Abstract
Adenosine, a nucleoside derived primarily from the extracellular hydrolysis of adenine nucleotides, is a potent regulator of inflammation. Adenosine mediates its effects on inflammatory cells by engaging one or more cell-surface receptors. The expression and function of adenosine receptors on different cell types change during the course of rheumatic diseases, such as rheumatoid arthritis. Targeting adenosine receptors directly for the treatment of rheumatic diseases is currently under study; however, indirect targeting of adenosine receptors by enhancing adenosine levels at inflamed sites accounts for most of the anti-inflammatory effects of methotrexate, the anchor drug for the treatment of rheumatoid arthritis. In this Review, we discuss the regulation of extracellular adenosine levels and the role of adenosine in regulating the inflammatory and immune responses in rheumatic diseases such as Rheumatoid Arthritis, psoriasis and other types of inflammatory arthritis. In addition, adenosine and its receptors are involved in promoting fibrous matrix production in the skin and other organs and the role of adenosine in fibrosis and fibrosing diseases will also be discussed.
Introduction
Many different intercellular signals maintain homeostasis in tissues and organs. One of the first of these signals identified in the regulation of a large number of physiological and pathological processes was adenosine. First identified as a potent vasodilator in 1929 by Drury and Szent-Gyorgi1, adenosine was subsequently shown to mediate its effects on cells via engagement of specific receptors (A1, A2a, A2b and A3 2,3). Indeed, adenosine has been described as a ‘retaliatory metabolite’ 4 that is released from cells in response to hypoxia, metabolic stress or injury and promotes the processes required to alleviate these noxious stimuli. Among its other functions, adenosine is an endogenous regulator of inflammation that mediates the transition from inflammation to healing. Pathological changes in, or pharmacological manipulation of, adenosine metabolism or adenosine receptor expression and/or function might have a role in both the pathogenesis and therapy of rheumatic diseases. We review the metabolic changes that regulate adenosine levels in inflamed tissue, the receptors that mediate the pharmacological and pathological effects of adenosine and their role in rheumatic diseases, as well as the potential role for therapeutic targeting of adenosine and its receptors.
Adenosine in inflammation and fibrosis
Inflammation of the joints, connective tissue, muscle and bone are the most common manifestations of the rheumatic diseases; both the innate and adaptive immune systems can contribute to the inflammation observed in diseases such as rheumatoid arthritis (RA) and psoriatic arthritis. In most inflammatory arthritides neutrophils are the most abundant cells in synovial fluids whereas T cells, B cells and macrophages predominate in the synovial tissues. At inflamed sites a large number of intercellular messengers and effector molecules secreted by the various cells are present, ranging in size from low molecular weight products (such as nitric oxide and prostaglandins among many others) to proteins (cytokines, growth factors, proteolytic enzymes and others). The cells (for example, synovial fibroblast-like cells and vascular endothelial cells) of the tissues that comprise the joint also contribute to injury and destruction of the structures of the joint; osteoclasts are critical to bone destruction, and chondrocytes secrete enzymes that destroy cartilage in inflamed joints. Adenosine, acting at its receptors, regulates the activation of all of these cell types and their secretion of intercellular messengers and effector molecules of all classes.
A common end point of inflammation is fibrosis or scarring, which can disrupt the appropriate functioning of the organ affected. Thus, joint contractures are also characteristic of inflammatory arthritis, and fibrosis characterizes organs affected in extra-articular RA, such as in the lung. The major manifestations of systemic sclerosis (SSc) are fibrosis of the skin and internal organs. Adenosine and its receptors also have a role in the pathogenesis of fibrosis in many different organs and might have a role in SSc as well.
Sources of adenosine
Intracellular ATP, the most abundant molecule in the cell, serves as the reservoir for the production of adenosine; however, most adenosine is formed in the extracellular space as a result of the sequential dephosphorylation of adenine nucleotides to adenosine (Figure 1). A number of transporters are involved in the export of ATP, including the proteins connexin-43 (also known as gap junction alpha-1 protein) 5, progressive ankylosis protein homolog (ANK) 6, pannexin-1 and pannexin-3 7, and probably others as well, including P2×78. Under resting conditions some ATP is dephosphorylated to adenosine but injury, hypoxia or other metabolic assault can trigger increased rates of intracellular conversion of ATP to adenosine or, more commonly, stimulate the release of adenine nucleotides into the extracellular space where they are dephosphorylated to adenosine by ectoenzymes at the cell surface (ecto-5’nucleotidase [CD73] and ecto-nucleoside triphosphate phosphohydrolase [CD39]) and by enzymes in blood or other extracellular fluids (for example, alkaline or acid phosphatases, including tissue nonspecific alkaline phosphatase [TNAP]). Once formed or released into the extracellular space adenosine can be deaminated to inosine and, in humans, ultimately to uric acid or taken up directly by cells by specific nucleoside transporters (ENT1 and ENT2) 9 and re-phosphorylated to ATP (Figure 1) 6,9-16.
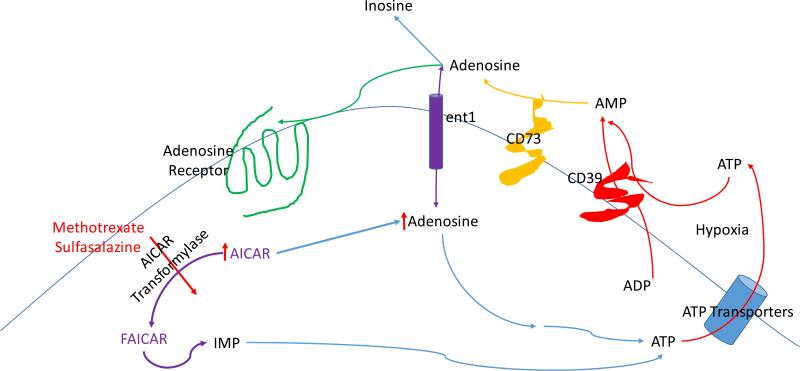
Figure 1. Cellular adenosine production in response to hypoxia and medications.
Adenosine is produced extracellularly from the hydrolysis of adenine nucleotides (ATP, ADP and AMP) by the ectoenzymes nucleoside triphosphate phosphohydrolase (NTPP or CD39) and ecto- 5’nucleotidase (CD73) whereas adenosine levels in the extracellular space are reduced by cellular uptake via the extracellular nucleoside transporter (ent1) and breakdown extracellularly to inosine by adenosine deaminase. Both methotrexate and sulfasalazine diminish the activity of aminoimidazolecarboxamido ribonucleotide (AICAR) transformylase leading to accumulation of AICAR and reduction of its metabolite formyl AICAR (FAICAR). Intracellular accumulation of AICAR leads to increased ATP release into the extracellular space.
Inflammation in the joint has long been known to lead to hypoxia due, in part, to pressure from synovial exudates, the disordered vasculature that develops in chronically inflamed synovium and the marked influx of inflammatory cells 17-19. Although acute hypoxia leads to increased adenosine release that helps to ameliorate tissue injury following ischemia–reperfusion (reviewed elsewhere20), in settings of chronic hypoxia and inflammation the ongoing cellular injury results in mitochondrial dysfunction21. In addition to release of inflammatory reactants from dysfunctional mitochondria, reduced ATP production probably leads to a reduction of ATP and/or adenosine levels in extracellular fluid (Corciulo and Cronstein, unpublished observations. Despite upregulation of adenosine receptors at inflamed sites (see below) the diminished adenosine level present in chronically inflamed joints probably permits further activation of inflammatory cells in the synovium 21. Hypoxia contributes to both acute and chronic inflammation by other mechanisms as well 21.
Adenosine receptors
Four subtypes of adenosine receptor exist, named in order of discovery: A1, A2a, A2b and A3. These receptors are all members of the G-protein-coupled family of 7-transmembrane spanning receptors 3 (FIGURE 2). A1 and A2a are high affinity receptors with activity in the low to mid-nanomolar range whereas A2b has a substantially lower affinity for adenosine (micromolar). Considerable species-dependent variability in A3 exists22. Adenosine receptors are widespread in their expression and are important regulators of many different types of physiological and pathological processes. A1 and A3 signal via Gαi signalling proteins, which downregulate cAMP expression, whereas A2a and A2b are linked to GαS proteins, which trigger increases in cellular cAMP content. A2b also signals through Gq proteins23.
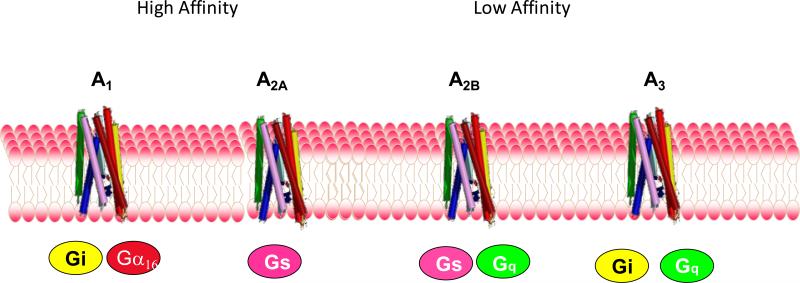
Figure 2. Adenosine receptors.
Adenosine receptors are all G protein-coupled receptors whose signal transduction is mediated by activation of intracellular G proteins. ADORA1 has the highest affinity for adenosine and is activated by high picomolar to low nanomolar adenosine concentrations; ADORA2A is activated by concentrations of adenosine in the mid-nanomolar range. By contrast, ADORA2B and ADORA3 are activated by adenosine concentrations in the micromolar range.
Expression of adenosine receptors is regulated by a number of stimuli, including inflammatory stimuli. In particular, A2a is upregulated by agents that stimulate activation of NFκB (a central transcriptional regulator in the inflammatory process), such as TNF, IL-1 and endotoxin, 24-28 and acts, as described below, as a feedback inhibitor of inflammation. Evidence from patients with RA confirms these observations: increased expression of A2a on peripheral white blood cells in these patients is reduced by treatment with anti-TNF agents29,30. In addition to regulation of A2a expression, TNF and other proinflammatory cytokines increase the function of these receptors by preventing receptor desensitization31, further downregulating inflammation. By contrast, interferon-γ (IFNγ) downregulates both the expression and function of A2a 32-34. This downregulation (which occurs following the increase in both extracellular adenosine levels and A2a expression and function after cellular injury or necrosis) indicates the potent role of adenosine and its receptors as feedback regulators of inflammation and innate immune responses.
Similarly, A3 adenosine receptor expression is increased on peripheral blood white cells in patients with RA, and treatment with anti-TNF antibodies reduces adenosine receptor overexpression29,35-37.
