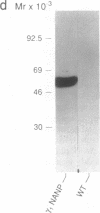
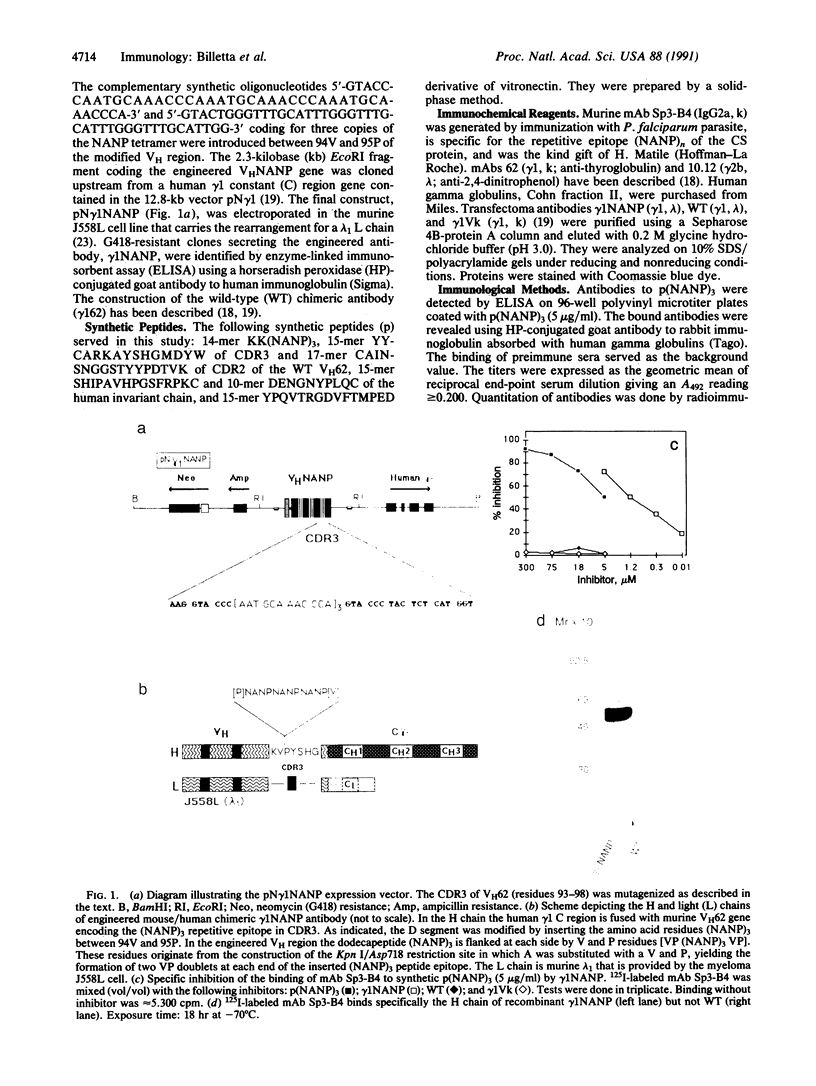
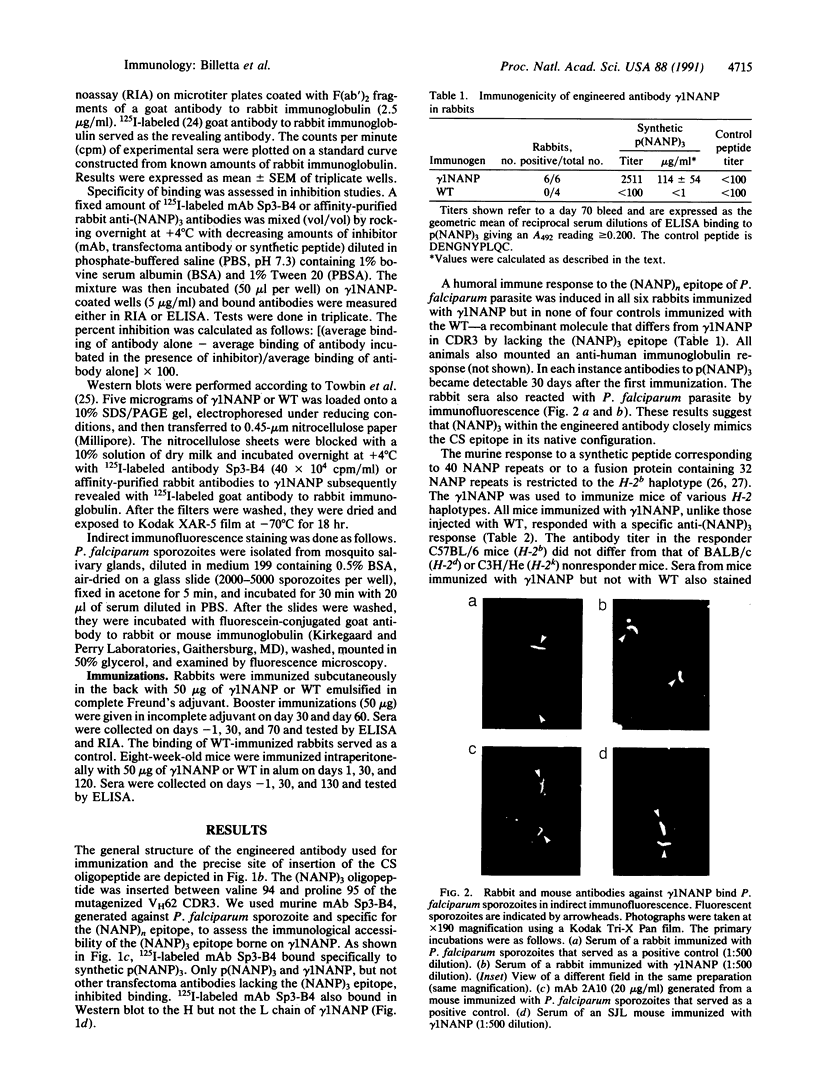
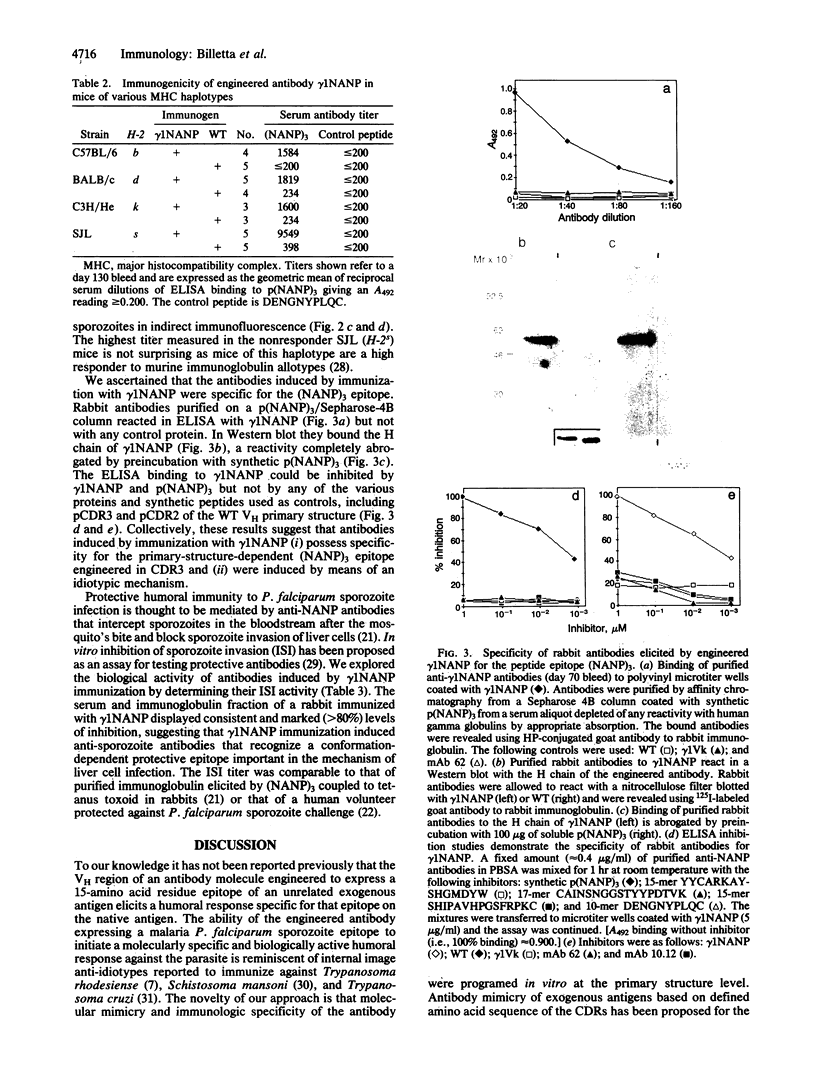
Abstract
We engineered an antibody expressing in the third complementarity-determining region of its heavy chain variable region a "foreign" epitope, the repetitive tetrapeptide Asn-Ala-Asn-Pro (NANP) of the circumsporozoite protein of Plasmodium falciparum parasite, one of the etiologic agents of malaria in humans. A monoclonal antibody to P. falciparum specific for the (NANP)n amino acid sequence bound to the engineered antibody, and a synthetic (NANP)3 peptide blocked this interaction. Immunization of rabbits and mice with the engineered antibody resulted in the elicitation of a humoral response to (NANP)3 synthetic peptide and P. falciparum parasite. In mice, in which immunity to the (NANP)n epitope is highly restricted by immune response genes, antibodies were induced in responder and nonresponder haplotypes of the major histocompatibility complex. Rabbit antibodies efficiently inhibited the in vitro invasion of cultured liver cells by P. falciparum parasite. Collectively, this study indicates that immunity to malaria in the absence of the parasite can be induced using antibody variable regions engineered to mimic the parasite's molecular structure. In general terms, the results suggest that antibody (idiotype) mimicry of an exogenous antigen is possible and may only require a discrete stretch of identity between the two molecules. The implication for the preparation of antibody-based vaccines and idiotype regulation of immunity are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bona C. A., Heber-Katz E., Paul W. E. Idiotype-anti-idiotype regulation. I. Immunization with a levan-binding myeloma protein leads to the appearance of auto-anti-(anti-idiotype) antibodies and to the activation of silent clones. J Exp Med. 1981 Apr 1;153(4):951–967. doi: 10.1084/jem.153.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona C., Moran T. Idiotype vaccines. Ann Inst Pasteur Immunol. 1985 May-Jun;136C(3):299–312. doi: 10.1016/s0769-2625(85)80002-7. [DOI] [PubMed] [Google Scholar]
