Abstract
Most hyphenated analytical approaches that rely on liquid chromatography-MS require relatively long separation times, produce incomplete resolution of oligosaccharide mixtures, use eluents that are incompatible with electrospray ionization, or require oligosaccharide derivatization. Here we demonstrate the analysis of heparin oligosaccharides, including disaccharides, ultra low molecular weight heparin, and a low molecular weight heparin, using a novel electrokinetic pump-based CE-MS coupling eletrospray ion source. Reverse polarity CE separation and negative mode electrospray ionization were optimized using a volatile methanolic ammonium acetate electrolyte and sheath fluid. The on-line CE hyphenated negative-ion electrospray ionization MS on an LTQ Orbitrap mass spectrometer was useful in disaccharide compositional analysis, bottom-up and top-down analysis of low molecular weight heparin. The application of this CE-MS method to ultra low molecular heparin suggest that a charge state distribution and the low level of sulfate group loss that is achieved make this method useful on-line tandem MS analysis of heparins.
Keywords: Hyphenated techniques, CE-MS, heparin, low molecular weight heparin, oligosaccharides, glycosaminoglycan
Graphical abstract
Introduction
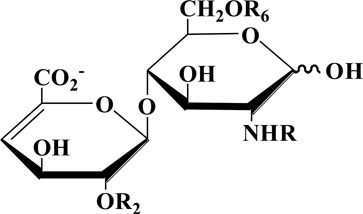
Glycosaminoglycans (GAGs) are polydisperse, linear, polyanionic, polysaccharides that are particularly challenging to analyze[1, 2]. Heparin is a particularly important GAG as it is a widely used clinical anticoagulant[3, 4]. Heparin and the structurally related GAG, heparan sulfate, has a highly variable sulfated structure comprised of a repeating disaccharide unit of →4) α-D-glucosamine (GlcN) (1→4) hexuronic acid (1→, where the GlcN residue can modified with N-acetyl (Ac) or N-sulfo (S) groups and 6-O-sulfo and/or 3-O-sulfo groups and the hexuronic acid can be β-D-glucuronic acid or α-L-iduronic acid residues that can be modified with 2-O-sulfo groups[1, 5]. The analysis of heparin and low molecular weight (LMW) heparins have become increasingly important since the contamination of the heparin supply chain in 2007–8[6, 7].
Heparin based drug products are becoming more dependent on mass spectrometry (MS) for high sensitivity, high throughput analyses. In particular, hyphenated MS methodologies, such as liquid chromatography (LC)-MS[4, 8–13] and more recently capillary electrophoresis (CE)-MS, are taking a leading role in these analyses[14–18]. These methods can provide enhanced resolution for the analysis of complex mixtures of heparin polysaccharide and oligosaccharide chains without loss of detection sensitivity. There are three types of routinely performed heparin and LMW heparin structural analysis that utilize hyphenated MS methodologies, disaccharide compositional analysis [4, 10, 11], bottom-up analysis [9, 12], and top-down analysis [8, 13]. In the case of disaccharide bottom-up analysis and compositional analysis, heparin sample is first treated with one or more heparin lyases [4, 9–12] to afford either oligosaccharides or disaccharides for subsequent LC or CE separation and MS analysis. Top-down analysis examines the whole intact GAG chain and has been limited to either small GAG chains, such as bikunin [19], or LMW heparins[8, 13]. Electrophoresis, while offering extremely high-resolution separations is particularly complicated for on-line coupling to MS. Sheathless CE-MS interfaces points to a potentially promising direction, transferring this post-electrophoresis pre-electrospray reaction directly to sheathless CE-MS system. Online sheathless CE-MS has been successfully applied to the analysis of GAG derived, chondroitin and dermatan sulfate oligosaccharides[17]. Alternatively, sheathed CE-MS interface, mixes a sheath liquid to the capillary output, owning advantages of CE eluent modification and analyte derivatization before it enters the mass spectrometer. Numerous studies also suggest that negatively charged heparin oligosaccharides represent ideal precursor ions for tandem MS analysis by reducing the level of sequence non-informative fragmentation through sulfate loss[20–22]. However, negative-mode CE-MS interface, which is more suitable and applicable to on-line analysis of negatively charged heparins, remains a major technical challenge. The current study relies on a reverse polarity separation with the analyte injected at the cathode (Figure 1) and a coated sheath capillary interface to promote the electrokinetic pumping of negatively charged heparin oligosaccharides for MS analysis.