Adenosine receptors and innate immunity
Neutrophils
The anti-inflammatory effects of adenosine were first suggested in 1983 when the capacity of extracellular adenosine to inhibit stimulated neutrophil superoxide anion generation was demonstrated 38, effects subsequently confirmed and expanded upon by many others 39-42. Later studies revealed that these effects were mediated by adnesoine receptor A2a 43-45. Thus, A2a engagement inhibits phagocytosis.
Before neutrophils can destroy invading bacteria or clear debris from a wound they must be recruited from the vasculature into the extravascular space, a process mediated by a series of adhesive interactions among neutrophils, endothelial cells and matrix. Numerous studies revealed that engagement of adenosine receptor A2a and A2b inhibited adhesion of neutrophils to both endothelial cells and other surfaces 46-50 by inhibiting both selectin and integrin-mediated adhesive interactions. Interestingly, in vitro studies demonstrated that A1 enhanced neutrophil adhesion to endothelial cells via α4 integrins 49. This same group had reported, based on studies with pharmacological inhibitors, that stimulation of A1 enhanced neutrophil chemotaxis; 43,51 however, later studies using knockout mice and their neutrophils demonstrated that, in fact, it was A3 that mediated enhanced chemotaxis 52,53.
One of the mechanisms by which inflammatory cell activation is terminated or downregulated is by engulfment of apoptotic cells, such as occurs at sites of crystal-induced inflammation. A2a engagement promotes engulfment-mediated neutrophil downregulation, whereas A3 engagement diminishes engulfment-mediated downregulation of proinflammatory actions (TABLE 1) 54,55.
TABLE 1.
Adenosine receptors regulate the function of inflammatory cells
| Inflammatory cell | Adenosine receptor | |||
|---|---|---|---|---|
| ADORA1 | ADORA2A | ADORA2B | ADORA3 | |
| Neutrophil | Increases adhesion to matrix49 | Inhibits superoxide anion generation43-45 Inhibits adhesion and recruitment Increases engulfment-mediated downregulation of neutrophil function46-50 |
Unknown | Chemotaxis52,53 |
| Macrophage | Increases giant cell formation91 Increases osteoclast differentiation92,93 |
Promotes M1 to M2 transition62,71 Inhibits cytokine expression61-72 Inhibits osteoclast formation94-96 |
Inhibits osteoclast formation94-96 Promotes M1 to M2 transition75-77 |
Inhibits cytokine expression70,78-86 |
| T cell | Unknown | Inhibits TCR-triggered activation103,104 Inhibits activation-induced cell death112 Inhibits Fas/FasL-mediated cell death112 |
Stimulates TH17 differentiation by increasing dendritic cell IL-6 production87-89 | Unknown |
| Endothelial cell | Unknown | Increases angiogenesis123 Increases barrier integrity (prevents oedema) 123,125 |
Increases angiogenesis123 Increases oedema formation in arthritis90 Promotes clearance of pulmonary oedema126 |
Unknown |
| Fibroblast | Unknown | Stimulates fibroblast production of collagen I and III130-133 Promotes skin, lung and hepatic fibrosis130-144 |
Stimulates collagen production130-133 | Unknown |
It has often been noted that imitation is the most sincere form of flattery and this adage is no less true for imitation of critical biological mechanisms by pathogens. Although most of the adenosine that mediates suppression of inflammation is produced endogenously some is produced by pathogens as a virulence factor. Invasive forms of Candida albicans, Staphylococcus aureus and Streptococcus suis have all been reported to produce adenosine as a virulence factor to protect against phagocyte-mediated injury 56-58. The hijacking of adenosine-mediated immunosuppression by pathogens provides strong evidence for the importance of this biological mechanism.
Monocytes and macrophages
Macrophages have critical roles in initiating the innate immune response against microbes and in eliminating debris at sites of injury. In addition to destroying most microbes, macrophages produce a variety of cytokines and mediators involved in regulating inflammatory responses and present antigens to T cells in order to orient the adaptive immune response. Macrophages are antigen presenting cells that promote the selective expansion and differentiation of lymphocytes specific for invaders or cancer cells and, in the rheumatic diseases, direct immune response to self-antigens. Two types of macrophages have been identified: M1 or classical macrophages and M2 or alternatively activated macrophages 59,60. In diseases such as RA, M1 macrophages have an important role by releasing a variety of proinflammatory cytokines (including TNF, IFNγ and IL-12 among many others), as well as oxidants, nitric oxide and other small-molecule mediators of tissue injury. M2 macrophages have a role in terminating inflammation after ingestion of apoptotic cells and promote wound healing by producing angiogenic and profibrotic cytokines.
Adenosine, acting at A2a, suppresses production of proinflammatory cytokines and stimulates expression of anti-inflammatory mediators such as IL-10 and vascular endothelial growth factor (VEGF) 61-72. More generally, A2a stimulation induces a switch from an M1 to a modified M2 phenotype 62,71. One mechanism by which A2a ligation alters macrophage function is via stimulating expression of the orphan nuclear receptor NR4A, which diminishes activation of NFκB-dependent gene expression 73,74. A2b also stimulates the switch from an M1 to an M2 phenotype 75-77. The role of A3 in regulation of inflammatory macrophage function is less clear; A3 stimulation clearly suppresses cytokine expression and release by macrophages and diminishes inflammation in mouse models of arthritis and inflammatory bowel disease 70,78-86. Indeed, the relatively poor anti-infective function of macrophages from human neonates is thought to reflect the dominance of A3 expressed by these cells 80. By contrast, studies have indicated that A3 ligation diminishes the suppression of inflammation induced by engulfment of apoptotic cells 54. In patients with RA, A3 expression is a marker of disease severity and can predict the efficacy of treatment of symptoms with a selective adenosine receptor A3 ligand (TABLE 1) 35.
The effect of A2b activation on the overall immune response remains ambiguous. As noted above, A2b promotes a phenotypical switch from M1 to M2 macrophages, 75-77 but also stimulates the differentiation of dendritic cells, which can promote type 17 T helper cells (TH17) immune responses via production of IL-6 87-89. Indeed, in in vivo studies high concentrations of adenosine exacerbated arthritis and tissue destruction in rats via an A2b mechanism 90.
Multinucleated giant cells, formed from the fusion of macrophages, are characteristic accompaniments of inflammation in response to foreign bodies or certain pathogens (most notably tuberculosis) and their presence in inflamed vessels gives Giant Cell Arteritis its name.. Adenosine receptors have a critical role in the formation of these syncytia as A1 stimulation is essential for formation of giant cells in vitro 91. Osteoclasts, a specialized form of multinucleated giant cell involved in bone remodelling and turnover, also depend on A1 to complete differentiation by a mechanism involving the signalling molecule TRAF6 92,93. By contrast, A2a and A2b stimulation inhibits osteoclast differentiation; mice lacking these receptors have osteopaenia 94-96 due, in part, to increased osteoclast-mediated bone resorption. Owing to its effects on osteoclasts and macrophages and production of proinflammatory cytokines, A2a also inhibits inflammatory bone damage induced by prosthetic wear particles97. Interestingly, both A2a and A2b stimulate bone formation in vivo 98 although only the direct stimulatory effects of A2b on osteoblast function have been well characterized 99,100.
The adaptive immune system
Regulation of T cells by A2a
Effector functions of T cells in RA synovium are triggered by the T cell receptor (TCR) recognition event followed by transmembrane signalling of the TCR–CD3 complex and by multiple interconnected intracellular biochemical pathways; therefore, it is important to explore the therapeutic opportunities of targeting T cells in both hypoxic and adenosine-rich microenvironments and normoxic and adenosine-poor tissue microenvironments of inflamed joints in RA. Studies of adenosine receptors on T cells were largely motivated by the need to understand their role in anti-pathogen and anti-tumour immunity, with a primary focus, until recently, on A2a 101,102. The high-affinity A2a, but not A1 or A3, was identified as the predominant adenosine receptor on the surface of mouse T cells103,104. Expression and functional coupling of A2a in mouse peripheral T cells and B cells correlated with adenosine-induced cAMP accumulation in lymphocytes and with adenosine-induced inhibition of TCR-triggered T cell activation103,104. The capacity of A2a stimulation to inhibit effector functions of different subsets of mouse T cells was subsequently confirmed105 and in vivo genetic and pharmacological evidence was provided showing that A2a is critical and non-redundant in controlling the extent of T-cell-mediated immune responses, inflammation and tissue damage in different models of antipathogen immune responses and antitumour immune responses 101,106. Non-redundance of A2a in T cells and myeloid cells was demonstrated by the observation that none of the other cAMP-elevating G-protein-coupled receptors on immune cells could replace A2a and compensate for the A2a-mediated attenuation of T-cell-driven immune responses and inflammatory damage in models of autoimmune and viral hepatitis101.
ADORA2A gene–dose effect
Naive T cells express low levels of A2a 103,104 but following TCR-triggered activation of T cells ADORA2A mRNA and A2a-mediated signalling rapidly increase105. Other studies have demonstrated a clear gene–dose effect for extracellular adenosine signalling at A2a and that no receptor reserve exists on these cells 107. The lack of ‘spare’ immunosuppressive A2a on T cells points toward the importance of every individual A2a molecule on these cells in maintaining the balance between immunosuppression and immunostimulation and the need for the precise regulation of numbers of A2a molecules to avoid autoimmunity without tolerization of T cells. Extracellular adenosine is short-lived in vivo but its immunosuppressive effects on T cells are prolonged due to the intracellular biochemical ‘memory’ of exposure of T cells to adenosine104.
In view of the direct evidence for the effect of A2a on T cells and the role of TH17 and T regulatory (Treg) cells in the pathogenesis of RA it would be of significant interest to investigate the genetic differences in ADORA2A between individual patients with RA and to search for correlations between the number of A2a molecules per immune cell and severity of the disease. ADORA2A gene polymorphisms, as well as the number of A2a molecules per immune cell, might also predispose a patient to developing RA or, equally importantly, to respond to adenosine-dependent therapies such as methotrexate (see below). An interesting experimental approach to testing this hypothesis was presented in studies of functional changes caused by genetic variation of ADORA2A that used personalized lymphoblastoid cell lines from twins as a model system108. This study demonstrated that there was altered potency of a partial A2A agonist for A2A receptor activation in cells expressing a receptor in which there was a previously described polymorphism associated with caffeine-induced sleep disorders.