- Brooks B. R., Pastor R. W., Carson F. W. Theoretically determined three-dimensional structure for the repeating tetrapeptide unit of the circumsporozoite coat protein of the malaria parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4470–4474. doi: 10.1073/pnas.84.13.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck C., Co M. S., Slaoui M., Gaulton G. N., Smith T., Fields B. N., Mullins J. I., Greene M. I. Nucleic acid sequence of an internal image-bearing monoclonal anti-idiotype and its comparison to the sequence of the external antigen. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6578–6582. doi: 10.1073/pnas.83.17.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave P. A. Idiotypic-anti-idiotypic regulation of antibody synthesis in rabbits. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5122–5125. doi: 10.1073/pnas.74.11.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Tramontano A., Levitt M., Smith-Gill S. J., Air G., Sheriff S., Padlan E. A., Davies D., Tulip W. R. Conformations of immunoglobulin hypervariable regions. Nature. 1989 Dec 21;342(6252):877–883. doi: 10.1038/342877a0. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Davie J. M., Seiden M. V., Greenspan N. S., Lutz C. T., Bartholow T. L., Clevinger B. L. Structural correlates of idiotopes. Annu Rev Immunol. 1986;4:147–165. doi: 10.1146/annurev.iy.04.040186.001051. [DOI] [PubMed] [Google Scholar]
- Del Giudice G., Cooper J. A., Merino J., Verdini A. S., Pessi A., Togna A. R., Engers H. D., Corradin G., Lambert P. H. The antibody response in mice to carrier-free synthetic polymers of Plasmodium falciparum circumsporozoite repetitive epitope is I-Ab-restricted: possible implications for malaria vaccines. J Immunol. 1986 Nov 1;137(9):2952–2955. [PubMed] [Google Scholar]
- Gibson K. D., Scheraga H. A. Predicted conformations for the immunodominant region of the circumsporozoite protein of the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5649–5653. doi: 10.1073/pnas.83.15.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Berzofsky J. A., Maloy W. L., Hayashi Y., Fujii N., Hockmeyer W. T., Miller L. H. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986 Aug 1;164(2):655–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzych J. M., Capron M., Lambert P. H., Dissous C., Torres S., Capron A. An anti-idiotype vaccine against experimental schistosomiasis. Nature. 1985 Jul 4;316(6023):74–76. doi: 10.1038/316074a0. