A large, biologically plausible neuronal network model of cortical tissue, featuring gross hemispheric organization, myelinated callosal axons, and distance-dependent conduction delays, was developed and used for studying the effects of callosal injury on cortical dynamics. The model captures several clinical observations, such as injury-dependent “slowing down” of network rhythms, altered response to stimulation, and increased population reaction time. This novel model provides a computational framework for studying the involvement of callosal injury in neurobehavioral sequelae.
Keywords: axonal injury, traumatic brain injury, EEG, brain rhythms, computational model
Abstract
Mild traumatic brain injury (mTBI) often results in neurobehavioral aberrations such as impaired attention and increased reaction time. Diffusion imaging and postmortem analysis studies suggest that mTBI primarily affects myelinated axons in white matter tracts. In particular, corpus callosum, mediating interhemispheric information exchange, has been shown to be affected in mTBI. Yet little is known about the mechanisms linking the injury of myelinated callosal axons to the neurobehavioral sequelae of mTBI. To address this issue, we devised and studied a large, biologically plausible neuronal network model of cortical tissue. Importantly, the model architecture incorporated intra- and interhemispheric organization, including myelinated callosal axons and distance-dependent axonal conduction delays. In the resting state, the intact model network exhibited several salient features, including alpha-band (8–12 Hz) collective activity with low-frequency irregular spiking of individual neurons. The network model of callosal injury captured several clinical observations, including 1) “slowing down” of the network rhythms, manifested as an increased resting-state theta-to-alpha power ratio, 2) reduced response to attention-like network stimulation, manifested as a reduced spectral power of collective activity, and 3) increased population response time in response to stimulation. Importantly, these changes were positively correlated with injury severity, supporting proposals to use neurobehavioral indices as biomarkers for determining the severity of injury. Our modeling effort helps to understand the role played by the injury of callosal myelinated axons in defining the neurobehavioral sequelae of mTBI.
NEW & NOTEWORTHY
A large, biologically plausible neuronal network model of cortical tissue, featuring gross hemispheric organization, myelinated callosal axons, and distance-dependent conduction delays, was developed and used for studying the effects of callosal injury on cortical dynamics. The model captures several clinical observations, such as injury-dependent “slowing down” of network rhythms, altered response to stimulation, and increased population reaction time. This novel model provides a computational framework for studying the involvement of callosal injury in neurobehavioral sequelae.
despite the rising numbers of mild traumatic brain injury (mTBI) of concussive type, little is known about the mechanisms linking this injury mode to physiological disruption of brain function and neurobehavioral aberrations. In the clinical setting, mTBI-induced alterations in brain function are often assessed using electroencephalographic (EEG) methods (Arciniegas 2011; Rapp et al. 2015). This allows quantification of the relative prevalence of different brain rhythms that are believed to be linked to different functional brain states (Buzsàki 2006). Thus analysis of mTBI-induced changes in EEG activity patterns may assist in understanding the connection between physiological mechanisms of brain injury and their neurobehavioral sequelae (Arciniegas 2011; Tebano et al. 1988; Thatcher 2006; Thatcher et al. 2001; West et al. 1982).
EEG recordings obtained from animal models and human clinical cases of mTBI (Rapp et al. 2015) suggest that the primary effect of concussive injury is to induce “slowing down” of cortical rhythms by shifting the spectrum of oscillations recorded during awake nonattending states toward lower frequencies. In particular, mTBI induces a reduction in the beta (12–30 Hz)- and alpha-band power (8–12 Hz) (Gosselin et al. 2009; Tebano et al. 1988) and an increase in the delta (1–4 Hz)- (Gosselin et al. 2009; Lu et al. 2011) and theta-band power (4–8 Hz) (Chen et al. 2006; Korn et al. 2005; MacFlynn et al. 1984; Moeller et al. 2011; Watson et al. 1995). These injury-induced spectral changes likely reflect alterations in the dynamics of underlying brain networks; however, the underlying mechanisms remain elusive (Haneef et al. 2013).
A large body of literature suggests that mTBI symptoms include impaired attention (Bonnelle et al. 2011; Malojcic et al. 2008; Niogi et al. 2008b; van Donkelaar et al. 2005), manifested as a longer reaction time of mTBI patients compared with healthy controls. Increased reaction time following mTBI is commonly assumed to reflect injury-induced deficits of central information processing, which depends on the architecture of neural structures and functional pathways that mediate a particular cognitive ability (Laughlin and Sejnowski 2003; Salinas and Sejnowski 2001). Yet the relation between mTBI-induced alterations in neural structures and functional pathways and mTBI sequelae remains elusive.
The corpus callosum, the largest white matter tract in the brain, is often reported in diffusion tensor imaging (DTI) (Hulkower et al. 2013) and histological studies (Blumbergs et al. 1995) as one of the structures affected in mTBI, along with the frontal lobe, internal capsule, and cingulum. In addition, finite element modeling-based analysis of concussive impacts sustained by national football league players suggests that largest impact-induced strains, often induced by violent head motion, are likely to occur in the corpus callosum, fornix, and midbrain (Viano et al. 2005). Hence, we adopted callosal axonal injury as an injury paradigm for addressing aforementioned issues in our computational modeling study.
Animal models of axonal injury suggest that traumatic stretching of axons induces strain intensity-dependent alterations in axonal function, which are manifested as changes in the compound axonal potential amplitude (likely reflecting spike amplitude changes in individual axons) and changes in the propagation time of compound axonal potentials (likely reflecting spike latency changes in individual axons) (Bain et al. 2001; Rickett et al. 2011; Shi and Blight 1996; Shi and Pryor 2002; Singh et al. 2009). Furthermore, in myelinated white matter axons significant strain-induced damage occurs at nodes of Ranvier, which are not covered by myelin, contain a high density of voltage-gated tetrodotoxin-sensitive sodium channels, and are critical for action potential transmission through myelinated axons (Maxwell 1996; Reeves et al. 2010). Several routes of injury affecting nodal excitability have been identified, including coupled left shift of nodal voltage-gated sodium channel activation and inactivation kinetics (Boucher et al. 2012; Wang et al. 2009), paranode demyelination (Sun et al. 2012), and mechanoporation of nodal membrane (Kilinc et al. 2009; Smith and Meaney 2000). On the basis of some of this evidence, we have previously used a computational model of a myelinated axon to propose that mild (below axotomy threshold) stretch injury can acutely affect the two key characteristics of axonal function: 1) spike latency and 2) spike amplitude (Volman and Ng 2013, 2014, 2016).
In the present study, we have devised a large-scale model of cortical tissue, incorporating model callosal axons with realistic axonal conduction delays and short-term synaptic depression. Activity in the intact model network was characterized by low neuronal spiking rates and an alpha-band (8–12 Hz) collective rhythm. The results regarding stretch-injury induced axonal spike amplitude and latency changes, obtained from our previously published myelinated axon model (Volman and Ng 2013, 2014, 2016), were incorporated into the network model to study the network-level implications of callosal injury. Quiescent injured model networks exhibited increased neuronal activity and injury-dependent alterations in the distribution of collective rhythms. Localized stimulation of the model network yielded injury-dose-dependent changes in the network dynamics. Population response time (a modeling proxy of reaction time) was positively correlated with the extent of model axonal injury, consistent with the clinical observations of increased reaction time following mTBI (Norris et al. 2013; Talavage et al. 2014). Our modeling results are consistent with clinical data (Bonnelle et al. 2011, 2012; Chen et al. 2006; Niogi et al. 2008a; Smits et al. 2009; Watson et al. 1995) and, if combined with the analysis of head motion, may help formulate criteria for injury severity assessment in mTBI. In addition, results of the present study provide a first step toward unveiling potential biophysical mechanisms mediating the effects of mTBI on brain function.