A2a and subsets of human T cells
Further characterization of which T cell subsets express A2a and whether and how their functions are regulated is required, although early studies clearly demonstrated differential expression and function of this receptor on T cell subsets109,110. CD4+ T helper cells express A2a which, when stimulated by adenosine or an A2A agonist, inhibits the proinflammatory functions of these cells110,111. Similar to mouse T cells, human T helper cells increased ADORA2A mRNA levels as soon as 4 hours after activation, and A2a was the predominant subtype of adenosine receptor expressed by these cells. Activation of A2a on T cells is of great consequence not only to T cell function but also to the fate of T cells, memory T cells and T-cell homeostasis as A2a counteracts processes leading to activation-induced cell death and Fas–Fas-ligand-mediated cell death in CD4+ T lymphocytes112. (TABLE 1) These original studies were carried out on mouse CD4+ hybridomas and human Jurkat cells (a transformed T cell line and were subsequently expanded to in vivo studies of naive T cell development and peripheral maintenance, which is the overall outcome of extracellular signalling to T cells in different tissue microenvironments113. The possibility of A2a involvement in T-cell selection processes was also the implication of early studies of immature T cells114.
A2a signalling in T cells
A2a is a Gs-linked receptor that stimulates an increase in intracellular cAMP levels, which in turn activates protein kinase A (PKA). cAMP–PKA signalling directly inhibits all known TCR-triggered effector functions104,106 because cAMP–PKA is the ‘high fidelity’ immunosuppressor that intercepts both early and late events in the TCR-triggered intracellular T cell activation pathway 115,116. Moreover, signalling mediated by A2a, cAMP, PKA, cAMP response element (CRE) and cAMP response element binding (CREB) protein can redirect proinflammatory T cell responses toward an immunosuppressive phenotype. This change in phenotype also occurs because the extracellular adenosine accumulation driven by hypoxia and hypoxia-inducible factor (HIF)-1α results in hypoxia response element (HRE) and CRE-dependent redirection of CD8 and CD4 and myeloid-cell-mediated immune responses toward production of immunosuppressive cytokines and molecules such as TGFβ, IL-10 and CTLA-4, and promotes the development of Treg cells and their inhibition of effector T cells117,118. Taken together, any interpretation of the effects of adenosine on T cells should consider A2a signalling regulating T cell development, maintenance and effector functions.
Regulation of T cells by A2b
Compared with adenosine receptor A2a, much less is known about the low affinity A2b on T cells, but—of relevance to RA—evidence indicates that activation of A2b by synthetic agonists could have TH17-promoting effects by redirecting the differentiation of bone marrow cells to a CD11c+GR1+ dendritic cell subset89,119. (TABLE 1) Moreover, A2b stimulation might also promote TH17 differentiation by stimulating IL-6 expression in a cAMP-independent manner88,119. Others have reported that A2b promotes Treg-cell differentiation and limits inflammatory injury120. A2b signals by multiple pathways; not only does A2b increase cAMP via coupling to Gs proteins but they might also signal via Gq and Gi proteins, thereby explaining their cAMP-independent effects on generation of IL-6121.
ADORA1 and ADORA3 in T cells
The expression and function of A1 and A3 on T cells and their subsets is not fully established. A report indicates that A3 is expressed on Jurkat cells but its expression and function in primary cells has not been established122.
Other cell types in inflammation
New blood vessel formation is a characteristic of the inflamed synovium, and hypoxia is a major driving force for neovascularization. Adenosine and its receptors have direct and indirect roles in stimulating new vessel formation. Acting at both A2a and A2b, adenosine stimulates both angiogenesis and production of such proangiogenic mediators as VEGF123. Moreover, both A2a and A2b directly stimulate endothelial cells to form new vessels123. (TABLE 1)
The effects of adenosine receptor stimulation on the formation and resolution of vascular oedema seems to vary with the tissue studied. A2a has been reported to promote vascular leakage in healing wounds and inflamed sites as a result of the increase in new blood vessels that form at these sites124. By contrast, the barrier integrity of human umbilical vein endothelial cells is enhanced by A2a stimulation125. Interestingly, A2b stimulation promotes clearance of pulmonary oedema126. A2b stimulation might have opposing effects in other vascular beds: excess adenosine generation, acting via A2b, can increase inflammation and inflammatory oedema in rat models of arthritis127. Thus, targeting adenosine receptors in the prevention of angiogenesis and vascular oedema in inflammation is complex.
Similar to the effects on oedema formation and angiogenesis, the effects of adenosine and adenosine receptors on collagen and matrix production by fibroblasts and fibroblast-like cells varies from tissue to tissue. Adenosine, acting at A2a, directly stimulates dermal fibroblasts to produce collagen and also stimulates the production of factors, such as IL-13 and connective tissue growth factor, that are capable of further amplifying collagen production 128. By contrast, A2b stimulation inhibits collagen production by some tissue fibroblasts (cardiac fibroblasts) but stimulates collagen production by fibroblasts in other tissues 128.
Adenosine and resolution of inflammation
The inflammatory response is critical for clearance of debris and protection from invading microorganisms; inflammation sets the stage for tissue repair. As with suppression of inflammation, adenosine, acting at its receptors, promotes tissue repair and regeneration. As noted above, A2a stimulation promotes the transformation of macrophages to an M2 phenotype, which is associated with lower levels of production of TNF, IL-12 and other proinflammatory mediators than the M1 phenotype, and with increased production of IL-10, IL-13 and VEGF,59,60 which promote angiogenesis and fibrosis. Moreover, A2a and A2b stimulation promotes angiogenesis and generation of matrix by directly stimulating endothelial cells, endothelial cell precursors and fibroblasts 123,129.
Adenosine receptors and fibrosis
Adenosine mediates the transition from inflammation to wound healing but an excess of wound healing might result in fibrosis, which can disrupt organ architecture and function. Fibrous contractions of the joints and fibrosis in the lungs and other tissues are common complications of RA and other rheumatic diseases, and fibrosis characterizes SSc. Results from animal models of scarring or diffuse fibrosis, a manifestation of SSc, indicate a role for adenosine and its receptors in fibrosis 130-133. Moreover, mice lacking adenosine deaminase have high levels of adenosine in the skin and spontaneously develop diffuse dermal fibrosis 131. Elevated adenosine levels in the tissue of mice lacking adenosine deaminase also lead to pulmonary fibrosis by engaging both A2a and A2b 134-141. In the liver, extracellular adenosine production with A2b stimulation mediates hepatic fibrosis in mouse models, a phenomenon that is inhibited by caffeine, a nonselective adenosine receptor antagonist142-144. This latter finding might account for the well-documented observation that coffee drinking and caffeine ingestion reduces deaths from liver disease 145-149. Peritoneal fibrosis also depends on A2a stimulation in animal models150. In the setting of priapism, in which the chronically engorged penis becomes hypoxic, adenosine release leads to both vasodilation and fibrosis via activation of A2b 151,152. Thus, although adenosine and its receptors generally have a beneficial role in terminating inflammation, these same receptors, if persistently stimulated, lead to enhanced fibrosis with resulting tissue and organ dysfunction.
Regulation of extracellular adenosine
Under some pathological conditions tissue adenosine levels are markedly increased. Perhaps the best studied condition under which adenosine levels increase is hypoxia. As noted above, adenosine was first described as a coronary vasodilator in 19291 and in subsequent studies it became increasingly clear that the hyperaemic response to hypoxia was attributable, in large part, to adenosine release153. Presumably the breakdown of ATP and release of adenine nucleotides into the extracellular space has a role in the generation of increased extracellular adenosine; HIF-1α and adenosine kinase were shown to have an important role in this pathway154. In addition, ENT1-mediated uptake of adenosine is downregulated by increases in intracellular cAMP155, and increases in extracellular adenosine drive further increases in adenosine levels by diminishing ENT1 function via adenosine-receptor-mediated increases in cAMP 156. It was recognized quite early that inflamed joints tend to be chronically hypoxic as a result of the increased cellular bulk, tissue oedema (with resulting compression of the microvasculature) and the pressure of increased synovial fluid17-19,157,158. Nonetheless, estimating the actual level of adenosine in synovial fluid is difficult as cells present in synovial fluid might undergo lysis, thus releasing ATP (which is converted to adenosine during processing); furthermore, adenosine is hydrolyzed by the adenosine deaminase present in synovial fluid159-161.
Extracellular adenosine levels can also fluctuate in other settings. A number of drugs can regulate adenosine levels in the extracellular fluid. Most notable among these are dipyridamole and ticagrelor, which are agents that inhibit ENT1-mediated adenosine uptake 156,162,163. Ethanol, which is metabolized as a lipid and requires ATP hydrolysis in order to be metabolized to acetyl-CoA, also causes adenosine release as a result of ATP breakdown156,164-167. More recently, it has been appreciated that extracellular adenosine levels might reach extremely high concentrations in the setting of rapid cellular breakdown and hypoxia, such as occurs in tumours, leading to consequences such as immunosuppression by tumours101,102,168.
Therapeutic targeting of adenosine
Although no drugs have been developed to treat rheumatic diseases that directly target adenosine and its receptors, adenosine-based targeting has been reported for a number of commonly used drugs. Anti-inflammatory and antirheumatic drugs that mediate at least some of their anti-inflammatory effects via targeting adenosine metabolism include methotrexate, sulfasalazine and high-dose aspirin.
Methotrexate
Weekly, low-dose methotrexate remains the gold standard therapy for patients with rheumatic diseases. Although its mechanism of action has not been fully elucidated (Box 1), it seems that enhancing adenosine release at inflamed sites has a major role in the anti-inflammatory actions of low-dose methotrexate. The enzyme inhibited best by intracellular methotrexate polyglutamates is aminoimidazolecarboxamidoribonucleotide (AICAR) transformylase; intracellular accumulation of AICAR leads to adenosine release169 (Figure 1). In 1991, the first results were published suggesting that methotrexate promotes adenosine release, which diminishes inflammation in vitro170; it was subsequently demonstrated that adenosine mediates the anti-inflammatory effects of methotrexate in an in vivo model in which increased intracellular AICAR levels at an inflamed site were documented171. Adenosine mediates the anti-inflammatory effects of methotrexate via occupancy of A2a and A3169. Moreover, adenosine receptor antagonists, including caffeine, block the anti-inflammatory effects of methotrexate treatment in an animal model of arthritis172 and in patients with Rheumatoid Arthritis. in prospective studies173,174. A large retrospective study of patients taking methotrexate did not support the contention that caffeine ingestion reversed the anti-inflammatory effects of weekly low-dose methotrexate; however, given that patients in this study had been successfully treated for RA for a number of years, those who had not responded to methotrexate were excluded from the analysis175. Nonetheless, other studies provide further evidence for the role of adenosine in the mechanism of action of methotrexate; for example, the expression of CD39, an ectoenzyme that catalyzes the dephosphorylation of ATP and ADP to AMP (which is critical for extracellular adenosine production), is a biomarker for methotrexate efficacy in patients with RA176. Other mechanisms of action have been described for the anti-inflammatory actions of methotrexate in the treatment of Rheumatoid Arthritis and other rheumatic conditions that do not include a role for adenosine169,177-179.