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Clyde D. F., Losonsky G., Cortesia M., Murphy J. R., Davis J., Baqar S., Felix A. M., Heimer E. P., Gillessen D. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987 Jul 16;328(6127):257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- Hollingdale M. R., Nardin E. H., Tharavanij S., Schwartz A. L., Nussenzweig R. S. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells; an in vitro assay of protective antibodies. J Immunol. 1984 Feb;132(2):909–913. [PubMed] [Google Scholar]
- Jerne N. K., Roland J., Cazenave P. A. Recurrent idiotopes and internal images. EMBO J. 1982;1(2):243–247. doi: 10.1002/j.1460-2075.1982.tb01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Jones P. T., Dear P. H., Foote J., Neuberger M. S., Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. 1986 May 29-Jun 4Nature. 321(6069):522–525. doi: 10.1038/321522a0. [DOI] [PubMed] [Google Scholar]
- Kelsoe G., Isaak D., Cerny J. Thymic requirement for cyclical idiotypic and reciprocal anti-idiotypic immune responses to a T-independent antigen. J Exp Med. 1980 Feb 1;151(2):289–300. doi: 10.1084/jem.151.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman R., Humphrey W., Jr Association of H-2 types with genetic control of immune responsiveness to IgA allotypes in the mouse. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2510–2513. doi: 10.1073/pnas.68.10.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. Radioiodination of proteins by the use of the chloramine-T method. Methods Enzymol. 1980;70(A):210–213. doi: 10.1016/s0076-6879(80)70050-2. [DOI] [PubMed] [Google Scholar]
- Morrison S. L. Transfectomas provide novel chimeric antibodies. Science. 1985 Sep 20;229(4719):1202–1207. doi: 10.1126/science.3929380. [DOI] [PubMed] [Google Scholar]
- Ollier P., Rocca-Serra J., Sommé G., Thèze J., Fougereau M. The idiotypic network and the internal image: possible regulation of a germ-line network by paucigene encoded Ab2 (anti-idiotypic) antibodies in the GAT system. EMBO J. 1985 Dec 30;4(13B):3681–3688. doi: 10.1002/j.1460-2075.1985.tb04135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann L., Clark M., Waldmann H., Winter G. Reshaping human antibodies for therapy. Nature. 1988 Mar 24;332(6162):323–327. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Esser K. M., Sher A. Immunization of mice against African trypanosomiasis using anti-idiotypic antibodies. J Exp Med. 1982 Apr 1;155(4):1108–1119. doi: 10.1084/jem.155.