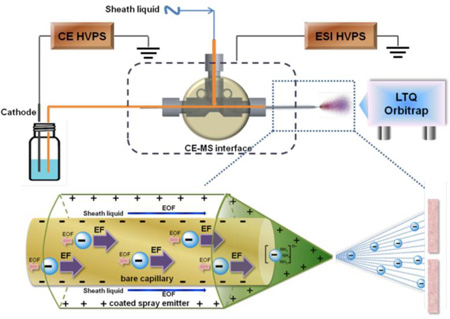
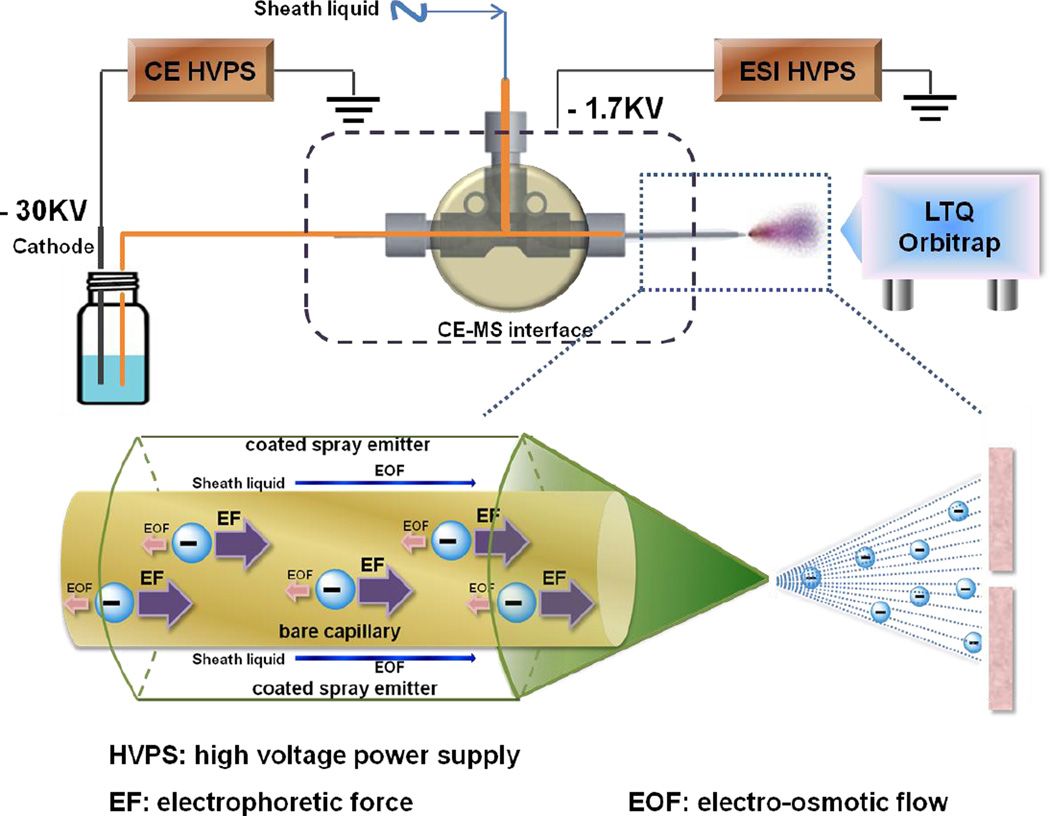
Figure 1.
Schematic representation of negative mode CE-MS system. A reverse polarity separation under a dominant electrophoretic force (EF) and low electroosmotic flow (EOF) is used to move analyte down a bare separation capillary. The cathode end of the separation capillary is capped with a protein-coated spray emitter sheath capillary with sheath liquid pumped by EOF, mixing with separation flow and affording a stable electrospray of negatively charged analyte that is introduced into an LTQ Orbitrap for MS analysis.
Experimental
Reagents
Heparin/heparan sulfate disaccharide standards were puchased from Iduron, Manchester, UK. Arixtra® and Lovenox® were purchased from Sanofi, Bridgewater, New Jersey. Ammonium acetate (HPLC Grade), calcium chloride (HPLC Grade), acetic acid (HPLC Grade) and methanol (HPLC Grade) were purchased from Fisher Scientific, Springfield, New Jersey. Bare separation capillary (360/150 µm OD × 50 µm ID × 50 cm) and coated spray emitter (1.0 mm OD × 0.75 mm ID, E-BS-CC1-750-1000-10u-B20) were obtained from CMP Scientific, Brooklyn, NY. Escherichia coli expression and purification of the recombinant Flavobacterium heparinum heparin lyase I, II, III (Enzyme Commission (EC) numbers 4.2.2.7, 4.2.2.x, 4.2.2.8) were performed in our laboratory as previously described[23].
Sample preparation
Eight heparin/heparan sulfate disaccharide standards, unsulfated (0S, ΔUA(1→4)GlcNAc, where ΔUA is 4-deoxy-α-l-threo-hex-4-enopyranosyluronic acid), monosulfated (2S, ΔUA2S(1→4)GlcNAc; 6S, ΔUA(1→4)GlcNAc6S; NS, ΔUA(1→4)GlcNS), disulfated (2S6S, ΔUA2S(1→4)GlcNAc6S; NS6S, ΔUA(1→4)GlcNS6S; NS2S, ΔUA2S(1→4)GlcNS), and trisulfated (TriS, ΔUA2S(1→4)GlcNS6S) (Table 1) were prepared as stock solutions with a concentration of 1 µg/µL. A standard mixture solution containing eight disaccharides (each with a final concentration of 100 µg/mL) was used in method development studies.
Table 1.
Structures of heparin/heparan sulfate disaccharide standards.
Desalted and lyophilized Arixtra® and Lovenox® were re-dissolved in distilled water at a concentration of 2 µg/µL and 1 µg/µL, respectively. Lovenox® (100 µg in 10 µL of distilled water) was added to 100 µL digestion buffer (50 mM NH4Ac, 2 mM CaCl2, pH 7.0). Heparin lyase I, II and III (10 mU each in Tris-HCl buffer, pH 7.0) were added and mixed well. The solution was placed in incubator at 37 °C overnight. The enzymatic digestion was terminated by removing the enzymes using a 3-kDa molecular weight cut-off (MWCO) spin-column. The filtrate containing disaccharides from the completely depolymerized Lovenox® was lyophilized and re-dissolved in 100 µL of distilled water at a concentration of 1 µg/µL.
CE-MS analysis
The CE separation was performed on a CMP ECE-001 capillary electrophoresis instrument equipped with a CMP EMASS-II CE-MS Ion Source (CMP Scientific, Brooklyn, NY). 10 mM ammonium acetate (AA) in 80% aqueous methanol (pH~7.5) was used as background electrolyte and applied in bare separation capillary (360/150 µm OD × 50 µm ID) at 25 °C.