BOX 1.
Methotrexate was originally developed as a folic acid antagonist to prevent the de novo synthesis of purines and pyrimidines in the treatment of cancer and leukaemia, which remain indications for use of this drug. Although high-dose aminopterin, an analogue of methotrexate, was reported to be useful in the treatment of rheumatoid arthritis (RA) in the early 1950s190, methotrexate was not widely used to treat inflammatory arthritis until anecdotal reports and uncontrolled trials were carried out in the 1970s followed by placebo-controlled trials in the 1980s and approval for the treatment of RA in 1989191. Although initially thought to block lymphocyte proliferation via inhibition of purine and pyrimidine synthesis, the inability of folic acid supplementation to reverse the efficacy of methotrexate treatment while preventing folate-dependent toxicities did not support this effect for methotrexate used at doses up to 1/100th of the dose given to treat malignancy192-194. In the treatment of Rheumatoid Arthritis, psoriasis and other inflammatory conditions methotrexate is administered, in low doses (5-25mg) once per week and has a half-life that is relatively short. It is undetectable in the circulation after 18 h but is widely distributed and present in tissues as long-lived polyglutamates, which remain potent inhibitors of a number of enzymes involved in the synthesis of purines and pyrimidines. The enzyme inhibited most potently by methotrexate polyglutamates is aminoimidazole carboxamidoribonucleotide (AICAR) transferase (AICART); inhibition of this enzyme leads to intracellular AICAR accumulation (see Figure 1). Increased intracellular AICAR promotes adenosine release, and blockade or deletion of adenosine receptors reverses the anti-inflammatory effects of methotrexate in animal models and patients with Rheumatoid Arthritis 169.
Sulfasalazine
Sulfasalazine was first developed as a specific therapy for RA in the 1940s and remains in use today. Sulfasalazine is broken down by gut bacteria to 5-aminosalicylate and sulfapyridine; sulfasalazine itself and 5-aminosalicylate are poorly absorbed from the gut and sulfapyridine acts as an antifolate once absorbed. Indeed, folate deficiency is a recognized side effect of sulfasalazine180. Among its other effects sulfasalazine promotes accumulation of intracellular AICAR and release of adenine nucleotides into the extracellular space where they are dephosphorylated to adenosine; blockade or loss of ecto-5’nucleotidase prevents adenosine release induced by sulfasalazine both in vitro and in vivo and reverses the adenosine-receptor-dependent anti-inflammatory effects of the drug in a mouse model of inflammation181,182 (Figure 1). These results suggest that, like methotrexate, adenosine mediates at least some of the anti-inflammatory effects of sulfasalazine.
High-dose aspirin
Although not commonly used anymore for the treatment of RA and other rheumatic diseases, high-dose aspirin was a mainstay of antirheumatic therapy until the development of less-toxic NSAIDs in the 1970s. Before the recognition that aspirin and other NSAIDs mediated many of their actions by inhibiting cyclooxygenases, it was thought that one mechanism by which high-dose aspirin mediated its therapeutic and toxic effects was by uncoupling oxidative phosphorylation and loss of intracellular ATP 183,184. Results of studies in human neutrophils indicated that some of the effects of high-dose salicylates on stimulated neutrophil function resulted from ATP loss and extracellular conversion to adenosine 185. Subsequent studies in mice confirmed that adenosine mediates the anti-inflammatory effects of high-dose aspirin in vivo as well 186. The doses of aspirin required verge on the toxic and are almost never used at present.
Antirheumatic drugs in development
On the basis of their anti-inflammatory activity in a variety of animal models [187, A3 agonists are currently under development for the treatment of RA. Indeed, a phase II clinical trial of CF101, an A3 agonist, reduced RA activity although the improvement did not achieve statistical significance188. Interestingly, an increased clinical response to CF101 was seen in patients in whom A3 was overexpressed on peripheral blood mononuclear cells 188. CF101 has also been administered to patients for the treatment of psoriasis and an early phase study demonstrates clear evidence of efficacy for this indication 189.
Conclusions
The role of adenosine and its receptors in regulating inflammation and immune responses has been well established. Adenosine, released by hypoxic or injured tissues or following treatment with drugs such as methotrexate, suppresses inflammation and immune responses via stimulation of its receptors on inflammatory and immune cells. Although this mechanism of action is useful for the treatment of diseases such as RA, in the setting of tumours the immunosuppression that occurs might be deleterious. The conditional benefits of modulating inflammation and immunity dictate that targeting adenosine receptors with agonists or antagonists will require individual tailoring.
Key Points.
Adenosine, generated from the extracellular hydrolysis of ATP, is a potent endogenous regulator of inflammation and immune reactions via interaction with one or more cell surface receptors.
The principal adenosine receptor involved in regulation of adaptive T cell responses is ADORA2A.
ADORA2A, ADORA2B and ADORA3 downregulate macrophage-mediated inflammatory actions, although ADORA2B might indirectly stimulate type 17 T helper cell immune responses via increased IL-6 production.
Adenosine mediates the anti-inflammatory effects of low-dose methotrexate treatment as well as some of the anti-inflammatory effects of sulfasalazine.
Acknowledgments
The work of B.N.C. is supported by grants from the NIH (R01 AR056672-07, R01 AR068593-02, 1UL1TR001445-02), Arthritis Foundation and Celgene. The work of M.S. is supported by a grant from the NIH (2R01 CA 111985-10).
Biography
Bruce Cronstein is the Paul R. Esserman Professor of Medicine at NYU School of Medicine where he directs the Clinical and Translational Science Institute. Trained as a physician and Rheumatologist, he first described the role of adenosine and its receptors in inflammation and demonstrated that adenosine mediates the anti-inflammatory effects of low-dose methotrexate in the treatment of rheumatic diseases. In other studies he has demonstrated the role of adenosine and its receptors in bone and liver physiology and the pathologic role of adenosine in fibrosis of the skin and liver.
Michail Sitkovsky is the E.W. Black Professor of Immunophysiology and Director of the New England Inflammation and Tissue Protection Institute at Northeastern University, Boston, Massachusetts, USA. Trained as a biophysicist and immunologist, he uncovered the non-redundant and domineering role of A2A adenosine receptor and hypoxia-inducible factor 1α in pathophysiological immunosuppression and redirection of immune response. These findings led to the conceptually novel repurposing of several classes of drugs in order to weaken the immunosuppressive hypoxia–A2A adenosine receptor axis and thereby enable the use of immunotherapies for cancer and infectious diseases.
Footnotes
Competing interests
B.N.C. has acted as a consultant for Bristol–Myers Squibb and AstraZeneca; he has received grants from AstraZeneca, Celgene and Takeda and has equity in Can-Fite Biopharma. M.S. declares no competing interests.
Author contributions
Both authors contributed to all aspects of this manuscript.
References
- 1.Drury AN, Szent-Gyorgi A. The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. Journal of Physiology. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sattin A, Rall TW. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3′, 5′-phosphate content of guinea pig cerebral cortex slices. Molecular pharmacology. 1970;6:13–23. [PubMed] [Google Scholar]
- 3.Fredholm BB, AP IJ, Jacobson KA, Linden J, Muller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacological reviews. 2011;63:1–34. doi: 10.1124/pr.110.003285. doi:10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newby AC, Holmquist CA, Illingworth J, Pearson JD. The control of adenosine concentration in polymorphonuclear leucocytes, cultured heart cells and isolated perfused heart from the rat. Biochemical Journal. 1983;214:317–323. doi: 10.1042/bj2140317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyer EC, Steinberg TH. Evidence that the gap junction protein connexin-43 is the ATP-induced pore of mouse macrophages. The Journal of biological chemistry. 1991;266:7971–7974. [PubMed] [Google Scholar]
- 6.Rosenthal AK, et al. The progressive ankylosis gene product ANK regulates extracellular ATP levels in primary articular chondrocytes. Arthritis research & therapy. 2013;15:R154. doi: 10.1186/ar4337. doi:10.1186/ar4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. doi:10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Iglesias R, et al. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. American journal of physiology. Cell physiology. 2008;295:C752–760. doi: 10.1152/ajpcell.00228.2008. doi:10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldwin SA, et al. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–743. doi: 10.1007/s00424-003-1103-2. doi:10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- 10.Dahl G, Muller KJ. Innexin and pannexin channels and their signaling. FEBS Lett. 2014;588:1396–1402. doi: 10.1016/j.febslet.2014.03.007. doi:10.1016/j.febslet.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Adamson SE, Leitinger N. The role of pannexin1 in the induction and resolution of inflammation. FEBS Lett. 2014;588:1416–1422. doi: 10.1016/j.febslet.2014.03.009. doi:10.1016/j.febslet.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alberto AV, et al. Is pannexin the pore associated with the P2X7 receptor? Naunyn Schmiedebergs Arch Pharmacol. 2013;386:775–787. doi: 10.1007/s00210-013-0868-x. doi:10.1007/s00210-013-0868-x. [DOI] [PubMed] [Google Scholar]
- 13.Ambrosi C, et al. Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J Biol Chem. 2010;285:24420–24431. doi: 10.1074/jbc.M110.115444. doi:10.1074/jbc.M110.115444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anselmi F, et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci U S A. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. doi:10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beckel JM, et al. Mechanosensitive release of adenosine 5′-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: a mechanism for purinergic involvement in chronic strain. Glia. 2014;62:1486–1501. doi: 10.1002/glia.22695. doi:10.1002/glia.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwamoto T, et al. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J Biol Chem. 2010;285:18948–18958. doi: 10.1074/jbc.M110.127027. doi:10.1074/jbc.M110.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levick JR. Hypoxia and acidosis in chronic inflammatory arthritis; relation to vascular supply and dynamic effusion pressure. The Journal of rheumatology. 1990;17:579–582. [PubMed] [Google Scholar]