Some of the presented results have been reported in the form of a conference abstract (Cui et al. 2015).
MATERIALS AND METHODS
Single-compartment conductance-based models were used in the present study for describing the dynamics of excitatory and inhibitory neurons. The advantage of these models is that they allow including the relevant biophysical features (e.g., basic ion channel dynamics) while being less computationally demanding than the detailed multicompartmental models of Hodgkin-Huxley variety. The model was implemented in ANSI C and was numerically solved using the Runge-Kutta method with the time step of 0.05 ms.
Pyramidal neurons.
The dynamics of excitatory pyramidal (PY) neurons were described using the model that was first introduced by Morris and Lecar (1981) and later modified by Prescott et al. (2008) to account for the spike generation mechanism in cortical PY neurons:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
The definitions and values of the model parameters are listed in Table 1. Synaptic currents (ISyn) and external currents (IExt) are described below (see Synaptic transmission and Network stimulation).
Table 1.
Neuronal and synaptic model parameters
| Parameter Description | Parameter Symbol | Parameter Value |
|---|---|---|
| Na conductance, PY neurons | GNaPY | 10 mS/cm2 |
| K conductance, PY neurons | GKPY | 10 mS/cm2 |
| Leak conductance, PY neurons | GLPY | 1.2 ± 0.1 mS/cm2 |
| Adaptation conductance, PY neurons | GAPY | 3 mS/cm2 |
| Na conductance, FS neurons | GNaFS | 35 mS/cm2 |
| K conductance, FS neurons | GKFS | 9 mS/cm2 |
| Leak conductance, FS neurons | GLFS | 0.1 ± 0.05 mS/cm2 |
| Capacitance (PY and FS neurons) | Cm | 1 μF/cm2 |
| Peak AMPA conductance, PY to PY | g̃AMPAPY←PY | 0.12 mS/cm2 |
| Peak AMPA conductance, PY to FS | g̃AMPAFS←PY | 0.02 mS/cm2 |
| Peak GABA conductance, FS to PY, ipsilateral | g̃GABAPY←FS | 5 mS/cm2 |
| Peak GABA conductance, FS to FS, ipsilateral | g̃GABAFS←FS | 0.003 mS/cm2 |
| Peak GABA conductance, FS to PY, contralateral | g̃GABAPY←FS | 1.25 mS/cm2 |
| Peak GABA conductance, FS to FS, contralateral | g̃GABAFS←FS | 0.00075 mS/cm2 |
| Peak NMDA conductance, PY to PY | g̃NMDAPY←PY | 0.048 mS/cm2 |
| Peak NMDA conductance, PY to FS | g̃NMDAFS←PY | 0.001 mS/cm2 |
| Peak GABA conductance, callosal FS to PY | g̃GABA,CCPY←FS | 1.25 mS/cm2 |
| Peak GABA conductance, callosal FS to FS | g̃GABA,CCFS←FS | 0.00075 mS/cm2 |
| AMPA decay time, PY to PY | τAMPAPY←PY | 5.0 ms |
| NMDA decay time, PY to PY | τNMDAPY←PY | 100.0 ms |
| GABA decay time, FS to PY | τGABAPY←FS | 10.0 ms |
| AMPA decay time, PY to FS | τAMPAFS←PY | 2.0 ms |
| NMDA decay time, PY to FS | τNMDAFS←PY | 50 ms |
| GABA decay time, FS to FS | τGABAFS←FS | 10.0 ms |
| Recovery time, Glu synapse | τR,Glu | 800.0 ms |
| Recovery time, GABA synapse | τR,GABA | 400.0 ms |
| Synaptic depression, Glu synapse | uGlu | 0.5 |
| Synaptic depression, GABA synapse | uGABA | 0.2 |
| Canonic AMPA/NMDA conductance, PY neurons | GPY | 130.0 mS/cm2 |
| Canonic AMPA/NMDA conductance, FS neurons | GFS | 10.0 mS/cm2 |
FS, fast spiking; PY, pyramidal.
Inhibitory interneurons.
The dynamics of fast-spiking (FS) inhibitory interneurons were described using the Wang-Buzsàki model (Wang and Buzsàki 1996):
| (6) |
The Na+ channel current was
| (7) |
with the steady-state value of the fast-activation variable
| (8) |
| (9) |
and the inactivation variable
| (10) |
| (11) |
The K+ current was given by IK = GKFSn4(Vm + 90), with the activation variable evolving as dn/dt = 5[αn(1 − n) − βnn], with αn(Vm) = −0.01(Vm + 34)/(exp(−0.1(Vm +34)) − 1) and βn(Vm) = 0.125exp[−(Vm + 44)/80]. The leak current was given by IL = GLFS(Vm + 65). The definitions and values of the parameters are listed in Table 1.
Synaptic transmission.
Neurons in the model network were connected by AMPA, NMDA, and GABAA synapses. Slow GABAB receptor-mediated inhibition has been shown to be important in cortical ON-OFF transitions (Mann et al. 2009). However, correct modeling of GABAB receptor action requires modeling of its interaction with neuronal K+ conductance; thus, to reduce the model's complexity, we did not consider GABAB receptor activation in the present model. The dynamics of synaptic conductance were described by
| (12) |
where g̃i,c is the peak per-spike synaptic conductance of type c (AMPA, NMDA, or GABAA) at the i-th neuron, and τi,c is the characteristic time of that conductance decay; the depression factor at the synapse from the j-th neuron to the i-th neuron, Kij(t), is given below. The parameters are listed in Table 1.
Short-term synaptic depression was described with the Tsodyks-Markram type model (Tsodyks and Markram 1997), assuming a phenomenological “synaptic resource” X that is reduced after each successful release of synaptic neurotransmitter and recovers exponentially within time τR:
| (13) |
| (14) |
where the function fij(AS, LS) represents the fractional amount of synaptic resource released at a synapse from the j-th to the i-th neuron per spike of amplitude AS and latency LS, and serves to parameterize the per-spike depression in relation to the presynaptic action potential amplitude, as described below. Recovery time values are listed in Table 1. Mechanistically, short-term synaptic depression arises at relatively small-size synaptic boutons (with a relatively small readily releasable pool of neurotransmitter), which is characteristic of central synapses (Dobrunz and Stevens 1997). Thus short-term synaptic depression was implemented at all model synapses, albeit parameters of resource (transmitter) usage and recovery were different for different types of connections.
Diffusion imaging studies suggest that mTBI may be associated with strain-induced damage to axon fibers in the corpus callosum (Hulkower et al. 2013), and animal models point at a critical involvement of tetrodotoxin-sensitive voltage-gated Na+ channels in traumatic axonal injury (Iwata et al. 2004; Rosenberg et al. 1999; Wang et al. 2009). In our previous modeling studies (Volman and Ng 2013, 2014), we suggested that stretch injury of tetrodotoxin-sensitive voltage-gated Na+ channels at nodes of Ranvier in myelinated axons might result in reduced amplitudes of axonal action potentials, consistent with findings from animal models of axonal stretch injury (Bain et al. 2001; Shi and Blight 1996; Shi and Pryor 2002). We further suggested that such a reduction in the axonal action potential amplitude might compromise the release of synaptic transmitter. To model the effect of axonal injury on synaptic transmission, in the present study we have adopted and modified the model of Destexhe et al. (1994), following which the fractional amount of synaptic resource released per spike of amplitude AS that was generated at the j-th neuron at time tSpike,j and arrived at the synapse between the j-th and i-th neurons at time tSpike,j + LS, was
| (15) |
In Eq. 15, uij captures the short-term synaptic depression attributable to the limited amount of synaptic resource (neurotransmitter) available for release at any given moment of time. The product uijXij is proportional to the peak per-spike concentration of synaptic transmitter in the synaptic cleft. The values of uij were different for glutamatergic and GABAergic model synapses and are listed in Table 1.