The sheath liquid was 10 mM ammonium acetate (AA) in 80% aqueous methanol. The sample was injected under 0.2 psi pressure for 5 s. The applied CE separation voltage was 30 kV with reversed polarity. The electrospray voltage at the CE-MS interface[24, 25] was −1.7 kV. The spray emitters were 1.0 mm OD × 0.75 mm ID, borosilicate glass with a 10 µm tip, which were coated by CMP using proprietary method (E-BS-CC1-750-1000-10u-B20, CMP Scientific, Corp.). The distance from emitter tip to mass spectrometer was adjusted to 4 mm with the help of a microscope camera.
A Thermo Fisher Scientific (San-Jose, California) LTQ-Orbitrap XL was coupled online to the CE. The MS analysis was under negative ion mode. The capillary voltage was −43 V. The capillary temperature was 200 °C and the tube lens voltage was −100 V. The Fourier transform (FT) MS resolution was 30,000 and all other parameters were set as default.
Data analysis and bioinformatics
The data analysis was performed on Thermo Xcalibur software. Intact chian automaticly processing was done as published before[8, 26] and oligosaccharides up to dodecasaccharide are focused on.
Results and discussion
CE-MS interface
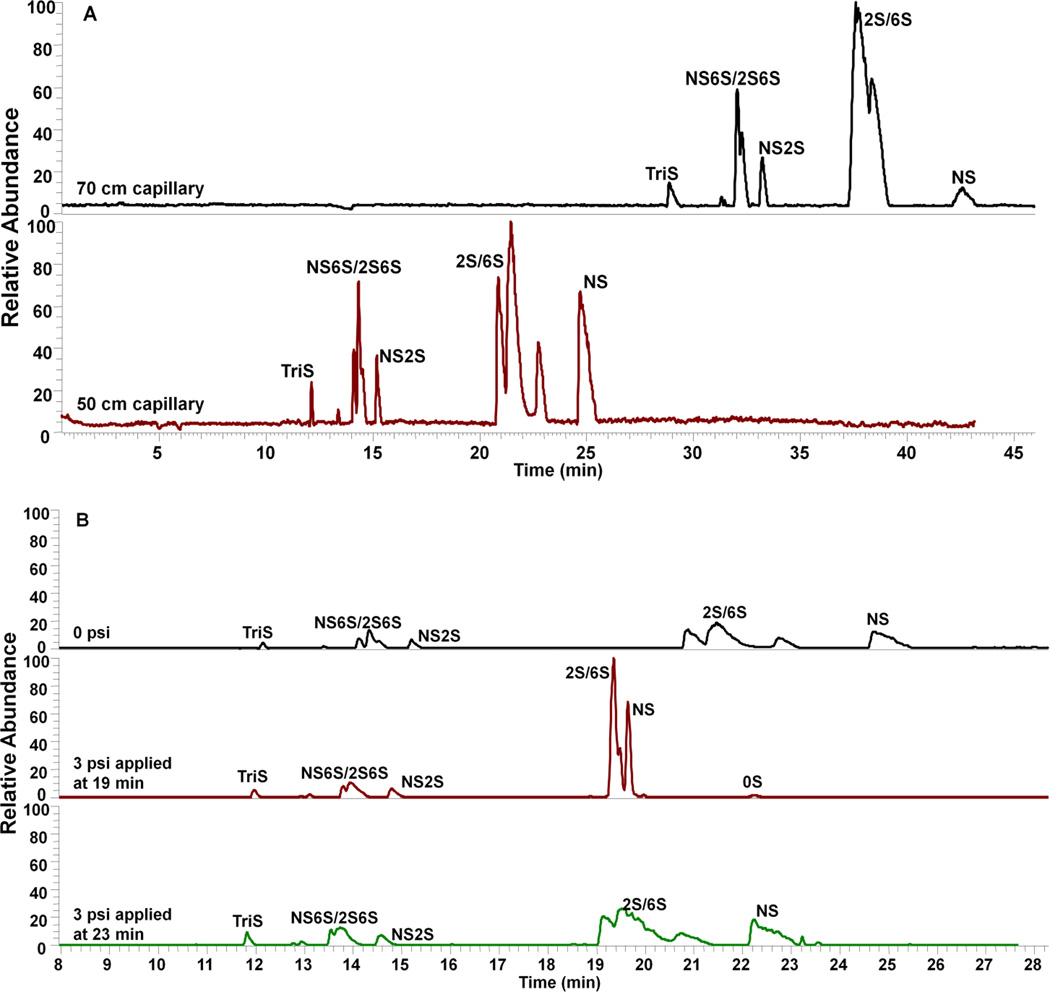
A reverse polarity separation of heparin oligosaccharides used an uncoated capillary with 10 mM aqueous ammonium acetate buffer containing 80% methanol (Figure 1). The presence of 80% methanol in the separation capillary reduces solute-wall interaction. In 10 mM ammonium acetate buffer containing 80% methanol, the bare fused silica wall is negatively charged with a diffuse layer of cations adjacent to the capillary wall silanol anions. When a reverse polarity electric field is applied, the cations in the diffuse layer migrate towards cathode. When these cations are hydrated, this migration drags solvent with them. While, when these cations are surrounded by mostly methanol, it is hard to drag solvent with the migration. This phenomenon is mainly due to the viscosity of methanol as well as the weakened interaction between the anions on the surface silanol and the cations in the diffuse layer. The length of the capillary (Figure 2A) and applied pressure (Figure 2B) were investigated by using a mixture of unsulfated, monosulfated, disulfated and trisulfated (TriS) heparin/heparan sulfate disaccharides to provide the optimal disaccharide separation. Based on the results, the optimun capillary length and pressure were 50 cm and 3 psi applied at 19 min, respectively. Under reversed polarity, the dominant electrophoretic force (EF) with a low electroosmotic flow (EOF) moves analyte down a bare separation capillary to the cathode with the most highly negatively charged analyte, TriS, moving fastest. The end of the separation capillary is capped with a protein-coated spray emitter sheath capillary with sheath liquid pumped by EOF, mixing with separation flow and affording a stable electrospray (see Electronic Supplementary Material (ESM) Fig. S2) of negatively charged analyte that is introduced into an LTQ Orbitrap for MS analysis (ESM Fig. S1).