- 18.Geborek P, Forslind K, Wollheim FA. Direct assessment of synovial blood flow and its relation to induced hydrostatic pressure changes. Annals of the rheumatic diseases. 1989;48:281–286. doi: 10.1136/ard.48.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kofoed H. Synovitis causes hypoxia and acidity in synovial fluid and subchondral bone. Injury. 1986;17:391–394. doi: 10.1016/0020-1383(86)90078-1. [DOI] [PubMed] [Google Scholar]
- 20.Grenz A, Homann D, Eltzschig HK. Extracellular adenosine: a safety signal that dampens hypoxia-induced inflammation during ischemia. Antioxid Redox Signal. 2011;15:2221–2234. doi: 10.1089/ars.2010.3665. doi:10.1089/ars.2010.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fearon U, Canavan M, Biniecka M, Veale DJ. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nature reviews. Rheumatology. 2016;12:385–397. doi: 10.1038/nrrheum.2016.69. doi:10.1038/nrrheum.2016.69. [DOI] [PubMed] [Google Scholar]
- 22.Borea PA, et al. The A3 adenosine receptor: history and perspectives. Pharmacological reviews. 2015;67:74–102. doi: 10.1124/pr.113.008540. doi:10.1124/pr.113.008540. [DOI] [PubMed] [Google Scholar]
- 23.Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacological Reviews. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 24.Khoa ND, Montesinos CM, Williams AJ, Kelly M, Cronstein BN. Th1 cytokines regulate adenosine receptors and their downstream signalling elements in human microvascular endothelial cells. J. Immunol. 2003;171:3991–3998. doi: 10.4049/jimmunol.171.8.3991. [DOI] [PubMed] [Google Scholar]
- 25.Khoa ND, et al. Inflammatory Cytokines Regulate Function and Expression of Adenosine A2A Receptors in Human Monocytic THP-1 Cells. Journal of immunology. 2001;167:4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- 26.Bshesh K, et al. The A2A receptor mediates an endogenous regulatory pathway of cytokine expression in THP-1 cells. Journal of Leukocyte Biology. 2002;72:1027–1036. [PubMed] [Google Scholar]
- 27.Sun WC, et al. Lipopolysaccharide and TNF-alpha modify adenosine A(2A) receptor expression and function in equine monocytes. Veterinary immunology and immunopathology. 2010;135:289–295. doi: 10.1016/j.vetimm.2009.12.001. doi:10.1016/j.vetimm.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Napieralski R, Kempkes B, Gutensohn W. Evidence for coordinated induction and repression of ecto-5'-nucleotidase (CD73) and the A2a adenosine receptor in a human B cell line. Biol Chem. 2003;384:483–487. doi: 10.1515/BC.2003.054. [DOI] [PubMed] [Google Scholar]
- 29.Varani K, et al. Normalization of A2A and A3 adenosine receptor up-regulation in rheumatoid arthritis patients by treatment with anti-tumor necrosis factor alpha but not methotrexate. Arthritis and rheumatism. 2009;60:2880–2891. doi: 10.1002/art.24794. doi:10.1002/art.24794. [DOI] [PubMed] [Google Scholar]
- 30.Vincenzi F, et al. A(2A) adenosine receptors are differentially modulated by pharmacological treatments in rheumatoid arthritis patients and their stimulation ameliorates adjuvant-induced arthritis in rats. PloS one. 2013;8:e54195. doi: 10.1371/journal.pone.0054195. doi:10.1371/journal.pone.0054195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khoa ND, Postow M, Danielsson J, Cronstein BN. Tumor necrosis factor-alpha prevents desensitization of Galphas-coupled receptors by regulating GRK2 association with the plasma membrane. Mol Pharmacol. 2006;69:1311–1319. doi: 10.1124/mol.105.016857. doi:10.1124/mol.105.016857. [DOI] [PubMed] [Google Scholar]
- 32.Khoa ND, et al. Inflammatory cytokines regulate function and expression of adenosine A(2A) receptors in human monocytic THP-1 cells. J Immunol. 2001;167:4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen DK, Montesinos MC, Williams AJ, Kelly M, Cronstein BN. Th1 cytokines regulate adenosine receptors and their downstream signaling elements in human microvascular endothelial cells. Journal of immunology. 2003;171:3991–3998. doi: 10.4049/jimmunol.171.8.3991. [DOI] [PubMed] [Google Scholar]
- 34.Block ET, Cronstein BN. Interferon-gamma inhibits adenosine A2A receptor function in hepatic stellate cells by STAT1-mediated repression of adenylyl cyclase. International journal of interferon, cytokine and mediator research : IJIM. 2010;2010:113–126. doi: 10.2147/ijicmr.s8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fishman P, Cohen S. The A adenosine receptor (AAR): therapeutic target and predictive biological marker in rheumatoid arthritis. Clinical rheumatology. 2016 doi: 10.1007/s10067-016-3202-4. doi:10.1007/s10067-016-3202-4. [DOI] [PubMed] [Google Scholar]
- 36.Ochaion A, et al. The anti-inflammatory target A(3) adenosine receptor is over-expressed in rheumatoid arthritis, psoriasis and Crohn's disease. Cell Immunol. 2009;258:115–122. doi: 10.1016/j.cellimm.2009.03.020. doi:10.1016/j.cellimm.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Ochaion A, et al. Methotrexate enhances the anti-inflammatory effect of CF101 via up-regulation of the A3 adenosine receptor expression. Arthritis research & therapy. 2006;8:R169. doi: 10.1186/ar2078. doi:10.1186/ar2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cronstein BN, Kramer SB, Weissmann G, Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. Journal of Experimental Medicine. 1983;158:1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cronstein BN, Rosenstein ED, Kramer SB, Weissmann G, Hirschhorn R. Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. Journal of immunology. 1985;135:1366–1371. [PubMed] [Google Scholar]
- 40.Marone G, Petracca R, Vigorita S. Adenosine receptors on human inflammatory cells. Int.Arch.Allergy.Appl.Immunol. 1985;77:259–263. doi: 10.1159/000233805. [DOI] [PubMed] [Google Scholar]
- 41.Pasini FL, Capecchi PL, Orrico A, Ceccatelli L, DiPierri T. Adenosine inhibits polymorphonuclear leukocyte in vitro activation: a possible role as an endogenous calcium entry blocker. J.Immunopharm. 1985;7:203–215. doi: 10.3109/08923978509047634. [DOI] [PubMed] [Google Scholar]
- 42.Roberts PA, Morgan BP, Campbell AK. 2-Chloroadenosine inhibits complement-induced reactive oxygen metabolite production and recovery of human polymorphonuclear leukocytes attacked by complement. Biochemical and biophysical research communications. 1985;126:692–697. doi: 10.1016/0006-291x(85)90240-2. [DOI] [PubMed] [Google Scholar]
- 43.Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest. 1990;85:1150–1157. doi: 10.1172/JCI114547. doi:10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gurden MF, et al. Functional characterization of three adenosine receptor types. British journal of pharmacology. 1993;109:693–698. doi: 10.1111/j.1476-5381.1993.tb13629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fredholm BB, Zhang Y, van der Ploeg I. Adenosine A2A receptors mediate the inhibitory effect of adenosine on formyl-Met-Leu-Phe-stimulated respiratory burst in neutrophil leucocytes. Naunyn-Schmiedebergs Archives of Pharmacology. 1996;354:262–267. doi: 10.1007/BF00171056. [DOI] [PubMed] [Google Scholar]
- 46.Thiel M, et al. Effects of adenosine on the functions of circulating polymorphonuclear leukocytes during hyperdynamic endotoxemia. Infection and immunity. 1997;65:2136–2144. doi: 10.1128/iai.65.6.2136-2144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bullough DA, Magill MJ, Mullane KM, Firestein GS. Carbohydrate- and CD18-dependent neutrophil adhesion to cardiac myocytes: effects of adenosine. Cardiovascular research. 1996;32:328–334. doi: 10.1016/0008-6363(96)00052-1. [DOI] [PubMed] [Google Scholar]
- 48.Firestein GS, et al. Inhibition of neutrophil adhesion by adenosine and an adenosine kinase inhibitor. The role of selectins. Journal of immunology. 1995;154:326–334. [PubMed] [Google Scholar]
- 49.Cronstein BN, et al. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol. 1992;148:2201–2206. [PubMed] [Google Scholar]
- 50.Cronstein BN, Levin RI, Belanoff J, Weissmann G, Hirschhorn R. Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J Clin Invest. 1986;78:760–770. doi: 10.1172/JCI112638. doi:10.1172/JCI112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rose FR, Hirschhorn R, Weissmann G, Cronstein BN. Adenosine promotes neutrophil chemotaxis. J Exp Med. 1988;167:1186–1194. doi: 10.1084/jem.167.3.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inoue Y, Chen Y, Hirsh MI, Yip L, Junger WG. A3 and P2Y2 receptors control the recruitment of neutrophils to the lungs in a mouse model of sepsis. Shock. 2008;30:173–177. doi: 10.1097/shk.0b013e318160dad4. doi:10.1097/shk.0b013e318160dad4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. doi:10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 54.Duro E, Pallai A, Koroskenyi K, Sarang Z, Szondy Z. Adenosine A3 receptors negatively regulate the engulfment-dependent apoptotic cell suppression of inflammation. Immunology letters. 2014;162:292–301. doi: 10.1016/j.imlet.2014.06.014. doi:10.1016/j.imlet.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 55.Koroskenyi K, et al. Involvement of adenosine A2A receptors in engulfment-dependent apoptotic cell suppression of inflammation. Journal of immunology. 2011;186:7144–7155. doi: 10.4049/jimmunol.1002284. doi:10.4049/jimmunol.1002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smail EH, et al. In vitro, Candida albicans releases the immune modulator adenosine and a second, high-molecular weight agent that blocks neutrophil killing. Journal of immunology. 1992;148:3588–3595. [PubMed] [Google Scholar]
- 57.Thammavongsa V, Kern JW, Missiakas DM, Schneewind O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. The Journal of experimental medicine. 2009;206:2417–2427. doi: 10.1084/jem.20090097. doi:10.1084/jem.20090097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu P, et al. Streptococcus suis adenosine synthase functions as an effector in evasion of PMN-mediated innate immunit. The Journal of infectious diseases. 2014;210:35–45. doi: 10.1093/infdis/jiu050. doi:10.1093/infdis/jiu050. [DOI] [PubMed] [Google Scholar]