The above model differs from the model of Destexhe et al. (1994) in two aspects: 1) the neurotransmitter release is described in this work as a discrete pulse-like event by adding the delta function dependence (thus the temporal profile of cleft neurotransmitter is not accounted for); and 2) on the basis of observations of axonal propagation failure for spikes with peak depolarization below −20 mV, it is assumed that no neurotransmitter release occurs below this spike-induced axonal membrane depolarization. Note that the spike latency LS is a function of Euclidean distance between neurons. In addition, both AS and LS change in axonal injury, as explained in Modeling injury-induced alterations in axonal properties.
The overall synaptic current to a model neuron was (sum running over all synapses to the neuron)
| (16) |
| (17) |
| (18) |
Network architecture.
The model network consisted of 6,400 neurons, 80% (5,120 neurons) of which were excitatory PY neurons and the remaining 20% (1,280 neurons) inhibitory FS interneurons. The model neurons were distributed on an 80 × 80 square lattice, with every fifth cell being an inhibitory FS neuron.
Gross hemispheric organization was modeled by dividing the model network into two equally sized symmetric subnetworks (representing the 2 hemispheres). Inside each one of those hemispheric subnetworks, model PY and FS neurons projected to, and received synaptic contacts from, other neurons found within their synaptic footprint (a 10 × 10 square region around a neuron). These connections model local intracolumnar cortical connectivity. The parameters of network connectivity are listed in Table 2.
Table 2.
Network architecture parameters
| Parameter Description | Parameter Symbol | Parameter Value |
|---|---|---|
| Ipsilateral connection probability | PIpsi | 0.0064 |
| Contralateral connection probability | PContra | 0.2 |
| Local connection probability, PY to PY | PPY←PY | 0.4 |
| Local connection probability, PY to FS | PFS←PY | 0.4 |
| Local connection probability, FS to PY | PPY←FS | 0.4 |
| Local connection probability, FS to FS | PFS←FS | 0.4 |
In addition to these local connections, longer range PY-PY and PY-FS ipsilateral connections were probabilistically established within the same hemisphere (Houzel et al. 2002). The probability PIpsi (= 0.0064) to establish such longer range ipsilateral connections was much lower than the probability PF (= 0.4) to establish an “in footprint” connection. These numbers were chosen to comply with the inverse power law principle of the distribution of the wire length in communication networks (Laughlin and Sejnowski 2003).
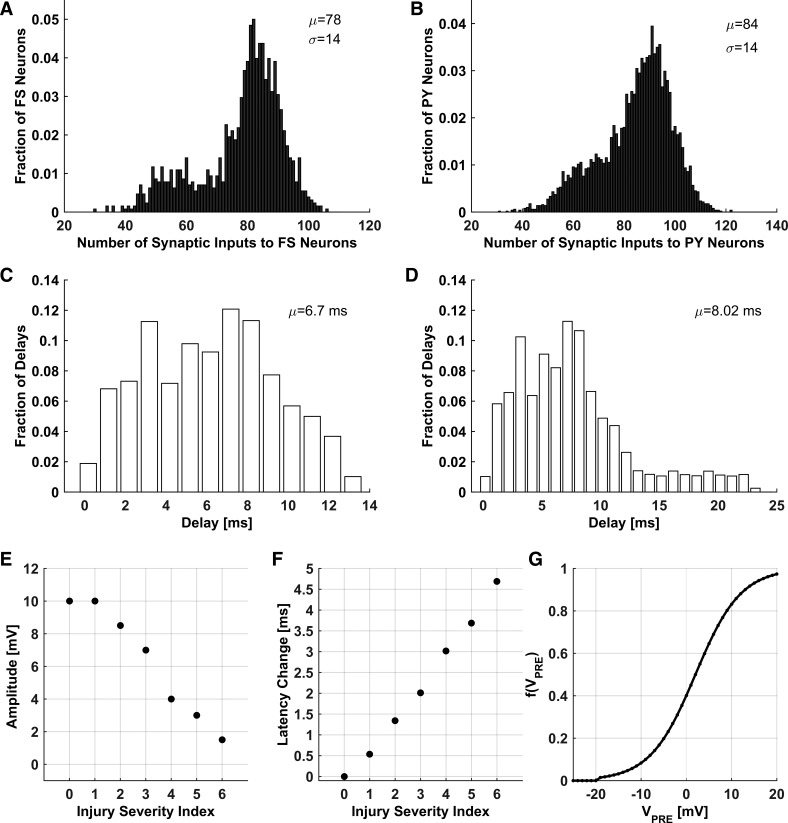
Interhemispheric communication via corpus callosum axons was modeled by establishing cross-hemispheric connections between model neurons (both PY and FS model neurons). Synaptic conductance of cross-hemispheric FS synapses was set to 25% of the ipsilateral GABA synaptic conductance, to comply with the observations of the lower presence of inhibitory contralateral projections (Tamamaki and Tomioka 2010). Each model neuron established an exact homotopic connection with its counterpart in the opposite “hemisphere.” In addition, each model neuron established a number of loose homotopic connections within the footprint of its contralateral counterpart (Aboitiz and Montiel 2003; Gazzaniga 2000; Hofer and Frahm 2006; Jarbo et al. 2012; Suárez et al. 2014) to reflect the role of callosal axons in connecting homologous cortical regions; these connections were established with probability PContra (= 0.2) for both PY and FS model neurons (Fame et al. 2011; Swadlow 1990; Tamamaki and Tomioka 2010). Thus the probabilities PIpsi and PContra respectively determined the relative impact of ipsilateral and callosal connections. Each neuron received, on average, 40 local, 20 ipsilateral, and 20 loose homotopic connections, as well as exactly 1 connection from its symmetric counterpart in the opposite hemisphere. The relative numbers of ipsilateral and callosal connections were set to reflect the anatomical data (Innocenti 1986). Distributions of synaptic inputs to model FS and PY neurons are shown in Fig. 1, A and B, respectively. The distributions are somewhat bimodal, reflecting smaller synaptic connectivity for model neurons on the lattice boundary. In a separate set of simulations (results not shown), we supplemented these boundary neurons with an additional input consistent with the network activity and verified that the results pertaining to injury-induced changes in the network dynamics do not depend on the lattice boundary conditions.
Fig. 1.
Characteristics of network structure. A and B: synaptic input distributions to FS (A) and PY (B) neurons. C: initial distribution of axonal conduction delays. D: distribution of axonal conduction delays after callosal injury (injury severity index = 6). E and F: axonal spike amplitude (E) and change in the average axonal spike latency (F) vs. injury severity index. G: synaptic event amplitude [f(VPre)] vs. presynaptic voltage (VPre).