Figure 2.
Optimization of (A) capillary length and (B) applied pressure on CE-MS analysis of 8 disaccharide standards. Background electrolyte and sheath liquid: 10 mM ammonium acetate (AA) in 80% aqueous methanol; separation voltage: −30 kV; electrospray voltage: −1.7 kV.
CE-MS of heparin/heparan sulfate disaccharides
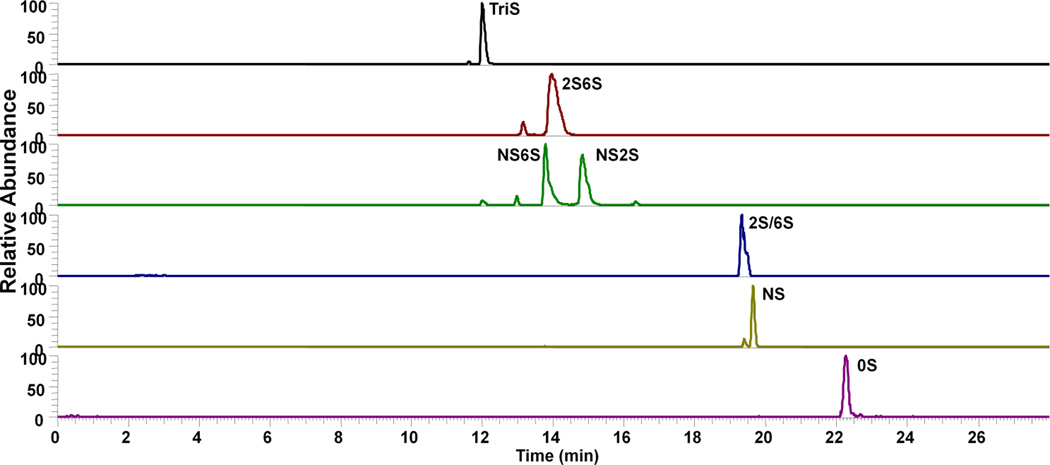
Eight heparin/heparan sulfate disaccharides were separated under optimized conditions and analyzed by negative-ion MS. Their extracted ion electropherograms (XIEs) are shown in Figure 3. These peaks were identified using standards. The observed charge states and mass/charge (m/z) ratios of these eight disaccharides are provided in the table in Figure 5. CE-MS shows sufficient resolution of the 8 disaccharide components for disaccharide compositional analysis of heparins and LMW heparins. The results suggest that on-line analysis of heparins can be achieved through the negative-mode CE-MS platform.
Figure 3.
Extracted ion electropherograms (XIE) of 8 disaccharide standards under optimal experimental conditions. Background electrolyte and sheath liquid: 10 mM ammonium acetate (AA) in 80% aqueous methanol; separation voltage: −30 kV; electrospray voltage: −1.7 kV; separation pressure (3psi) was applied at 19 min..
Figure 5.
Extracted Ion Chromatography of building blocks of LMWH detected in CE-MS. (Compositions are shown in the combined table).
CE-MS of Arixtra®
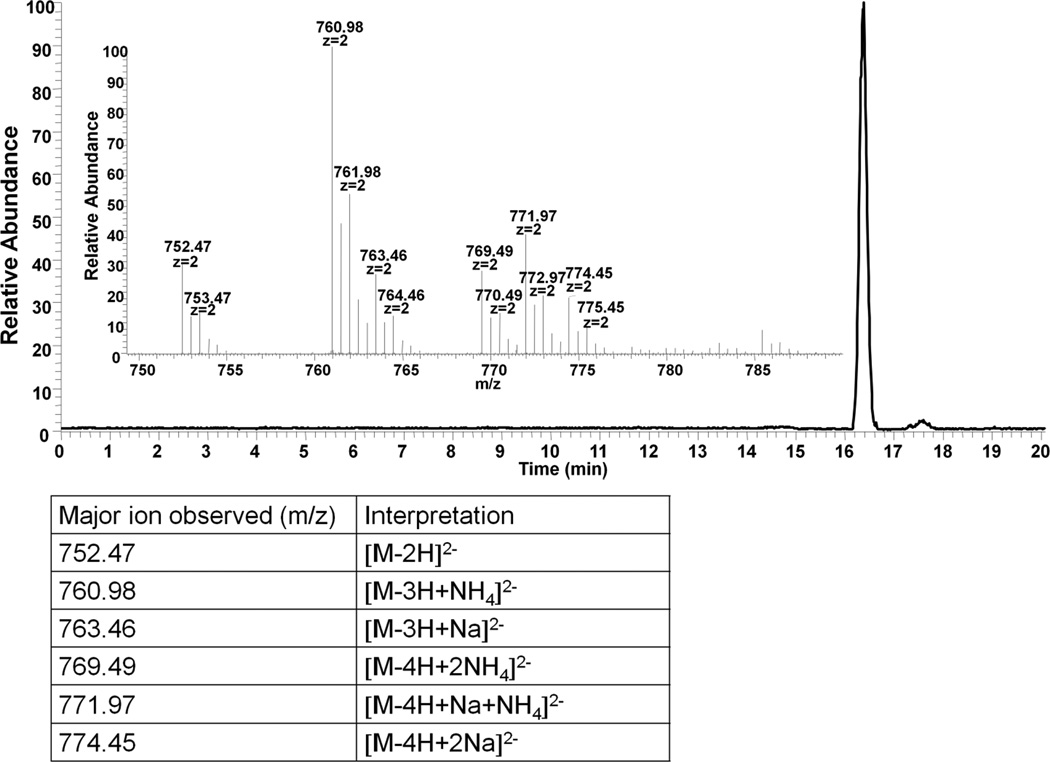
Arixtra® is an ultra low molecular weight (ULMW) heparin, a chemically synthesized octasulfated pentasaccharide (ESM Fig. S3), which is used clinically as a specific anti-Factor Xa agent[27, 28]. Arixtra® poses a specific challenge for MS analysis as it is highly charged and prone, under many MS conditions, to lose sulfo groups (SO3−). Under specific MS conditions, parent ions can be generated at an optimal charge state to allow the application of tandem MS using collisionally-induced dissociation (CID) with a low loss in sulfo groups and an enhanced number of sequence-informative fragments[29, 30]. Thus, we selected Arixtra® as a CE-MS analyte to observe the quality of MS analysis and the distribution of charge states. The XIE and the MS of Arixtra® and the distribution of charge states under CE-MS analysis are presented in Figure 4. The intact molecular ion is observed as a doubly-charged ion, along with ammonium and sodium exchange with protons. Based on the quality of these data, future work will investigate the application of CID tandem MS analysis for sequencing oligosaccharides eluting from the capillary.