- 59.Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72:4111–4126. doi: 10.1007/s00018-015-1995-y. doi:10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laria A, et al. The macrophages in rheumatic diseases. J Inflamm Res. 2016;9:1–11. doi: 10.2147/JIR.S82320. doi:10.2147/JIR.S82320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Csoka B, et al. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood. 2007;110:2685–2695. doi: 10.1182/blood-2007-01-065870. doi:10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferrante CJ, et al. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation. 2013;36:921–931. doi: 10.1007/s10753-013-9621-3. doi:10.1007/s10753-013-9621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grinberg S, Hasko G, Wu D, Leibovich SJ. Suppression of PLCbeta2 by endotoxin plays a role in the adenosine A(2A) receptor-mediated switch of macrophages from an inflammatory to an angiogenic phenotype. The American journal of pathology. 2009;175:2439–2453. doi: 10.2353/ajpath.2009.090290. doi:10.2353/ajpath.2009.090290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hasko G, et al. Adenosine inhibits IL-12 and TNF-alpha production via adenosine A2a receptor-dependent and independent mechanisms. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 65.Hasko G, et al. Adenosine receptor agonists differentially regulate Il-10, TNF-a, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. Journal of immunology. 1996;157:4634–4640. [PubMed] [Google Scholar]
- 66.Nemeth ZH, et al. Adenosine stimulates CREB activation in macrophages via a p38 MAPK-mediated mechanism. Biochemical and biophysical research communications. 2003;312:883–888. doi: 10.1016/j.bbrc.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 67.Nemeth ZH, et al. Adenosine Augments IL-10 Production by Macrophages through an A2B Receptor-Mediated Posttranscriptional Mechanism. Journal of immunology. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pinhal-Enfield G, et al. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors. The American journal of pathology. 2003;163:711–721. doi: 10.1016/S0002-9440(10)63698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramanathan M, et al. Differential regulation of HIF-1alpha isoforms in murine macrophages by TLR4 and adenosine A(2A) receptor agonists. Journal of leukocyte biology. 2009;86:681–689. doi: 10.1189/jlb.0109021. doi:jlb.0109021 [pii] 10.1189/jlb.0109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szabo C, et al. Suppression of macrophage inflammatory protein (MIP)-1alpha production and collagen-induced arthritis by adenosine receptor agonists. British journal of pharmacology. 1998;125:379–387. doi: 10.1038/sj.bjp.0702040. doi:10.1038/sj.bjp.0702040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leibovich S, et al. Synergistic up-regulation of vascular endothelial growth factor (VEGF) expression in murine macrophages by adenosine A2A receptor agonists and endotoxin. Am.J.Path. 2002;160:2231–2244. doi: 10.1016/S0002-9440(10)61170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams AJ, Cronstein BN. The Effect of A(2A) Adenosine Receptor Activation on C-C Chemokine Receptor 7 Expression in Human THP1 Macrophages During Inflammation. Inflammation. 2011 doi: 10.1007/s10753-011-9353-1. doi:10.1007/s10753-011-9353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crean D, et al. Adenosine Modulates NR4A Orphan Nuclear Receptors To Attenuate Hyperinflammatory Responses in Monocytic Cells. Journal of immunology. 2015;195:1436–1448. doi: 10.4049/jimmunol.1402039. doi:10.4049/jimmunol.1402039. [DOI] [PubMed] [Google Scholar]
- 74.Murphy EP, Crean D. Molecular Interactions between NR4A Orphan Nuclear Receptors and NF-kappaB Are Required for Appropriate Inflammatory Responses and Immune Cell Homeostasis. Biomolecules. 2015;5:1302–1318. doi: 10.3390/biom5031302. doi:10.3390/biom5031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tian Y, Piras BA, Kron IL, French BA, Yang Z. Adenosine 2B Receptor Activation Reduces Myocardial Reperfusion Injury by Promoting Anti-Inflammatory Macrophages Differentiation via PI3K/Akt Pathway. Oxid Med Cell Longev. 2015;2015:585297. doi: 10.1155/2015/585297. doi:10.1155/2015/585297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sciaraffia E, et al. Human monocytes respond to extracellular cAMP through A2A and A2B adenosine receptors. Journal of leukocyte biology. 2014;96:113–122. doi: 10.1189/jlb.3A0513-302RR. doi:10.1189/jlb.3A0513-302RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koscso B, et al. Adenosine augments IL-10-induced STAT3 signaling in M2c macrophages. Journal of leukocyte biology. 2013;94:1309–1315. doi: 10.1189/jlb.0113043. doi:10.1189/jlb.0113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee HS, Chung HJ, Lee HW, Jeong LS, Lee SK. Suppression of inflammation response by a novel A(3) adenosine receptor agonist thio-Cl-IB-MECA through inhibition of Akt and NF-kappaB signaling. Immunobiology. 2011;216:997–1003. doi: 10.1016/j.imbio.2011.03.008. doi:10.1016/j.imbio.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 79.Ramakers BP, et al. The effect of adenosine receptor agonists on cytokine release by human mononuclear cells depends on the specific Toll-like receptor subtype used for stimulation. Cytokine. 2006;35:95–99. doi: 10.1016/j.cyto.2006.07.014. doi:10.1016/j.cyto.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 80.Levy O, et al. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mabley J, et al. The adenosine A3 receptor agonist, N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide, is protective in two murine models of colitis. European journal of pharmacology. 2003;466:323–329. doi: 10.1016/s0014-2999(03)01570-x. [DOI] [PubMed] [Google Scholar]
- 82.Knight D, et al. Adenosine A3 receptor stimulation inhibits migration of human eosinophils. Journal of leukocyte biology. 1997;62:465–468. doi: 10.1002/jlb.62.4.465. [DOI] [PubMed] [Google Scholar]
- 83.Bowlin TL, Borcherding DR, Edwards CK, 3rd, McWhinney CD. Adenosine A3 receptor agonists inhibit murine macrophage tumor necrosis factor-alpha production in vitro and in vivo. Cell Mol Biol (Noisy-le-grand) 1997;43:345–349. [PubMed] [Google Scholar]
- 84.Hasko G, et al. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. Journal of immunology. 1996;157:4634–4640. [PubMed] [Google Scholar]
- 85.McWhinney CD, et al. Activation of adenosine A3 receptors on macrophages inhibits tumor necrosis factor-alpha. European journal of pharmacology. 1996;310:209–216. doi: 10.1016/0014-2999(96)00272-5. [DOI] [PubMed] [Google Scholar]
- 86.Sajjadi FG, Takabayashi K, Foster AC, Domingo RC, Firestein GS. Inhibition of TNF-alpha expression by adenosine: role of A3 adenosine receptors. Journal of immunology. 1996;156:3435–3442. [PubMed] [Google Scholar]
- 87.Hasko G, Csoka B, Nemeth ZH, Vizi ES, Pacher P. A(2B) adenosine receptors in immunity and inflammation. Trends Immunol. 2009;30:263–270. doi: 10.1016/j.it.2009.04.001. doi:10.1016/j.it.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilson JM, et al. The A2B adenosine receptor promotes Th17 differentiation via stimulation of dendritic cell IL-6. Journal of immunology. 2011;186:6746–6752. doi: 10.4049/jimmunol.1100117. doi:10.4049/jimmunol.1100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liang D, et al. A2B adenosine receptor activation switches differentiation of bone marrow cells to a CD11c(+)Gr-1(+) dendritic cell subset that promotes the Th17 response. Immun Inflamm Dis. 2015;3:360–373. doi: 10.1002/iid3.74. doi:10.1002/iid3.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Teramachi J, et al. Adenosine abolishes MTX-induced suppression of osteoclastogenesis and inflammatory bone destruction in adjuvant-induced arthritis. Laboratory investigation; a journal of technical methods and pathology. 2011;91:719–731. doi: 10.1038/labinvest.2011.9. doi:10.1038/labinvest.2011.9. [DOI] [PubMed] [Google Scholar]
- 91.Merrill JT, et al. Adenosine A1 receptor promotion of multinucleated giant cell formation by human monocytes: a mechanism for methotrexate-induced nodulosis in rheumatoid arthritis. Arthritis Rheum. 1997;40:1308–1315. doi: 10.1002/1529-0131(199707)40:7<1308::AID-ART16>3.0.CO;2-M. doi:10.1002/1529-0131(199707)40:7<1308::AID-ART16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 92.Kara FM, et al. Adenosine A1 receptors (A1Rs) play a critical role in osteoclast formation and function. FASEB J. 2010;24:2325–2333. doi: 10.1096/fj.09-147447. doi:10.1096/fj.09-147447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He W, Cronstein BN. Adenosine A1 receptor regulates osteoclast formation by altering TRAF6/TAK1 signaling. Purinergic Signal. 2012;8:327–337. doi: 10.1007/s11302-012-9292-9. doi:10.1007/s11302-012-9292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He W, Mazumder A, Wilder T, Cronstein BN. Adenosine regulates bone metabolism via A1, A2A, and A2B receptors in bone marrow cells from normal humans and patients with multiple myeloma. FASEB J. 2013;27:3446–3454. doi: 10.1096/fj.13-231233. doi:10.1096/fj.13-231233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mediero A, Kara FM, Wilder T, Cronstein BN. Adenosine A(2A) receptor ligation inhibits osteoclast formation. Am J Pathol. 2012;180:775–786. doi: 10.1016/j.ajpath.2011.10.017. doi:10.1016/j.ajpath.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Corciulo C, Wilder T, Cronstein BN. Adenosine A2B Receptors Play an Important Role in Bone Homeostasis. Purinergic Signaling. 2016 doi: 10.1007/s11302-016-9519-2. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mediero A, Frenkel S, Wilder T, Cronstein BN. Activation of Adenosine A(2A) Receptors Prevents Wear Particle-Induced Osteolysis. Arthritis and rheumatism. 2011;63:S697–S698. [Google Scholar]
- 98.Mediero A, Wilder T, Perez-Aso M, Cronstein BN. Direct or indirect stimulation of adenosine A2A receptors enhances bone regeneration as well as bone morphogenetic protein-2. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29:1577–1590. doi: 10.1096/fj.14-265066. doi:10.1096/fj.14-265066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carroll SH, et al. A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo. The Journal of biological chemistry. 2012;287:15718–15727. doi: 10.1074/jbc.M112.344994. doi:10.1074/jbc.M112.344994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Strazzulla LC, Cronstein BN. Regulation of bone and cartilage by adenosine signaling. Purinergic signalling. 2016 doi: 10.1007/s11302-016-9527-2. doi:10.1007/s11302-016-9527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 102.Ohta A, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. doi:0605251103 [pii] 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- 104.Koshiba M, Kojima H, Huang S, Apasov S, Sitkovsky MV. Memory of extracellular adenosine A2A purinergic receptor-mediated signaling in murine T cells. The Journal of biological chemistry. 1997;272:25881–25889. doi: 10.1074/jbc.272.41.25881. [DOI] [PubMed] [Google Scholar]
- 105.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. Journal of immunology. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 106.Lukashev D, Ohta A, Apasov S, Chen JF, Sitkovsky M. Cutting edge: Physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. Journal of immunology. 2004;173:21–24. doi: 10.4049/jimmunol.173.1.21. [DOI] [PubMed] [Google Scholar]