Axonal conduction delays were implicitly incorporated into synaptic connections between all model neurons. Conduction delays between neurons were set by assuming that 1) only callosal axons in the model are myelinated ones (ipsilateral axons were assumed to be nonmyelinated); 2) the length of an axon connecting two model neurons is equal to the Euclidean distance between them, and the conduction delay is proportional to the axonal length; 3) per same axon length, conduction velocity in myelinated axons is 10-fold higher than that in nonmyelinated axons; and 4) conduction velocity in myelinated axons is ∼5.66 m/s, close to the estimated conduction velocity of 5.7 m/s for a 1-μm-diameter myelinated axon (Wang et al. 2008). The maximal possible conduction delay was set to 20 ms, and the actual distributions of axonal conduction delays for intact and injured networks are shown in Fig. 1, C and D. For the intact network, the range of axonal conduction delays was consistent with the range of interhemispheric conduction delays (9.2–20.6 ms) for an 80- to 180-mm length of callosal axons in humans (Ringo et al. 1994). Although axonal conduction velocity depends on many factors (Caminiti et al. 2013; Ringo et al. 1994; Waxman 1980) (e.g., the fiber diameter and thickness of the myelin sheath), we have simplified the model by omitting all these dependencies; the values we selected were broadly consistent with the conduction velocity values measured for thin (fiber diameter ≤1 μm) myelinated and nonmyelinated axons. The initial distribution of axonal conduction delays is shown in Fig. 1C. The mean initial conduction delay was ∼6.7 ms, consistent with the estimated range of conduction delay times (7.6–9.9 ms) for 1-μm-diameter myelinated callosal axons in the human brain (Aboitiz et al. 1992).
In biological cortical networks, the local synaptic footprint of PY neurons (a 10 × 10 region of lattice units in our model) spans the area of ∼1 mm2 (Beaulieu and Colonnier 1985, 1983; Laughlin and Sejnowski 2003; Meyer et al. 2010; Whittington et al. 1995); thus the separation between two adjacent neurons in our model network corresponds to the biological distance of ∼0.1 mm. Thus the electrical activity of each model neuron in our network could be viewed as an averaged activity of biological neurons within the ∼0.01-mm2 area. This choice of spatial scale was further rationalized by noting that in nonmyelinated axons, conduction velocity is ∼0.56 m/s (Aboitiz et al. 1992; Wang et al. 2008); thus axonal delays within the ∼0.01-mm2 area are expected to be ∼0.25 ms, comparable to the delays associated with synaptic transmission and dendritic integration. Thus the spatial scale of ∼0.1 mm appears to be the finest spatial scale below which axonal conduction delays are less likely to be important.
Network stimulation.
In addition to collateral synapses from other model neurons, each model neuron received external stimulation, collectively representing inputs from other cortical layers, other cortical areas, and thalamic afferents (Destexhe and Sejnowski 2003; Tokoro et al. 2015). This stimulation was needed for generating electrical activity in the model network. To reduce the computational complexity associated with explicit modeling of a large number of synaptic inputs that impinge on the neuron, we assumed that all of the external stimulation to a given neuron arrives through two canonic synapses. External stimulation of PY and FS model neurons was modeled as a Poisson process (100 Hz) that activated canonic excitatory conductance (AMPA and NMDA) at times tExt. This choice of the external stimulation frequency yielded a network-averaged PY firing rate of ∼0.41 Hz, consistent with typically low firing rates of PY neurons. The canonic conductance and the associated external currents for each neuron were
| (19) |
| (20) |
where the subscript c denotes AMPA and NMDA receptor types. The parameters are listed in Table 1.
Attention-like stimulation was simulated by transiently (duration 500 ms) increasing the external stimulation frequency in preselected (attending) subnetworks of model PY neurons (Borgers et al. 2005, 2008); in these simulations, the external stimulation frequency was typically increased twofold. This choice of attention-like stimulation frequency was rationalized by observations of strongest model network response when stimulated at this frequency relative to the external stimulation frequency of 100 Hz. In some simulations, stimulation was delivered to one subnetwork of model neurons that were in the same “hemisphere” (20 × 20 subnetwork, excluding FS neurons). In other simulations, a stimulation was delivered to two symmetrical subnetworks (20 × 20 each, excluding FS neurons) from different “hemispheres.” Attention-like stimulation was applied after the network reached a steady state. An increase in the external stimulation frequency during the time window of attention-like stimulation parameterized the “strength of attention stimuli.”
Modeling injury-induced alterations in axonal properties.
Experimental evidence from in vitro models suggests that axonal stretch injury likely involves damage to tetrodotoxin-sensitive voltage-gated Na+ channels at nodes of Ranvier (Yuen et al. 2009) as well as stretch-induced demyelination of paranodal/juxtaparanodal regions flanking the nodes (Sun et al. 2012). Using a computational model of a myelinated axon, we showed previously that these injury-induced alterations can affect axonal signal transmission characteristics (the axonal spike amplitude and axonal spike latency) in a way that depends on the injury severity (Volman and Ng 2013, 2014). In the present work, we incorporated these results to study the effect of callosal injury on the network activity. Relatively mild callosal injury, following which callosal axons exhibit altered functionality but do not degenerate, was parameterized by the “injury severity index,” which corresponded to amplitude-latency change pairs (ΔA, ΔT; see Table 3) that quantified the joint effect of nodal Na+ channels injury and paranode demyelination on axonal function. The values of these injury parameters were adopted from our previous studies (Volman and Ng 2013, 2014). Higher injury severity indexes corresponded to stronger injury-induced spike amplitude and latency changes (Table 3; Fig. 1, E and F) and more significant changes in the synaptic event amplitude (Fig. 1G). Axonal injury affected synaptic transmission (modeled according to Eq. 15) at synapses of both excitatory and inhibitory interhemispheric axons. In particular, following axonal injury, the spike amplitude of an interhemispheric synapse was set to AS = (1 − 0.01ΔA)AS, whereas the spike latency for that same axon was set to LS = (1 + 0.01·ΔT)LS. Note that LS is a function of Euclidean distance between neurons; therefore, callosal axonal injury affected the distribution of axonal conduction delays (Fig. 1D; compare with Fig. 1C for the distribution of conduction delays before callosal injury). The effect of severe callosal injury (following which callosal axons degenerate) was studied by assuming the axons to be severely damaged (nonconducting). This condition was modeled by removing the corresponding callosal connections from the model network. Note that in both scenarios (alterations in callosal spike conduction and removal of callosal connectivity), the functional connectivity of the model corpus callosum is affected; however, only in the second scenario (removal of callosal connectivity) is the anatomical connectivity affected, as well. Thus the outcomes of the two scenarios are expected to be qualitatively similar but quantitatively different.
Table 3.
Amplitude reduction and latency increase values for callosal injury scenarios of mild traumatic axonal stretch injury
| Injury Severity Index | ΔA, % | ΔT, % |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 0 | 8 |
| 2 | 15 | 20 |
| 3 | 30 | 30 |
| 4 | 60 | 45 |
| 5 | 70 | 55 |
| 6 | 85 | 70 |
Injury-dependent changes in spike amplitude (ΔA) and latency (ΔT) were calculated relative to the intact condition (represented by an injury severity index of 0). [Adapted from Volman and Ng (2014).]
In some simulations, callosal axonal amplitude and latency changes were decoupled and varied in an independent manner for elucidating the relative contributions of these parameters (spike amplitude and latency) to the injury-induced alterations in network dynamics.
Methods of analysis.
Spiking activities of individual model neurons were monitored by recording their membrane potential traces. To analyze the patterns of collective activity, we defined network-averaged population activity (NAPA) as an average over all membrane potential traces of all model neurons (PY and FS), and local-averaged population activity (LAPA) as an average over membrane potential traces of model neurons in a selected localized subnetwork. NAPA captures the global oscillatory trends in the model network, whereas LAPA allows assessing the effect of synaptic connectivity on the network activity (e.g., by analyzing activity in ipsilateral vs. contralateral subnetworks). Note that the LAPA measure we used in the present study should not be confused with experimentally measured local field potentials (LFPs). Anatomical and electrophysiological origins of experimentally recorded LFPs remain a subject of active research (Buzsàki et al. 2012; Einevoll et al. 2013), with a recent detailed modeling study delineating the conditions for computing the best LFP proxy in a three-dimensional network model of multicompartmental neurons with realistic morphology and realistic spatial distributions of cells and synapses (Mazzoni et al. 2015). Yet, the measure we used in the present study has been conventionally used by other modelers to estimate patterns of collective activity using EEG-like methods of spectral analysis (Bazhenov et al. 2001; Ursino and La Cara 2006).
