with very recent abacavir use (usually within 6 months of use), implying that if such an effect exists, it may be reversible with discontinued use of the drug (27). Furthermore, a hypothesized mechanism by which abacavir use may be linked to MI risk involves a net positive effect upon intravascular platelet activation, also potentially reversible and thereby not necessarily reflected in coronary plaque burden (28).
In this group of aging, virally suppressed HIV-infected men with extensive protease inhibitor exposure, no consistent associations between protease inhibitor use and subclinical coronary plaque presence and extent were apparent. Cumulative darunavir use was associated with a substantial increase in prevalence of calcified plaque and extent of CAC and total plaque score among the relatively few studied men (n=90) who were darunavir-exposed. Given this small sample size, as evidenced by the broad confidence intervals, our measurement of the effect size is probably not precise and inferences between darunavir use and coronary plaque risk are not possible from these data alone. Also, the associations might reflect the effect of longer duration of HIV infection and unsuppressed HIV viremia among darunavir-exposed men compared to non-darunavir-exposed men. In support of this interpretation, unlike some of the older protease inhibitors, darunavir use has not, per se, been demonstrated to constitute a distinct association with factors known to increase CVD risk in the general population, such as pro-atherogenic hyperlipidemia or insulin resistance (29, 30). Furthermore, in the absence of clinical outcome data linking darunavir to increased CVD events, our findings regarding association of its use with greater prevalence and amount of specific plaque types among the relatively small number of darunavir users in our cohort need to be interpreted with caution.
As was demonstrated in the SMART (Strategies for Management of Antiretroviral Therapy) trial, the benefits of continuous ART-related HIV suppression (and possibly immune repletion), regardless of the ART drugs used probably far outweigh any deleterious or CVD-predisposing effects of any specific ART use that might be mediated through classic CVD risk factors(16). HIV non-suppression is clearly associated with greater levels of systemic inflammation and adverse immune activation (7, 31), processes that have been linked to coronary atherogenesis in the general population (32). ART-induced HIV RNA suppression reduces systemic inflammation and immune activation, which in turn may decrease risk for coronary atherogenesis and/or plaque progression (31).
It is important to note that in the current study our focus was on subclinical coronary plaque measured by coronary CT and not on coronary artery disease (CAD)-related clinical events. In the general population, however, the extent of CAC is a strong predictor of CAD events (33). Also, the presence of noncalcified or mixed plaque has been associated with increased risk of coronary events in symptomatic patients (34). Our group recently reported that HIV infection was associated with a higher prevalence and extent of noncalcified plaque compared to HIV-uninfected men (4). Our current findings suggest that overall exposure to specific antiretroviral therapies is unlikely to be a significant driver of these associations. HIV parameters including CD4 nadir, CD4 count and levels of inflammatory biomarkers have been found to be independent risk factors for cardiovascular disease among HIV-infected individuals, suggesting that HIV infection itself contributes to cardiovascular disease risk (35). The association between duration of HAART use and coronary stenosis recently reported by our group (4) may indicate that HAART use duration is a surrogate marker for duration of HIV infection and less likely a discreet risk factor for coronary stenosis associated with specific antiretroviral drug use.
Our study has important limitations including the cross-sectional nature of the plaque data, and the fact that persons were exposed to many different ART therapies over time that were variably remote from the time of scanning. We attempted to control for confounders using multivariable analysis, however in this observational non-randomized study it is not possible to control for all potentially confounding variables. Also, our study included only men who have sex with men, therefore the findings might not be generalizable to women or to all men.
In this group of aging HIV-infected, largely virally-suppressed men with extensive exposure to ART, we found no consistent association between the use of specific ART drugs with both the presence and extent of coronary plaque, despite the fact that use of some of the studied drugs has been associated with clinical factors known to increase coronary plaque risk in the general population. We hypothesize that the lack of consistent associations between ART use and plaque presence and extent reflect that the durable benefits of prolonged HIV suppression that ART use affords offset any drug-associated risks for coronary plaque in this population. Future work should include systematic longitudinal evaluation of ART naive persons who initiate specific ART therapy and assessment for the development and progression of subclinical plaque as well as clinical CVD outcomes.