Figure 4.
Negative-mode CE-MS analysis of Arixtra®. The inset shows the major ions observed in the peak at 16.4 min. The interpretations are listed in the table below.
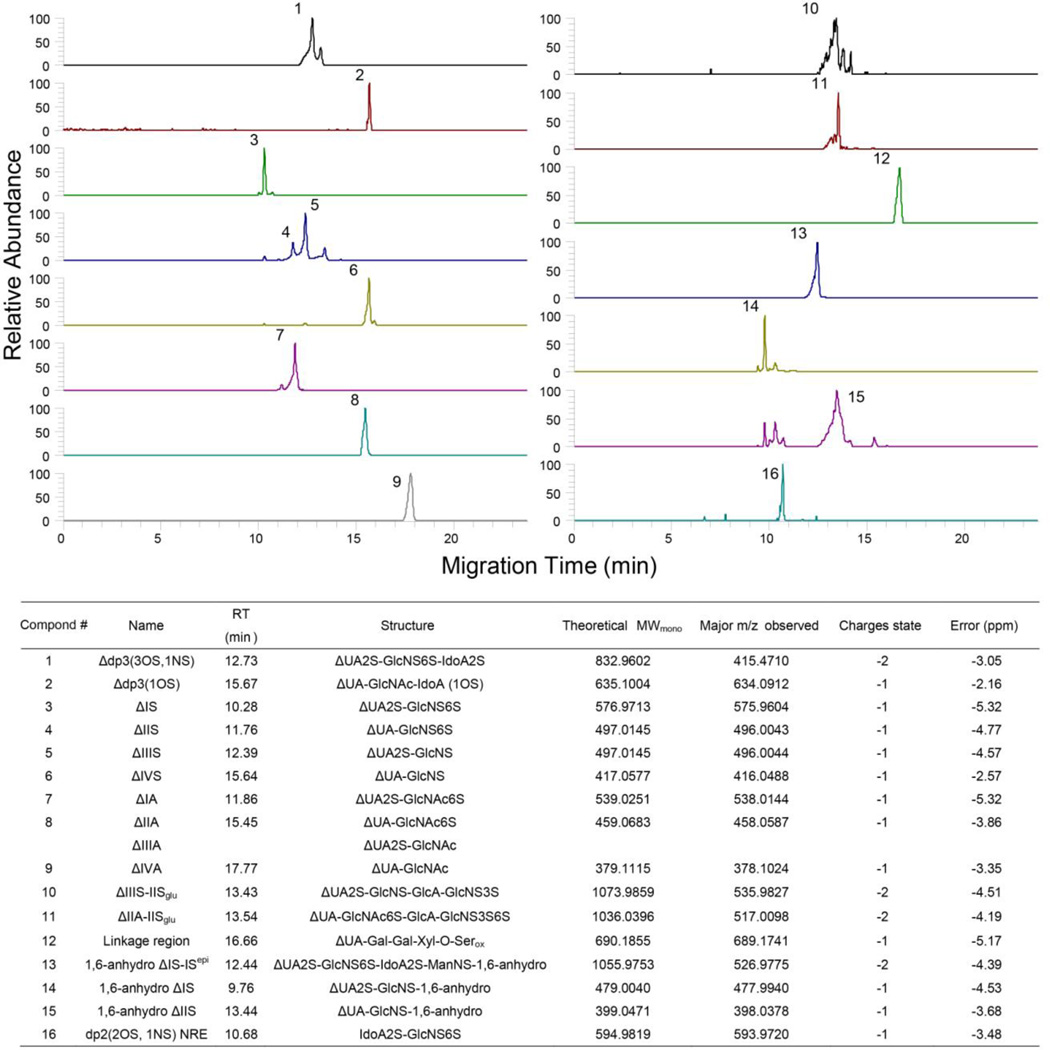
CE-MS of LMWH building blocks
Extracted ion electrophoresis of building blocks of LMW heparin, Lovenox®, produced by treatment with heparin lyases I, II and III, separated by CE and determined using bottom-up analysis with MS detection (Figure 5). This analysis was highly reproducible (ESM Fig. S6) and the composition is shown in the combined table of Figure 5. CE-MS could be completed within 20 min and 17 building blocks including modified structures were determined. All components except ΔIIA and ΔIIIA could be separated in extracted ion electropherograms. A signature building block of all enoxaparins, including Lovenox®, are oligosaccharides containing process artifacts, 1,6-anhydro oligosaccharides (ESM Fig. S4), are observed as disaccharide and tetrasaccharides[10]. In addition to normally encountered building blocks, a linkage region tetrasaccharide was also identified[9]. Moreover, 3-O-sulfo group containing tetrasaccharides that are remnants of the antithrombin pentasaccharide binding site, such as the one present within the structure of Arixtra®, can also be determined by CE-MS. Thus, CE-MS can be used for the rapid bottom-up analysis of LMW heparins.