Time-frequency spectrograms were computed on NAPA and LAPA traces (defined as described above) using wavelet transforms (complex Morlet wavelet), with a sampling rate of 20 kHz (corresponding to a numerical solution time step of 0.05 ms). For steady-state dynamics (network stimulation only by random external excitation), plots of spectral power vs. frequency were obtained by averaging the corresponding time-frequency spectrograms over time. For analysis of different rhythms, continuous time-averaged power spectra were partitioned into frequency bands as commonly used in EEG analysis (delta, 1–4 Hz; theta, 4–8 Hz; slow alpha, 8–10 Hz; fast alpha, 10–12 Hz; and beta, 12–30 Hz), and integrated spectral power for each band was analyzed.
Collective activity was also analyzed by constructing network raster plots, in which the x-axis was the time and the y-axis encoded the firing events of individual model neurons (both PY and FS). Raster plot data were used for constructing instantaneous population firing rate plots by partitioning the time into bins and calculating the network-averaged mean number of spikes fired during the corresponding time bins. Population response time to attention-like stimulation was defined as the time since the onset of stimulation at which the network exhibited maximal response (parameterized as the population-averaged firing rate). To obtain the results, simulation data were averaged over 60 independent statistical realizations. Results are presented as means ± SE.
RESULTS
Steady-state dynamics of the intact model neuronal network.
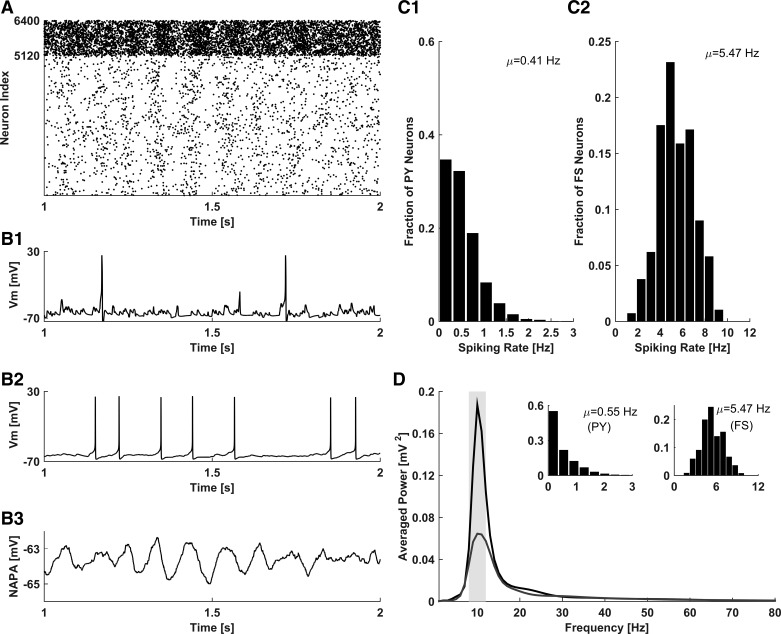
Figure 2A shows the raster plot of collective activity in the intact model network. Although the electrical activity of individual model PY neurons and individual model FS interneurons is mostly irregular, intermittent periods of weakly coherent population activity can be observed in the raster plot. The irregularity of individual neuronal firing was further confirmed by inspecting the membrane potential traces of a sample PY neuron (Fig. 2B1) and FS interneuron (Fig. 2B2). NAPA (Fig. 2B3), calculated by averaging over the membrane potential traces of all (PY and FS) model neurons in the network, disclosed the existence of population oscillations. Model PY neurons fired action potentials at an average rate of ∼0.41 Hz, whereas model FS interneurons fired action potentials at a higher average rate of ∼5.47 Hz (Fig. 2, C1 and C2). Spectral analysis of NAPA revealed a spectral power peak at the frequency of ∼10 Hz, corresponding to the alpha band (8–12 Hz; Fig. 2D). Thus, in the resting (nonattending) state, the activity of our model network was characterized by the low-frequency irregular spiking of PY and FS neurons and the existence of collective oscillations in the alpha band, consistent with the experimental EEG results for awake, nonattending activity in the intact brain (Klimesch 1999).
Fig. 2.
Characterization of intact network activity in the resting (nonattending) state. A: raster plot of spiking activity in the network (PY neurons indexes: 1–5,120; FS neurons indexes: 5,121–6,400). B: membrane potential of a representative PY neuron (B1), membrane potential of a representative FS neuron (B2); and NAPA, calculated as an average over membrane potentials of all PY and FS model neurons (B3). C: firing rate distributions of model PY (C1) and FS (C2) neurons. D: spectral power distribution of NAPA (black trace), exhibiting a peak in the alpha band (gray bar in 8- to 12-Hz range). Gray trace illustrates the spectral power distribution of NAPA for a network without callosal connections; inset indicates the firing rate distributions of model PY (left inset) and FS (right inset) neurons in this condition.
In our model, network activity was critically determined by the presence of callosal axons. Peak spectral power was dramatically reduced after the callosal axons were removed from the model network (Fig. 2D, gray curve with the lower peak for the model without the callosal axons). In the absence of the model callosal axons, the firing rate of the model PY neurons increased compared with the scenario with intact callosal connectivity, whereas the firing rate of the model FS interneurons was not significantly different (Fig. 2D, inset), consistent with the nondominance of model callosal FS connections, as explained in Network architecture. This modeling result is consistent with the suggestion that in the resting (awake, nonattending) condition, callosal connections may mediate inhibitory action (Bloom and Hynd 2005; Innocenti 1994; van der Knaap and van der Ham 2011) of cortical regions on their contralateral peers.
Response to attention-like stimulation in a model network with intact callosal connectivity.
Impaired attention is often observed in mTBI patients (Bonnelle et al. 2011; Malojcic et al. 2008; Niogi et al. 2008b; van Donkelaar et al. 2005), and white matter injury has been suggested as a possible anatomical correlate (Bush 2010; Ham and Sharp 2012). In particular, corpus callosum, connecting the cortical networks of the two hemispheres (Hofer and Frahm 2006; Innocenti 1986), is one of the most recognized mTBI loci (Hulkower et al. 2013). Given the importance of callosal axons in cortical communication, we hypothesized that the functionality of callosal axons could be reflected in the responses of our model network to attention-like stimulation. Thus we first studied the responses induced in the intact model network by attention-like stimulation, which was modeled as a temporary increase in the external stimulation rate (100 Hz above the baseline stimulation rate for 500 ms) of a preselected set of 20 × 20 PY neurons. The stimulation frequency during the attention-like stimulation window parameterized the “strength of attention stimuli.”