Supplementary Material
Acknowledgments
GPT analysed the data and wrote the manuscript; XL analysed the data and revised the manuscript; WSP, LPJ, MDW, TTB, LK and JPF designed the study and revised the manuscript; FJP designed the study, analysed the data and wrote the manuscript. The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute (grants UO1-AI-35042, UL1-RR025005, UM1-AI-35043, UO1-AI-35039, UO1-AI-35040, and UO1-AI-35041). This study is funded by the National Heart, Lung, and Blood Institute (grant RO1 HL095129 to W.S.P.), with additional support from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health and National Institutes of Health Roadmap for Medical Research (grant UL1 TR 001079).
Source of Funding: The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute (grants UO1-AI-35042, UL1-RR025005, UM1-AI-35043, UO1-AI-35039, UO1-AI-35040, and UO1-AI-35041). This study is funded by the National Heart, Lung, and Blood Institute (grant RO1 HL095129 to WSP), with additional support from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health and National Institutes of Health Roadmap for Medical Research (grant UL1 TR 001079)
Footnotes
Conflicts of Interest: TTB has served as a consultant to Gilead Sciences, Merck, ViiV Healthcare, Abbvie, EMD-Serono, Bristol Myers Squibb, and Theratechnologies; FJP serves as a consultant for and on speakers' bureau for Gilead Sciences, Janssen Pharmaceuticals, Merck and Co., and Bristol Myers Squibb; MDW has served as a consultant for Gilead Sciences. For the remaining authors no conflicts of interest were declared.
References
- 1.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 2.Feinstein MJ, Bahiru E, Achenbach C, Longenecker CT, Hsue P, So-Armah K, et al. Patterns of Cardiovascular Mortality for HIV-Infected Adults in the United States: 1999 to 2013. The American journal of cardiology. 2015 doi: 10.1016/j.amjcard.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA internal medicine. 2013;173(8):614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Post WS, Budoff M, Kingsley L, Palella FJ, Jr, Witt MD, Li X, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Annals of internal medicine. 2014;160(7):458–67. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. Aids. 2010;24(2):243–53. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Ascenzo F, Cerrato E, Calcagno A, Grossomarra W, Ballocca F, Omede P, et al. High prevalence at computed coronary tomography of non-calcified plaques in asymptomatic HIV patients treated with HAART: a meta-analysis. Atherosclerosis. 2015;240(1):197–204. doi: 10.1016/j.atherosclerosis.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. Aids. 2009;23(9):1059–67. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo J, Plutzky J. The biology of atherosclerosis: general paradigms and distinct pathogenic mechanisms among HIV-infected patients. The Journal of infectious diseases. 2012;205(Suppl 3):S368–74. doi: 10.1093/infdis/jis201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ, Jr, Kingsley LA, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. The Journal of infectious diseases. 2015;211(8):1219–28. doi: 10.1093/infdis/jiu594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351(9119):1881–3. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- 11.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Archives of internal medicine. 2005;165(10):1179–84. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 12.Riddler SA, Li X, Chu H, Kingsley LA, Dobs A, Evans R, et al. Longitudinal changes in serum lipids among HIV-infected men on highly active antiretroviral therapy. HIV medicine. 2007;8(5):280–7. doi: 10.1111/j.1468-1293.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- 13.Group DADS. Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, et al. Class of antiretroviral drugs and the risk of myocardial infarction. The New England journal of medicine. 2007;356(17):1723–35. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 14.Saint-Marc T, Partisani M, Poizot-Martin I, Bruno F, Rouviere O, Lang JM, et al. A syndrome of peripheral fat wasting (lipodystrophy) in patients receiving long-term nucleoside analogue therapy. Aids. 1999;13(13):1659–67. doi: 10.1097/00002030-199909100-00009. [DOI] [PubMed] [Google Scholar]