CE-MS of LMWH intact chains
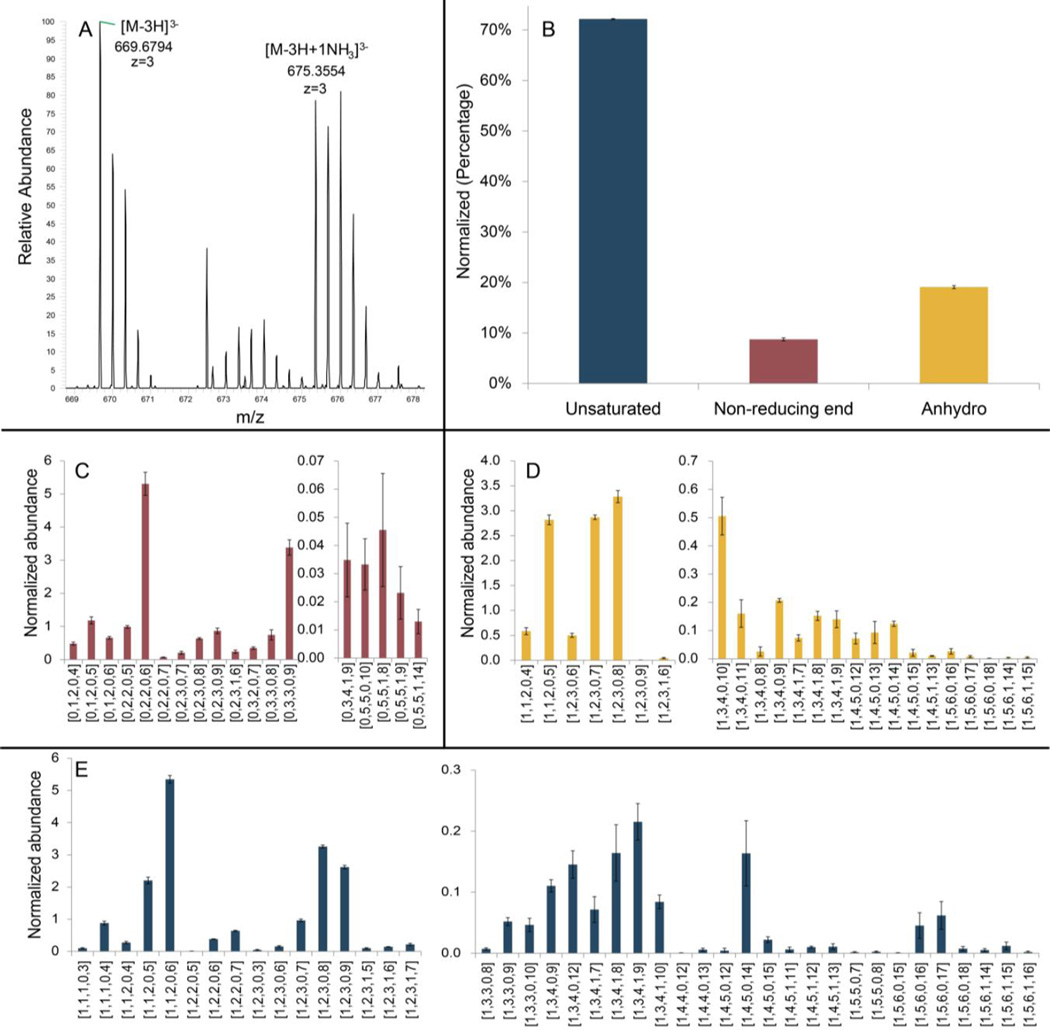
Finally, CE-MS was applied for the reproducible (ESM Fig. S7) top-down analysis of the LMW heparin, Lovenox® (Figure 6). Eighty-four oligosaccharides, ranging from disaccharides (degree of polymerization (dp) 2) to dodecasaccharides (dp12) could be detected. These experiments were performed in triplicate to determine the standard deviation of the relative quantity of each component chain measured. Ions with ammonium adduction are also observed and are shown in Figure 6A. CE-MS analysis could be completed in less than 30 min with a lower level of ammonium adduction than observed using hydrophilic interaction liquid chromatography (HILIC)-LC-MS[9], which results in less false positives, simplifying the data processing.
Figure 6.
Intact chain characterization of LMWH (Lovenox®). Oligosaccharide compositions are given as [ΔHexA, HexA, GlcN, Ac, SO3]. (A) NH3 adduction in CE-MS (1,6-anhydro dp8, 1Ac, 8SO3. M=2012.06). (B) Relative amount (percentage) of identified 3 major components of LMWH. (C) Compositional characterization of oligosaccharides with saturated non-reducing end. (D) Compositional characterization of oligosaccharides with 1,6-anhydro reducing end. (E) Compositional characterization of oligosaccharides with unsaturated non-reducing end.
Conclusions
The results obtained demonstrate the utility of CE with reverse polarity separation and a coated sheath capillary interface to promote the electrokinetic pumping of negatively charged heparin oligosaccharides for negative-ion MS analysis on an LTQ Orbitrap. This CE-MS method was useful for disaccharide compositional analysis, bottom-up analysis, and top-down analysis. The results obtained were comparable or better than those obtained when these analyses were performed using LC-MS[4, 8, 9]. Moreover, when compared to our previously published, normal polarity CE hyphenated positive-ion MS, the use of reverse polarity CE hyphenated negative-ion MS should facilitate its application for CID tandem MS to determine sequence of chains within LMW heparins or their lyase treated products.