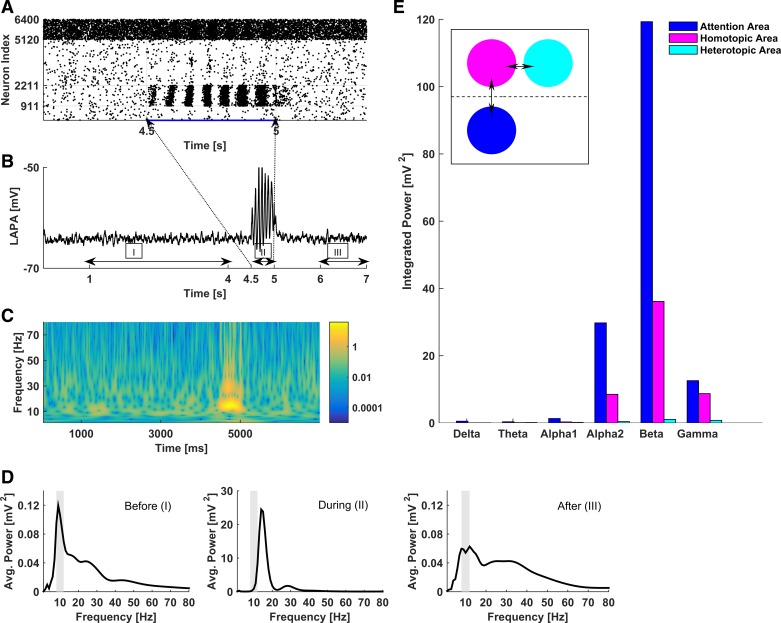
Figure 3A shows the raster plot of the intact network activity in response to unilateral attention-like stimulation (onset and offset times of attention-like stimuli are marked by arrows and indexes of stimulated PY model neurons are 911-2,211). The stimulated area responded to the attention-like stimulation with collective oscillations and increased neuronal spiking activity during the oscillation bouts. LAPA (Fig. 3B), calculated as an average over the membrane potentials of all model neurons in the stimulated area, also discloses strongly coherent collective oscillations, similar to the characteristics of synchronized activity that is often recorded in attending animals (Gray et al. 1989; Lima et al. 2010; von Stein et al. 1999; Wróbel 2000). The time-frequency plot (Fig. 3C) shows that, during the attention-like stimulation, the dominant LAPA frequency shifts toward the beta (12–30 Hz) band; the shift in the dominant frequency was nearly the same for stimulation frequencies in the range 100–400 Hz (simulation data are not shown). This spectral shift during the attention-like stimulation is further quantified in Fig. 3D, where we show the distribution of time-averaged spectral power before (left; time window 1–4 s), during (middle; time window 4.5–5 s), and after (right; time window 6–7 s) the attention-like stimulation. These modeling results are in line with a vast body of experimental observations regarding the shift toward higher frequency bands during attention stimulation (Gross et al. 2004; Womelsdorf and Fries 2007; Wróbel 2000). Our results are also consistent with animal models of attention that reveal increased presence of beta rhythms during top-down attention (Buschman and Miller 2007).
Fig. 3.
Response of the network to unilateral attention-like stimulation, modeled as a localized transient stimulation of model PY neurons (400 PY neurons subject to stochastic Poisson stimulation at 200 Hz for 500 ms; other simulation conditions are the same as in Fig. 1). A: raster plot of network activity in response to the stimulation at time 4.5 s, indicating locally coherent collective oscillations in the stimulated area. Blue trace under the raster plot represents the injected current. B: LAPA dynamics in the stimulated area before (I), during (II), and after (III) the stimulation. C: time-frequency plot of the LAPA in B. Logarithmic (base 10) scaling has been applied to accentuate the different responses during different time windows. D: spectral power distributions of LAPA in the stimulated area before, during, and after the stimulation, corresponding to I, II, and III in B, respectively. The dominance of beta rhythm during the stimulation should be compared with the dominance of alpha rhythm both before and after the stimulation. E: localized and specific activation of the contralateral region in response to unilateral attention-like stimulation. Bars show integrated spectral power across different bands during unilateral stimulation at 200 Hz, measured in the stimulated area (blue), the homotopic contralateral area (magenta), and the heterotopic contralateral area (cyan). Inset schematically shows the arrangement of these areas, with the double arrows indicating the connections among them.
To quantify the function of the model corpus callosum in relaying information between the two subnetworks that corresponded to the two hemispheres, we have computed the integrated spectral power in different frequency bands of different network regions during the attention-like stimulation. Figure 3E shows the integrated spectral power during the attention-like stimulation for the attending region and the contralateral homotopic and heterotopic regions that are associated with it (schematic in inset of Fig. 3E explains the different regions). Figure 3E shows that during the attention-like stimulation, both the stimulated region and its contralateral homotopic region respond with beta-band activity; however, the heterotopic region has a more leveled spectral distribution. This suggests that 1) the model callosal connections are significant in relaying information between the two “hemispheres”; 2) localized attention-like stimulation evokes spatially constrained responses that do not encompass the entire network; and 3) during strong attention-like stimulation, the model callosal connectivity mediates excitatory effects of cortical regions on their contralateral peers, consistent with the proposal by Bloom and Hynd (2005).
Slowing down of network rhythms following injury of callosal axons in the nonattending model network.
Several clinical studies have indicated that concussive mTBI could have a measurable effect on the EEG patterns of collective network activity (Chen et al. 2006; Korn et al. 2005; Moeller et al. 2011; Watson et al. 1995). In particular, it was found that slower frequency components of the EEG spectrum become more pronounced following mTBI (Korn et al. 2005; Moeller et al. 2011). The same conclusions were reached in animal models of concussion (West et al. 1982). This, along with the observations of white matter axonal damage following mTBI, prompted us to explore the role of callosal axons in brain rhythm alterations post-mTBI.
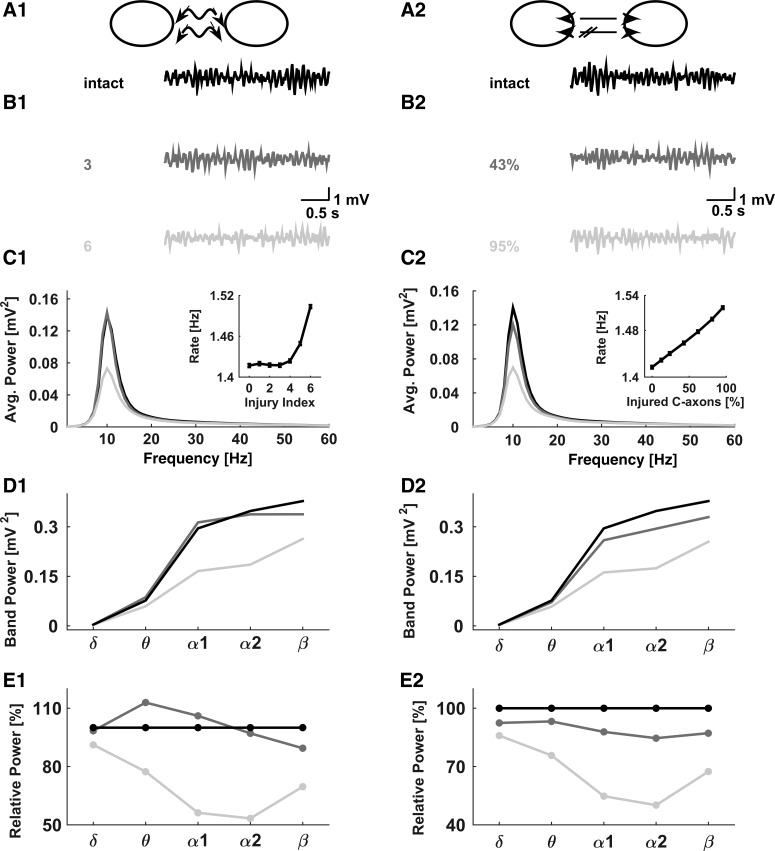
To explore the potential effect of callosal injury on the collective activity in our model network, we considered two scenarios: 1) traumatic axonal stretch injury, in which the model callosal axons were subjected to spike amplitude and spike latency changes that were adopted from our previous work on mild traumatic axonal stretch injury (Volman and Ng 2013, 2014) and were parameterized by the “injury severity index” as explained in materials and methods; and 2) a scenario in which only a fraction of callosal axons were severely damaged, to the extent that these model axons were not able to relay any action potentials. The first scenario can be thought of as representing a mild injury (following which the axonal conduction, albeit compromised, is retained), whereas the second is representative of a more severe injury (following which the axonal conduction is completely lost). Alternatively, the two scenarios can be loosely associated with acute (compromised conduction) and chronic (loss of conduction due to axonal degeneration) phases of mild injury. Figure 4, A1 and A2, shows the schematic presentations of these two conditions.