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., Kirchhoff L. V., Hieny S., Sher A. Molecular mimicry of a carbohydrate epitope on a major surface glycoprotein of Trypanosoma cruzi by using anti-idiotypic antibodies. J Immunol. 1985 Dec;135(6):4155–4159. [PubMed] [Google Scholar]
- Sege K., Peterson P. A. Use of anti-idiotypic antibodies as cell-surface receptor probes. Proc Natl Acad Sci U S A. 1978 May;75(5):2443–2447. doi: 10.1073/pnas.75.5.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollazzo M., Castiglia D., Billetta R., Tramontano A., Zanetti M. Structural definition by antibody engineering of an idiotypic determinant. Protein Eng. 1990 May;3(6):531–539. doi: 10.1093/protein/3.6.531. [DOI] [PubMed] [Google Scholar]
- Sollazzo M., Hasemann C. A., Meek K. D., Glotz D., Capra J. D., Zanetti M. Molecular characterization of the VH region of murine autoantibodies from neonatal and adult BALB/c mice. Eur J Immunol. 1989 Mar;19(3):453–457. doi: 10.1002/eji.1830190307. [DOI] [PubMed] [Google Scholar]
- Stein K. E., Söderström T. Neonatal administration of idiotype or antiidiotype primes for protection against Escherichia coli K13 infection in mice. J Exp Med. 1984 Oct 1;160(4):1001–1011. doi: 10.1084/jem.160.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain J., Wikler M., Franssen J. D., Collignon C. Idiotypic regulation of the immune system by the induction of antibodies against anti-idiotypic antibodies. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5126–5130. doi: 10.1073/pnas.74.11.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cleave V. H., Naeve C. W., Metzger D. W. Do antibodies recognize amino acid side chains of protein antigens independently of the carbon backbone? J Exp Med. 1988 Jun 1;167(6):1841–1848. doi: 10.1084/jem.167.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeyen M., Milstein C., Winter G. Reshaping human antibodies: grafting an antilysozyme activity. Science. 1988 Mar 25;239(4847):1534–1536. doi: 10.1126/science.2451287. [DOI] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Hollingdale M. R., Cochrane A. H., Quakyi I., Nussenzweig R. S., Nussenzweig V. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985 Jun 21;228(4706):1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]