Supplementary Material
Acknowledgments
The work was supported by Grants from the National Institutes of Health in the form of Grants HL125371, GM38060, GM103390, GM090127, HL096972, and HL10172.
Biographies

Jonathan Amster is Professor of Chemistry at the University of Georgia, and Head of the Department of Chemistry. His research interests include understanding the fundamentals of Fourier transform mass spectrometry and its application to bioanalytical chemistry, the development of methods for the structural analysis of glycosaminoglycan oligosaccharides, and the application of ion mobility for examining the three dimensional structure of glycan-protein complexes.

Lianli Chi is a Professor at the National Glycoengineering Research Center, Shandong University, China. He received his Ph.D. in analytical chemistry under the supervision of Professor Robert J. Linhardt from Rensselaer Polytechnic Institute. Currently he is studying in the field of proteomics and post-translational modification proteomics, as well as structural characterization of low molecular weight heparins.

Franklin E. Leach III is currently the lead scientist for research and development at Photochem Technologies in Athens, GA. His primary interests are the development of mass spectrometry based instrumentation and methodologies for the structural characterization of biomolecules.

Lei Lin is a Postdoctoral Research Fellow in Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute. Her research mainly focuses on analytical method development using mass spectrometry for heparin analysis.

Robert J. Linhardt is the Senior Constellation Professor of Biocatalysis and Metabolic Engineering at Rensselaer, holding appointments in Chemistry, Biology, Chemical Engineering & Biomedical Engineering. He holds an Adjunct Professor of Orthopaedics at the Icahn School of Medicine at Mount Sinai and an Adjunct Professor of Pharmacy at Albany College of Pharmacy. His research focuses on glycoscience and glycotechnology, with specific emphasis on heparin.

Xinyue Liu is a graduate student from Shandong University, attending an international program in Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute. Her research mainly focuses on mass spectrometry method developing and glycosaminoglycan structure analysis.

Fuming Zhang (PhD) is a Research Professor in Department of Chemical and Biological Engineering, Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute. His research focus is in glycomics, glycoengineering and molecular interactions using surface plasmon resonance (SPR).

James (Qiangwei) Xia (PhD) is the CEO and principal scientist of CMP Scientific, Corp. His current research interest is mainly the electrophoretic separation and mass spectrometric detection of analytes that are relevant to life science and biopharmaceutical industry. More specifically, he is devoted to make capillary electrophoresis-mass spectrometry (CE-MS) coupling technology simpler, faster, and more robust.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Compliance with ethical Standards
Ethical Approval This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Linhardt RJ, Toida T. Role of glycosaminoglycans in cellular communication. Acc Chem Res. 2004;37:431–438. doi: 10.1021/ar030138x. [DOI] [PubMed] [Google Scholar]
- 2.Heinegård D. Fell-muir lecture: proteoglycans and more-from molecules to biology. Int J Experim Pathol. 2009;90:575–586. doi: 10.1111/j.1365-2613.2009.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linhardt RJ. 2003 Claude S. Hudson Award Address in carbohydrate chemistry. Heparin: structure and activity. J Med Chem. 2003;46:2551–2564. doi: 10.1021/jm030176m. [DOI] [PubMed] [Google Scholar]
- 4.Yang B, Weyers A, Baik JY, Sterner E, Sharfstein S, Mousa SA, Zhang F, Dordick JS, Linhardt RJ. Ultra-performance ion-pairing liquid chromatography with on-line electrospray ion trap mass spectrometry for heparin disaccharide analysis. Anal Biochem. 2011;415:59–66. doi: 10.1016/j.ab.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao Z, Tappen BR, Ly M, Zhao W, Canova LP, Guan H, Linhardt RJ. Heparin mapping using heparin lyases and the generation of a novel low molecular weight heparin. J Med Chem. 2011;54:603–610. doi: 10.1021/jm101381k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerrini M, Beccati D, Shriver Z, Naggi A, Viswanathan K, Bisio A, Capila I, Lansing JC, Guglieri S, Fraser B, Al-Hakim A, Gunay NS, Zhang Z, Robinson L, Buhse L, Nasr M, Woodcock J, Langer R, Venkataraman G, Linhardt RJ, Casu B, Torri G, Sasisekharan R. Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nat Biotech. 2008;26:669–675. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Zhang Z, Linhardt RJ. Lessons learned from the contamination of heparin. Nat Prod Rep. 2009;26:313–321. doi: 10.1039/b819896a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Zhang F, Zaia J, Linhardt RJ. Top-down approach for the direct characterization of low molecular weight heparins using LC-FT-MS. Anal Chem. 2012;84:8822–8829. doi: 10.1021/ac302232c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li G, Steppich J, Wang Z, Sun Y, Xue C, Linhardt RJ, Li L. Bottom-up low molecular weight heparin analysis using liquid chromatography-fourier transform mass spectrometry for extensive characterization. Anal Chem. 2014;86:6626–6632. doi: 10.1021/ac501301v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Li D, Sun X, Bai X, Jin L, Chi L. Liquid chromatography–diode array detection–mass spectrometry for compositional analysis of low molecular weight heparins. Anal Biochem. 