Fig. 4.
Slowing down in networks with injured callosal axons. Left panels represent the scenario of strained callosal axons; right panels represent the scenario of partially eliminated callosal connections. A: schematics illustrating the straining (A1) and partial connectivity (A2) scenarios. B1: from top to bottom, typical NAPA traces represent intact model network (injury severity index = 0), mildly injured network (injury severity index = 3), and severely injured network (injury severity index = 4). B2: from top to bottom, typical NAPA traces represent intact model network (PContra = 0.2), mildly injured network (PContra = 0.11, 43% of callosal axons not conducting), and severely injured network (PContra = 0, 95% of callosal axons not conducting). C: spectral distributions for intact and injured networks for injury scenarios as described in B. Grayscale code is the same as in B. Insets show network-averaged firing rates for different injury scenarios. Data points are means ± SE (n = 60). D: spectral power in different oscillation bands for different injury scenarios. E: spectral power in different oscillation bands, normalized with respect to the intact network, for different injury scenarios. In C–E, the data are averaged over 60 statistically independent realizations.
Progressively increasing levels of injury that were parameterized either as an increasing injury severity index (Table 3) or as decreasing callosal connectivity caused small yet noticeable changes in the patterns of the network collective activity. Visual inspection of NAPA traces (Fig. 4, B1 and B2) suggests that both the amplitude and the frequency of collective rhythms might be affected by callosal injury. This is confirmed in Fig. 4C, where we show spectral power distributions for different injury scenarios and different injury severity levels. The insets in Fig. 4, C1 and C2, show that the network-averaged firing rate of model neurons increases as a function of the injury severity in both injury scenarios. This modeling result is consistent with the suggestion of inhibitory role of corpus callosum during low-frequency cortical activity (Bloom and Hynd 2005).
Figure 4, D1 and D2, shows the spectral power in different frequency bands (defined in materials and methods) calculated for different injury scenarios. Stronger injury (parameterized as an increasing injury severity index in Fig. 4D1 and as a higher fraction of injured model callosal axons in Fig. 4D2) yielded a general reduction in band power. However, the effect of injury might not be apparent because of the bands spanning unequal frequency intervals (e.g., beta band spans 18 Hz, from 12 to 30 Hz, whereas delta band spans only 3 Hz, from 1 to 4 Hz). To avoid conflating the effects of injury with the effects of band width, we normalized the band power values by their corresponding band power values obtained for the intact network. Thus, in this normalized view, all normalized band power values in the intact model network are at 100%. Figure 4, E1 and E2, shows the band-normalized spectral power plots for different injury scenarios in Fig. 4, D1 and D2. These plots reveal that in the model, callosal injury induced a significant reduction in the alpha-band power. These results are qualitatively consistent with the observations of reduced spectral power and a shift toward lower frequencies following mTBI (Chen et al. 2006; Korn et al. 2005; Moeller et al. 2011; West et al. 1982).
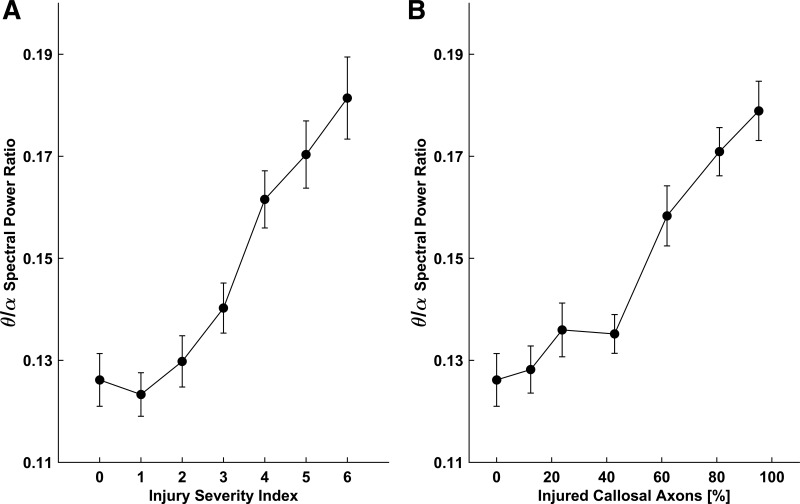
Increased theta-to-alpha-band power ratio has been reported in quantitative EEG studies of concussion in humans (Chen et al. 2006; Dow et al. 1944; Watson et al. 1995). Figure 5 shows that the theta-to-alpha ratio in our model network increases with increasing injury severity, for both injury scenarios. These simulation results are qualitatively consistent with the existing clinical data (Chen et al. 2006; Dow et al. 1944; Watson et al. 1995).
Fig. 5.
Theta-to-alpha (θ/α) spectral power ratio vs. injury severity index for straining (A) and partial connectivity (B) scenarios of callosal injury (schematic in Fig. 4A). Data points are means ± SE (n = 60).
In the present study we focused on the outcomes of mild TBI. Loss of callosal connectivity can occur acutely as a result of relatively severe injury (following which the axons are torn) or chronically, as a result of axonal degradation and disconnection. In the latter case, homeostatic regulation (Houweling et al. 2005; Turrigiano 1999) is likely to counter injury-induced perturbations. Therefore, to focus on mTBI scenarios and to avoid conflating the effects of axonal injury proper with the effects of homeostatic regulation, in what follows we confined ourselves to the studies of acute phase mTBI, during which axonal conduction is likely to be only partially compromised.
Callosal injury affects the population response to attention-like stimuli.
Oscillations in the alpha (8–12 Hz)- and beta (13–30 Hz)-frequency bands have been linked to performance on cognitive tasks and memory (Klimesch 1999). Neurobehavioral studies indicate that changes in top-down attention (Bonnelle et al. 2011; Halterman et al. 2006; Niogi et al. 2008a; Scheibel et al. 2007), reaction time (Bonnelle et al. 2011; Halterman et al. 2006; Niogi et al. 2008a; Scheibel et al. 2007), and working memory (Malojcic et al. 2008) are often observed following mTBI. Given the role of callosal axons in cortical information processing (Aboitiz and Montiel 2003; Gazzaniga 2000; Houzel et al. 2002; van der Knaap and van der Ham 2011), it is plausible to assume that mTBI sequelae might be at least partially triggered by callosal injury. We used our model network to test this hypothesis.