- 107.Armstrong JM, et al. Gene dose effect reveals no Gs-coupled A2A adenosine receptor reserve in murine T-lymphocytes: studies of cells from A2A-receptor-gene-deficient mice. The Biochemical journal. 2001;354:123–130. doi: 10.1042/0264-6021:3540123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hillger JM, et al. Getting personal: Endogenous adenosine receptor signaling in lymphoblastoid cell lines. Biochemical pharmacology. 2016 doi: 10.1016/j.bcp.2016.06.006. doi:10.1016/j.bcp.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 109.Koshiba M, Rosin DL, Hayashi N, Linden J, Sitkovsky MV. Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Molecular pharmacology. 1999;55:614–624. [PubMed] [Google Scholar]
- 110.Csoka B, et al. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:3491–3499. doi: 10.1096/fj.08-107458. doi:10.1096/fj.08-107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alam MS, et al. A2A adenosine receptor (AR) activation inhibits pro-inflammatory cytokine production by human CD4+ helper T cells and regulates Helicobacter-induced gastritis and bacterial persistence. Mucosal Immunol. 2009;2:232–242. doi: 10.1038/mi.2009.4. doi:10.1038/mi.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Himer L, et al. Adenosine A2A receptor activation protects CD4+ T lymphocytes against activation-induced cell death. FASEB J. 2010;24:2631–2640. doi: 10.1096/fj.10-155192. doi:10.1096/fj.10-155192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cekic C, Sag D, Day YJ, Linden J. Extracellular adenosine regulates naive T cell development and peripheral maintenance. The Journal of experimental medicine. 2013;210:2693–2706. doi: 10.1084/jem.20130249. doi:10.1084/jem.20130249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Apasov S, Chen JF, Smith P, Sitkovsky M. A(2A) receptor dependent and A(2A) receptor independent effects of extracellular adenosine on murine thymocytes in conditions of adenosine deaminase deficiency. Blood. 2000;95:3859–3867. [PubMed] [Google Scholar]
- 115.Takayama H, Trenn G, Sitkovsky MV. Locus of inhibitory action of cAMP-dependent protein kinase in the antigen receptor-triggered cytotoxic T lymphocyte activation pathway. The Journal of biological chemistry. 1988;263:2330–2336. [PubMed] [Google Scholar]
- 116.Sitkovsky MV, Trenn G, Takayama H. Cyclic AMP-dependent protein kinase as a part of the possible down-regulating pathway in the antigen receptor-regulated cytotoxic T lymphocyte conjugate formation and granule exocytosis. Annals of the New York Academy of Sciences. 1988;532:350–358. doi: 10.1111/j.1749-6632.1988.tb36352.x. [DOI] [PubMed] [Google Scholar]
- 117.Sitkovsky MV. T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol. 2009;30:102–108. doi: 10.1016/j.it.2008.12.002. doi:10.1016/j.it.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 118.Hatfield S, Belikoff B, Lukashev D, Sitkovsky M, Ohta A. The antihypoxia adenosinergic pathogenesis as a result of collateral damage by overactive immune cells. Journal of leukocyte biology. 2009;86:545–548. doi: 10.1189/jlb.0908577. doi:10.1189/jlb.0908577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen M, et al. An A2B Adenosine Receptor Agonist Promotes Th17 Autoimmune Responses in Experimental Autoimmune Uveitis (EAU) via Dendritic Cell Activation. PloS one. 2015;10:e0132348. doi: 10.1371/journal.pone.0132348. doi:10.1371/journal.pone.0132348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ehrentraut H, Westrich JA, Eltzschig HK, Clambey ET. Adora2b adenosine receptor engagement enhances regulatory T cell abundance during endotoxin-induced pulmonary inflammation. PloS one. 2012;7:e32416. doi: 10.1371/journal.pone.0032416. doi:10.1371/journal.pone.0032416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cohen MV, Yang X, Downey JM. A(2b) adenosine receptors can change their spots. British journal of pharmacology. 2010;159:1595–1597. doi: 10.1111/j.1476-5381.2010.00668.x. doi:10.1111/j.1476-5381.2010.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gessi S, et al. Pharmacological and biochemical characterization of A3 adenosine receptors in Jurkat T cells. British journal of pharmacology. 2001;134:116–126. doi: 10.1038/sj.bjp.0704254. doi:10.1038/sj.bjp.0704254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feoktistov I, Biaggioni I, Cronstein BN. Adenosine receptors in wound healing, fibrosis and angiogenesis. Handbook of experimental pharmacology. 2009:383–397. doi: 10.1007/978-3-540-89615-9_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Montesinos MC, et al. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am J Pathol. 2002;160:2009–2018. doi: 10.1016/S0002-9440(10)61151-0. doi:10.1016/S0002-9440(10)61151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hassanian SM, Dinarvand P, Rezaie AR. Adenosine regulates the proinflammatory signaling function of thrombin in endothelial cells. Journal of cellular physiology. 2014;229:1292–1300. doi: 10.1002/jcp.24568. doi:10.1002/jcp.24568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. doi:10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Teramachi J, et al. Adenosine abolishes MTX-induced suppression of osteoclastogenesis and inflammatory bone destruction in adjuvant-induced arthritis. Laboratory investigation; a journal of technical methods and pathology. 2011;91:719–731. doi: 10.1038/labinvest.2011.9. doi:10.1038/labinvest.2011.9. [DOI] [PubMed] [Google Scholar]
- 128.Shaikh G, Cronstein B. Signaling pathways involving adenosine A2A and A2B receptors in wound healing and fibrosis. Purinergic Signal. 2016;12:191–197. doi: 10.1007/s11302-016-9498-3. doi:10.1007/s11302-016-9498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cronstein BN. Adenosine receptors and fibrosis: a translational review. F1000 Biol Rep. 2011;3:21. doi: 10.3410/B3-21. doi:10.3410/B3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chan ES, et al. Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis and rheumatism. 2006;54:2632–2642. doi: 10.1002/art.21974. [DOI] [PubMed] [Google Scholar]
- 131.Fernandez P, et al. Pharmacological blockade of A2A receptors prevents dermal fibrosis in a model of elevated tissue adenosine. The American journal of pathology. 2008;172:1675–1682. doi: 10.2353/ajpath.2008.070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Katebi M, Fernandez P, Chan ES, Cronstein BN. Adenosine A2A receptor blockade or deletion diminishes fibrocyte accumulation in the skin in a murine model of scleroderma, bleomycin-induced fibrosis. Inflammation. 2008;31:299–303. doi: 10.1007/s10753-008-9078-y. doi:10.1007/s10753-008-9078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Perez-Aso M, Chiriboga L, Cronstein BN. Pharmacological blockade of adenosine A2A receptors diminishes scarring. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:4254–4263. doi: 10.1096/fj.12-209627. doi:10.1096/fj.12-209627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Blackburn MR, et al. Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway. The Journal of clinical investigation. 2003;112:332–344. doi: 10.1172/JCI16815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chunn JL, et al. Partially adenosine deaminase-deficient mice develop pulmonary fibrosis in association with adenosine elevations. Am J Physiol Lung Cell Mol Physiol. 2006;290:L579–587. doi: 10.1152/ajplung.00258.2005. [DOI] [PubMed] [Google Scholar]
- 136.Chunn JL, et al. Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. Journal of immunology. 2005;175:1937–1946. doi: 10.4049/jimmunol.175.3.1937. [DOI] [PubMed] [Google Scholar]
- 137.Ma B, et al. Adenosine metabolism and murine strain-specific IL-4-induced inflammation, emphysema, and fibrosis. The Journal of clinical investigation. 2006;116:1274–1283. doi: 10.1172/JCI26372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schneider DJ, Lindsay JC, Zhou Y, Molina JG, Blackburn MR. Adenosine and osteopontin contribute to the development of chronic obstructive pulmonary disease. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:70–80. doi: 10.1096/fj.09-140772. doi:10.1096/fj.09-140772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sun CX, et al. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. The Journal of clinical investigation. 2006;116:2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhou Y, Murthy JN, Zeng D, Belardinelli L, Blackburn MR. Alterations in adenosine metabolism and signaling in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. PLoS One. 2010;5:e9224. doi: 10.1371/journal.pone.0009224. doi:10.1371/journal.pone.0009224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhou Y, et al. Distinct roles for the A2B adenosine receptor in acute and chronic stages of bleomycin-induced lung injury. Journal of immunology. 2011;186:1097–1106. doi: 10.4049/jimmunol.1002907. doi:10.4049/jimmunol.1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chan ES, et al. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. British journal of pharmacology. 2006;148:1144–1155. doi: 10.1038/sj.bjp.0706812. doi:10.1038/sj.bjp.0706812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Peng Z, et al. Ecto-5'-nucleotidase (CD73) -mediated extracellular adenosine production plays a critical role in hepatic fibrosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008 doi: 10.1096/fj.07-100685. [DOI] [PubMed] [Google Scholar]
- 144.Robson SC, Schuppan D. Adenosine: tipping the balance towards hepatic steatosis and fibrosis. Journal of hepatology. 2010;52:941–943. doi: 10.1016/j.jhep.2010.02.009. doi:S0168-8278(10)00110-8 [pii] 10.1016/j.jhep.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Corrao G, et al. The effect of drinking coffee and smoking cigarettes on the risk of cirrhosis associated with alcohol consumption. A case-control study. Provincial Group for the Study of Chronic Liver Disease. Eur J Epidemiol. 1994;10:657–664. doi: 10.1007/BF01719277. [DOI] [PubMed] [Google Scholar]
- 146.Corrao G, Zambon A, Bagnardi V, D'Amicis A, Klatsky A. Coffee, caffeine, and the risk of liver cirrhosis. Ann Epidemiol. 2001;11:458–465. doi: 10.1016/s1047-2797(01)00223-x. [DOI] [PubMed] [Google Scholar]
- 147.Klatsky AL, Armstrong MA. Alcohol, smoking, coffee, and cirrhosis. American journal of epidemiology. 1992;136:1248–1257. doi: 10.1093/oxfordjournals.aje.a116433. [DOI] [PubMed] [Google Scholar]
- 148.Tverdal A, Skurtveit S. Coffee intake and mortality from liver cirrhosis. Ann Epidemiol. 2003;13:419–423. doi: 10.1016/s1047-2797(02)00462-3. [DOI] [PubMed] [Google Scholar]
- 149.Ferrari D, et al. Purinergic signaling in scarring. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30:3–12. doi: 10.1096/fj.15-274563. doi:10.1096/fj.15-274563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nakav S, et al. Blocking adenosine A2A receptor reduces peritoneal fibrosis in two independent experimental models. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24:2392–2399. doi: 10.1093/ndt/gfp041. doi:gfp041 [pii] 10.1093/ndt/gfp041. [DOI] [PubMed] [Google Scholar]