- 15.Neuhaus J, Angus B, Kowalska JD, La Rosa A, Sampson J, Wentworth D, et al. Risk of all-cause mortality associated with nonfatal AIDS and serious non-AIDS events among adults infected with HIV. Aids. 2010;24(5):697–706. doi: 10.1097/QAD.0b013e3283365356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strategies for Management of Antiretroviral Therapy Study G. El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, et al. CD4+ count-guided interruption of antiretroviral treatment. The New England journal of medicine. 2006;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 17.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. American journal of epidemiology. 1987;126(2):310–8. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 18.Hacioglu Y, Gupta M, Choi TY, George RT, Deible CR, Jacobson LP, et al. Use of cardiac CT angiography imaging in an epidemiology study - the Methodology of the Multicenter AIDS Cohort Study cardiovascular disease substudy. Anadolu kardiyoloji dergisi : AKD = the Anatolian journal of cardiology. 2013;13(3):207–14. doi: 10.5152/akd.2013.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 20.Holmberg SD, Moorman AC, Williamson JM, Tong TC, Ward DJ, Wood KC, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360(9347):1747–8. doi: 10.1016/S0140-6736(02)11672-2. [DOI] [PubMed] [Google Scholar]
- 21.Fontas E, van Leth F, Sabin CA, Friis-Moller N, Rickenbach M, d'Arminio Monforte A, et al. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? The Journal of infectious diseases. 2004;189(6):1056–74. doi: 10.1086/381783. [DOI] [PubMed] [Google Scholar]
- 22.Palella FJ, Jr, Gange SJ, Benning L, Jacobson L, Kaplan RC, Landay AL, et al. Inflammatory biomarkers and abacavir use in the Women's Interagency HIV Study and the Multicenter AIDS Cohort Study. Aids. 2010;24(11):1657–65. doi: 10.1097/QAD.0b013e3283389dfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. American journal of epidemiology. 2008;168(6):656–64. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schafer JL. Analysis of incomplete multivariate data. CRC press; 1997. [Google Scholar]
- 26.Llibre JM, Hill A. Abacavir and cardiovascular disease: A critical look at the data. Antiviral Res. 2016;132:116–21. doi: 10.1016/j.antiviral.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Worm SW, Sabin C, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. The Journal of infectious diseases. 2010;201(3):318–30. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 28.Satchell CS, O'Halloran JA, Cotter AG, Peace AJ, O'Connor EF, Tedesco AF, et al. Increased platelet reactivity in HIV-1-infected patients receiving abacavir-containing antiretroviral therapy. The Journal of infectious diseases. 2011;204(8):1202–10. doi: 10.1093/infdis/jir509. [DOI] [PubMed] [Google Scholar]
- 29.Saumoy M, Ordonez-Llanos J, Martinez E, Ferrer E, Domingo P, Ribera E, et al. Atherogenic properties of lipoproteins in HIV patients starting atazanavir/ritonavir or darunavir/ritonavir: a substudy of the ATADAR randomized study. The Journal of antimicrobial chemotherapy. 2015;70(4):1130–8. doi: 10.1093/jac/dku501. [DOI] [PubMed] [Google Scholar]
- 30.Martinez E, Gonzalez-Cordon A, Ferrer E, Domingo P, Negredo E, Gutierrez F, et al. Differential body composition effects of protease inhibitors recommended for initial treatment of HIV infection: a randomized clinical trial. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60(5):811–20. doi: 10.1093/cid/ciu898. [DOI] [PubMed] [Google Scholar]
- 31.Arildsen H, Sorensen KE, Ingerslev JM, Ostergaard LJ, Laursen AL. Endothelial dysfunction, increased inflammation, and activated coagulation in HIV-infected patients improve after initiation of highly active antiretroviral therapy. HIV medicine. 2013;14(1):1–9. doi: 10.1111/j.1468-1293.2012.01027.x. [DOI] [PubMed] [Google Scholar]
- 32.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 33.Tota-Maharaj R, Blaha MJ, Blankstein R, Silverman MG, Eng J, Shaw LJ, et al. Association of coronary artery calcium and coronary heart disease events in young and elderly participants in the multi-ethnic study of atherosclerosis: a secondary analysis of a prospective, population-based cohort. Mayo Clinic proceedings. 2014;89(10):1350–9. doi: 10.1016/j.mayocp.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmadi N, Nabavi V, Hajsadeghi F, Flores F, French WJ, Mao SS, et al. Mortality incidence of patients with non-obstructive coronary artery disease diagnosed by computed tomography angiography. The American journal of cardiology. 2011;107(1):10–6. doi: 10.1016/j.amjcard.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 35.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–55. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.