2014;451:35–41. doi: 10.1016/j.ab.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Yang B, Chang Y, Weyers AM, Sterner E, Linhardt RJ. Disaccharide analysis of glycosaminoglycan mixtures by ultra-high-performance liquid chromatography–mass spectrometry. J Chromatogr A. 2012;1225:91–98. doi: 10.1016/j.chroma.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X, Li D, Chi L, Du X, Bai X, Chi L. Fragment profiling of low molecular weight heparins using reversed phase ion pair liquid chromatography-electrospray mass spectrometry. Carbohydr Res. 2015;407:26–33. doi: 10.1016/j.carres.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Chi L, Jin L, Xu X, Du X, Ji S, Chi L. Mapping of low molecular weight heparins using reversed phase ion pair liquid chromatography–mass spectrometry. Carbohydr Polym. 2014;99:339–344. doi: 10.1016/j.carbpol.2013.08.074. [DOI] [PubMed] [Google Scholar]
- 14.Smith RD, Olivares JA, Nguyen NT, Udseth HR. Capillary zone electrophoresis-mass spectrometry using an electrospray ionization interface. Anal Chem. 1988;60:436–441. [Google Scholar]
- 15.Moini M. Simplifying CE−MS Operation. 2. Interfacing low-flow separation techniques to mass spectrometry using a porous tip. Anal Chem. 2007;79:4241–4246. doi: 10.1021/ac0704560. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Calero V, Moyano E, Puignou L, Galceran MT. Pressure-assisted capillary electrophoresis–electrospray ion trap mass spectrometry for the analysis of heparin depolymerised disaccharides. J Chromatogr A. 2001;914:277–291. doi: 10.1016/s0021-9673(00)01181-x. [DOI] [PubMed] [Google Scholar]
- 17.Zamfir A, Seidler DG, Schönherr E, Kresse H, Peter-Katalinić J. On-line sheathless capillary electrophoresis/nanoelectrospray ionization-tandem mass spectrometry for the analysis of glycosaminoglycan oligosaccharides. Electrophoresis. 2004;25:2010–2016. doi: 10.1002/elps.200405925. [DOI] [PubMed] [Google Scholar]
- 18.Sun X, Lin L, Liu X, Zhang F, Chi L, Xia Q, Linhardt RJ. Capillary electrophoresis–mass spectrometry for the analysis of heparin oligosaccharides and low molecular weight heparin. Anal Chem. 2016;88:1937–1943. doi: 10.1021/acs.analchem.5b04405. [DOI] [PubMed] [Google Scholar]
- 19.Chi L, Wolff JJ, Laremore TN, Restaino OF, Xie J, Schiraldi C, Toida T, Amster IJ, Linhardt RJ. Structural analysis of bikunin glycosaminoglycan. J Am Chem Soc. 2008;130:2617–2625. doi: 10.1021/ja0778500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chai W, Luo J, Lim CK, Lawson AM. Characterization of heparin oligosaccharide mixtures as ammonium salts using electrospray mass spectrometry. Anal Chem. 1998;70:2060–2066. doi: 10.1021/ac9712761. [DOI] [PubMed] [Google Scholar]
- 21.Korir A, Larive C. Advances in the separation, sensitive detection, and characterization of heparin and heparan sulfate. Anal Bioanal Chem. 2009;393:155–169. doi: 10.1007/s00216-008-2412-2. [DOI] [PubMed] [Google Scholar]
- 22.Saad OM, Leary JA. Delineating mechanisms of dissociation for isomeric heparin disaccharides using isotope labeling and ion trap tandem mass spectrometry. J Am Soc Mass Spectrom. 2004;15:1274–1286. doi: 10.1016/j.jasms.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Su H, Blain F, Musil RA, Zimmermann JJ, Gu K, Bennett DC. Isolation and expression in Escherichia coli of hepB and hepC, genes coding for the glycosaminoglycan-degrading enzymes heparinase II and heparinase III, respectively, from Flavobacterium heparinum. Appl Environ Microbiol. 1996;62:2723–2734. doi: 10.1128/aem.62.8.2723-2734.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun L, Zhu G, Zhao Y, Yan X, Mou S, Dovichi NJ. Ultrasensitive and Fast Bottom-up Analysis of Femtogram Amounts of Complex Proteome Digests. Angew Chem Int Edit. 2013;52:13661–13664. doi: 10.1002/anie.201308139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wojcik R, Dada OO, Sadilek M, Dovichi NJ. Simplified capillary electrophoresis nanospray sheath-flow interface for high efficiency and sensitive peptide analysis. Rapid Commun Mass Spectrom. 2010;24:2554–2560. doi: 10.1002/rcm.4672. [DOI] [PubMed] [Google Scholar]
- 26.Maxwell E, Tan Y, Tan Y, Hu H, Benson G, Aizikov K, Conley S, Staples GO, Slysz GW, Smith RD, Zaia J. GlycReSoft: a software package for automated recognition of glycans from LC/MS data. PLOS ONE. 2012;7:e45474. doi: 10.1371/journal.pone.0045474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauer KA, Hawkins DW, Peters PC, Petitou M, Herbert J-M, van Boeckel CAA, Meuleman DG. Fondaparinux, a synthetic pentasaccharide: the first in a new class of antithrombotic agents-the selective factor Xa inhibitors. Cardiovasc Drug Rev. 2002;20:37–52. doi: 10.1111/j.1527-3466.2002.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 28.Warkentin TE, Crowther MA. Reversing anticoagulants both old and new. Can J Anaesth. 2002;49:S11–S25. [PubMed] [Google Scholar]
- 29.Ly M, Leach FE, Laremore TN, Toida T, Amster IJ, Linhardt RJ. The proteoglycan bikunin has a defined sequence. Nat Chem Biol. 2011;7:827–833. doi: 10.1038/nchembio.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kailemia MJ, Ruhaak LR, Lebrilla CB, Amster IJ. Oligosaccharide analysis by mass spectrometry: a review of recent developments. Anal Chem. 2014;86:196–212. doi: 10.1021/ac403969n. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.