- 151.Mi T, et al. Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. The Journal of clinical investigation. 2008;118:1491–1501. doi: 10.1172/JCI33467. doi:10.1172/JCI33467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wen J, et al. Increased adenosine contributes to penile fibrosis, a dangerous feature of priapism, via A2B adenosine receptor signaling. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:740–749. doi: 10.1096/fj.09-144147. doi:10.1096/fj.09-144147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Berne RM, Belardinelli L. Effects of hypoxia and ischaemia on coronary vascular resistance, A-V node conduction and S-A node excitation. Acta Med Scand Suppl. 1985;694:9–19. doi: 10.1111/j.0954-6820.1985.tb08795.x. [DOI] [PubMed] [Google Scholar]
- 154.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111:5571–5580. doi: 10.1182/blood-2007-11-126763. doi:10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 155.Waidmann O, et al. Inhibition of the equilibrative nucleoside transporter 1 and activation of A2A adenosine receptors by 8-(4-chlorophenylthio)-modified cAMP analogs and their hydrolytic products. J Biol Chem. 2009;284:32256–32263. doi: 10.1074/jbc.M109.056622. doi:10.1074/jbc.M109.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. Journal of Biological Chemistry. 1990;265:1946–1951. [PubMed] [Google Scholar]
- 157.Rothschild BM, Masi AT. Pathogenesis of rheumatoid arthritis: a vascular hypothesis. Semin Arthritis Rheum. 1982;12:11–31. doi: 10.1016/0049-0172(82)90020-8. [DOI] [PubMed] [Google Scholar]
- 158.Richman AI, Su EY, Ho G., Jr. Reciprocal relationship of synovial fluid volume and oxygen tension. Arthritis Rheum. 1981;24:701–705. doi: 10.1002/art.1780240512. [DOI] [PubMed] [Google Scholar]
- 159.Zamani B, Jamali R, Ehteram H. Synovial fluid adenosine deaminase and high-sensitivity C-reactive protein activity in differentiating monoarthritis. Rheumatol Int. 2012;32:183–188. doi: 10.1007/s00296-010-1602-3. doi:10.1007/s00296-010-1602-3. [DOI] [PubMed] [Google Scholar]
- 160.Huang LF, Guo FQ, Liang YZ, Li BY, Cheng BM. Simple and rapid determination of adenosine in human synovial fluid with high performance liquid chromatography-mass spectrometry. J Pharm Biomed Anal. 2004;36:877–882. doi: 10.1016/j.jpba.2004.07.038. doi:10.1016/j.jpba.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 161.Ottonello L, et al. Synovial fluid from patients with rheumatoid arthritis inhibits neutrophil apoptosis: role of adenosine and proinflammatory cytokines. Rheumatology (Oxford) 2002;41:1249–1260. doi: 10.1093/rheumatology/41.11.1249. [DOI] [PubMed] [Google Scholar]
- 162.Cattaneo M, Schulz R, Nylander S. Adenosine-mediated effects of ticagrelor: evidence and potential clinical relevance. J Am Coll Cardiol. 2014;63:2503–2509. doi: 10.1016/j.jacc.2014.03.031. doi:10.1016/j.jacc.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 163.Armstrong D, et al. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Ther. 2014;19:209–219. doi: 10.1177/1074248413511693. doi:10.1177/1074248413511693. [DOI] [PubMed] [Google Scholar]
- 164.Nagy LE, et al. Adenosine is required for ethanol-induced heterologous desensitization. Molecular pharmacology. 1989;36:744–748. [PubMed] [Google Scholar]
- 165.Gordon AS, Nagy L, Mochly-Rosen D, Diamond I. Chronic ethanol-induced heterologous desensitization is mediated by changes in adenosine transport. Biochemical Society Symposia. 1990;56:117–136. [Review] [55 refs] [PubMed] [Google Scholar]
- 166.Diamond I, Nagy L, Mochly-Rosen D, Gordon A. The role of adenosine and adenosine transport in ethanol-induced cellular tolerance and dependence. Possible biologic and genetic markers of alcoholism. [Review] [31 refs]. Annals of the New York Academy of Sciences. 1991;625:473–487. doi: 10.1111/j.1749-6632.1991.tb33878.x. [DOI] [PubMed] [Google Scholar]
- 167.Peng Z, et al. Adenosine signaling contributes to ethanol-induced fatty liver in mice. The Journal of clinical investigation. 2009;119:582–594. doi: 10.1172/JCI37409. doi:37409 [pii] 10.1172/JCI37409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chouker A, et al. Critical role of hypoxia and A2A adenosine receptors in liver tissue-protecting physiological anti-inflammatory pathway. Mol Med. 2008;14:116–123. doi: 10.2119/2007-00075.Chouker. doi:10.2119/2007-00075.Chouker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chan ES, Cronstein BN. Methotrexate--how does it really work? Nature reviews. Rheumatology. 2010;6:175–178. doi: 10.1038/nrrheum.2010.5. doi:10.1038/nrrheum.2010.5. [DOI] [PubMed] [Google Scholar]
- 170.Cronstein BN, Eberle MA, Gruber HE, Levin RI. Methotrexate inhibits neutrophil function by stimulating adenosine release from connective tissue cells. Proceedings of the National Academy of Sciences (USA) 1991;88:2441–2445. doi: 10.1073/pnas.88.6.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate: increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. Journal of Clinical Investigation. 1993;92:2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Montesinos C, et al. Reversal of the antiinflammatory effects of methotrexate by the nonselective adenosine receptor antagonists theophylline and caffeine. Evidence that the antiinflammatory effects of methotrexate are mediated via multiple adenosine receptors in rat adjuvant arthritis. Arth. Rheum. 2000;43:656–663. doi: 10.1002/1529-0131(200003)43:3<656::AID-ANR23>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 173.Nesher G, Mates M, Zevin S. Effect of caffeine consumption on efficacy of methotrexate in rheumatoid arthritis. Arthritis and rheumatism. 2003;48:571–572. doi: 10.1002/art.10766. [DOI] [PubMed] [Google Scholar]
- 174.Silke C, et al. The effects of caffeine ingestion on the efficacy of methotrexate. Rheumatology. 2001;40(Suppl1):34. [Google Scholar]
- 175.Benito-Garcia E, et al. Dietary caffeine intake does not affect methotrexate efficacy in patients with rheumatoid arthritis. The Journal of rheumatology. 2006;33:1275–1281. doi:0315162X-33-1275 [pii] [PubMed] [Google Scholar]
- 176.Peres RS, et al. Low expression of CD39 on regulatory T cells as a biomarker for resistance to methotrexate therapy in rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2509–2514. doi: 10.1073/pnas.1424792112. doi:10.1073/pnas.1424792112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Olsen NJ, Spurlock CF, 3rd, Aune TM. Methotrexate induces production of IL-1 and IL-6 in the monocytic cell line U937. Arthritis research & therapy. 2014;16:R17. doi: 10.1186/ar4444. doi:10.1186/ar4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Spurlock CF, 3rd, Tossberg JT, Fuchs HA, Olsen NJ, Aune TM. Methotrexate increases expression of cell cycle checkpoint genes via JNK activation. Arthritis and rheumatism. 2012;64:1780–1789. doi: 10.1002/art.34342. doi:10.1002/art.34342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Spurlock CF, 3rd, et al. Methotrexate-mediated inhibition of nuclear factor kappaB activation by distinct pathways in T cells and fibroblast-like synoviocytes. Rheumatology. 2015;54:178–187. doi: 10.1093/rheumatology/keu279. doi:10.1093/rheumatology/keu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lindenbaum J. Drugs and vitamin B12 and folate metabolism. Curr Concepts Nutr. 1983;12:73–87. [PubMed] [Google Scholar]
- 181.Gadangi P, et al. The anti-inflammatory mechanism of sulfasalazine is related to adenosine release at inflamed sites. J Immunol. 1996;156:1937–1941. [PubMed] [Google Scholar]
- 182.Morabito L, et al. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J Clin Invest. 1998;101:295–300. doi: 10.1172/JCI1554. doi:10.1172/JCI1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mehlman MA, Tobin RB, Madappally MM, Hahn HK. Mode of action of aspirin. Effect of dietary aspirin on mitochondrial pyruvate metabolism in normal and thiamine-deficient rats. The Journal of biological chemistry. 1971;246:1618–1622. [PubMed] [Google Scholar]
- 184.Thompkins L, Lee KH. Studies on the mechanism of action of salicylates. IV. Effect of salicylates on oxidative phosphorylation. J Pharm Sci. 1969;58:102–105. doi: 10.1002/jps.2600580122. [DOI] [PubMed] [Google Scholar]
- 185.Cronstein BN, Van de Stouwe M, Druska L, Levin RI, Weissmann G. Nonsteroidal antiinflammatory agents inhibit stimulated neutrophil adhesion to endothelium: adenosine dependent and independent mechanisms. Inflammation. 1994;18:323–335. doi: 10.1007/BF01534273. [DOI] [PubMed] [Google Scholar]
- 186.Cronstein BN, Montesinos MC, Weissmann G. Salicylates and sulfasalazine, but not glucocorticoids, inhibit leukocyte accumulation by an adenosine-dependent mechanism that is independent of inhibition of prostaglandin synthesis and p105 of NFkappaB. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6377–6381. doi: 10.1073/pnas.96.11.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Baharav E, et al. Antiinflammatory effect of A3 adenosine receptor agonists in murine autoimmune arthritis models. The Journal of rheumatology. 2005;32:469–476. [PubMed] [Google Scholar]
- 188.Silverman MH, et al. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: data from a phase II clinical trial. The Journal of rheumatology. 2008;35:41–48. [PubMed] [Google Scholar]
- 189.David M, et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol. 2012;26:361–367. doi: 10.1111/j.1468-3083.2011.04078.x. doi:10.1111/j.1468-3083.2011.04078.x. [DOI] [PubMed] [Google Scholar]
- 190.Gubner R, August S, Ginsberg V. Therapeutic suppression of tissue reactivity. II. Effect of aminopterin in rheumatoid arthritis and psoriasis. American Journal of Medical Science. 1951;221:176–182. [PubMed] [Google Scholar]
- 191.Weinblatt ME. Methotrexate in rheumatoid arthritis: a quarter century of development. Trans Am Clin Climatol Assoc. 2013;124:16–25. [PMC free article] [PubMed] [Google Scholar]
- 192.Morgan SL, et al. Supplementation with folic acid during methotrexate therapy for rheumatoid arthritis. A double-blind, placebo-controlled trial. Annals of internal medicine. 1994;121:833–841. doi: 10.7326/0003-4819-121-11-199412010-00002. [DOI] [PubMed] [Google Scholar]
- 193.Morgan SL, Baggott JE, Koopman WJ, Krumdieck CL, Alarcon GS. Folate supplementation and methotrexate. Annals of the rheumatic diseases. 1993;52:315–316. doi: 10.1136/ard.52.4.315-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Morgan SL, et al. The effect of folic acid supplementation on the toxicity of low-dose methotrexate in patients with rheumatoid arthritis. Arthritis and rheumatism. 1990;33:9–18. doi: 10.1002/art.1780330102. [DOI] [PubMed] [Google Scholar]