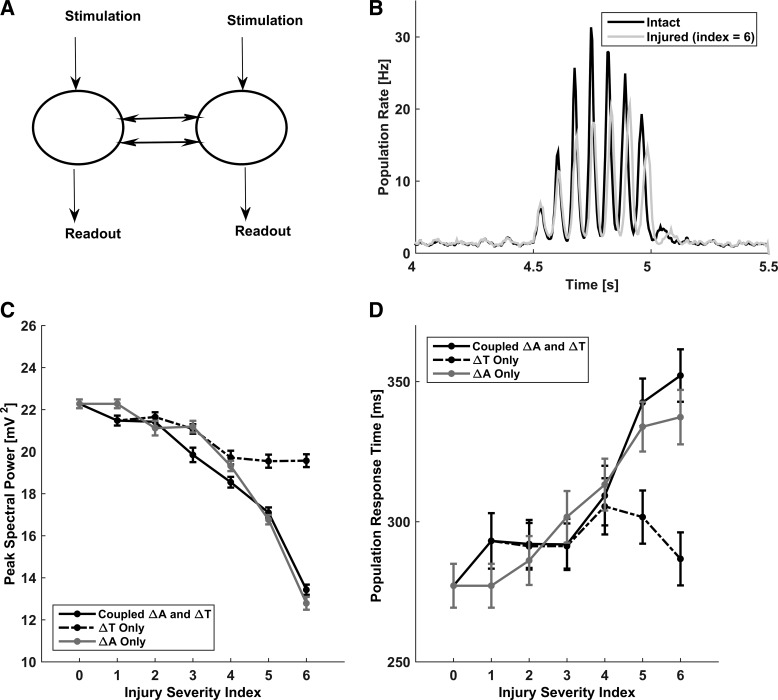
Figure 6A shows the schematic of the simulation setup that we used to test the response of our intact and injured model networks to attention-like stimulation. In these simulations, attention-like stimuli were modeled as described in materials and methods. In response to localized attention-like stimuli, the model networks exhibited a stereotypical response that was characterized by rhythmic bouts of highly correlated collective activity (Fig. 6B). Notably, in the model networks with injured callosal axons, the maximal response to attention-like stimuli developed at a later time compared with the intact model network. We quantified the effect of callosal injury by computing the peak spectral power of LAPA (sampled from the stimulated areas of model networks). Figure 6C shows that peak spectral power of response to attention-like stimulation decreases with increasing the injury severity index. Figure 6D plots the population response time against the injury severity index, clearly showing that the population response time increases with increasing the injury severity. These results are consistent with the clinical data regarding increased reaction time exhibited by mTBI subjects (Bonnelle et al. 2011; Halterman et al. 2006; Niogi et al. 2008a; Scheibel et al. 2007).
Fig. 6.
Effect of callosal injury on the network response to attention-like stimulation. A: schematic of the simulation setup. Two local subnetworks, connected by callosal axons, were stimulated at the same time by stochastic Poisson stimuli, and their response to the stimulation was measured for different levels of callosal injury. B: activity traces of population response to stimulation, averaged over all model neurons in the stimulated subnetworks. Black trace represents the intact network (injury scenario 0). Gray trace represents the injured network (injury scenario 6; ΔA = 85%, ΔT = 70%). C: peak spectral power during attention-like stimulation vs. injury severity index: coupled amplitude and latency changes (solid black), amplitude not varied (dashed black), and latency not varied (solid gray). D: population response time to attention-like stimulation vs. injury severity index for the different scenarios in C. In C and D, data points are means ± SE (n = 60).
Increased population response time with increasing injury severity might reflect injury-induced increased axonal conduction delays. Alternatively, it might reflect the weakening of callosal signal (as would be captured by injury-induced reduction in callosal signal amplitude). To dissect the dependence of the population response time on callosal injury parameters, we performed simulations in which the injury-induced changes in callosal spike amplitude and latency were decoupled. In one set of simulations (dashed black curves in Fig. 6, C and D), we only varied the spike latency parameter without varying the spike amplitude parameter. In another set of simulations (solid gray curves in Fig. 6, C and D), we only varied the spike amplitude parameter without varying the spike latency parameter. Results, shown in Fig. 6, C and D, along with the original results for the injured model networks, suggest that the population response time is primarily affected by the injury-induced axonal spike amplitude reduction. Thus delayed population response time in our model reflects injury-induced weakening of callosal signal.
DISCUSSION
The relative prevalence of different brain rhythms is believed to be linked to different functional brain states (Buzsàki 2006), with faster rhythms such as beta (12–30 Hz) and gamma (30–80 Hz) corresponding to more attentive states. Computational modeling and neuropharmacology studies have shown that network oscillations can be affected by a variety of factors, including intrinsic neuronal properties and synaptic parameters (Wang 2010). In the present study, we used computational modeling to assess the impact of mTBI-induced alterations in callosal axonal conduction parameters on collective activity in cortical networks. Our model captures several well-documented mTBI sequelae (such as an increased theta-to-alpha spectral power ratio and increased population response time), thus suggesting a mechanistic link between observed neurophysiological impairments (axonal injury) and altered neurobehavioral indices following mTBI of concussive type.
In the present model, we implicitly assumed that only callosal white matter axons are affected in mTBI. This assumption is clearly a simplification that was motivated by a large body of DTI data implying that mTBI predominantly affects the functional and structural integrity of white matter tracts (reviewed in Hulkower et al. 2013), as well as by an apparent focus of clinical literature on the corpus callosum, perhaps because it is the largest white matter tract in the brain and an important site of TBI pathology. This simplification allowed us to avoid conflating the effects of axonal injury with those of other injury modes. In reality, other structures, such as the frontal lobes, internal capsules, and cingulum, are also likely to be associated with injury. It is also conceivable that neuronal structures in gray matter are affected, although the possible modes of acute injury remain elusive. In vitro studies suggest that direct mechanical stimulation can elicit pathological responses in neurons and astrocytes (Geddes et al. 2003; Miller et al. 2009; Morrison et al. 2011; Tavalin et al. 1995; Toth et al. 1997); whether or not such stimulation intensity is feasible in the context of mTBI events remains to be determined using detailed biomechanical models of brain tissue dynamics in response to external stressors. In addition, neurobehavioral measures such as attention and reaction time are complex phenomena that involve several brain regions and neurotransmitters/neuromodulators. Proper operational definitions of these phenomena and their cellular correlates, allowing correct quantification, are still a subject of intensive research. One popular measure of quantifying phenomena such as attention and reaction time, which we adopted in the present study, is in terms of population activity (e.g., Borgers et al. 2005, 2008); however, this is by no means the only possible measure, and other methods may or may not yield different quantitative conclusions. The impact of attention/reaction time methodology and the contributions of the different structures affected in mTBI to neurobehavioral sequelae remain to be determined. Our present model provides a first step in this direction by considering the effects of mTBI on myelinated callosal axons, using simple measures of population activity.
The two different injury scenarios (injury-induced alteration of axonal spike amplitude and latency vs. injury-induced loss of callosal connectivity) may be thought of as reflecting mild and moderate-to-severe TBI, respectively. From the temporal point of view, injury-induced alteration of axonal spike amplitude and latency may correspond to the acute phase of mTBI, whereas the scenario in which callosal connectivity is partially lost may correspond to the chronic stage of mTBI during which some of callosal connections are lost due to slowly developing axonal degradation post injury. DTI data (Kraus et al. 2007) suggest functional axonal injury as an mTBI outcome, and in vitro studies (Sun et al. 2012; Wolf et al. 2001) indicate that mTBI-related axonal injury likely includes damage to voltage-gated Na+ channels (localized at axonal nodes of Ranvier in myelinated axons) as well as some primary (injury-induced) demyelination of node-flanking paranodes. These injury outcomes (channel pathophysiology and paranode demyelination) were implicitly incorporated in our model network on the basis of our earlier published computational model of axonal injury (Volman and Ng 2013, 2014), which targeted acute mTBI sequelae. Thus our conclusions regarding reaction time changes and increased theta-to-alpha spectral power ratio pertain more to the acute phase postinjury, although the effects of callosal injury were qualitatively the same for both injury modes (data not shown for population response time changes in model networks with reduced callosal connectivity). On the other hand, partial loss of callosal connectivity may reflect slowly developing degradation of injured axons, e.g., as a result of relatively slow pathological Ca2+ accumulation and protease activation (Saatman et al. 2010). To properly account for the effects of these slow processes on network dynamics, it is necessary to incorporate posttraumatic connectivity changes (Perez et al. 2014) and/or plasticity (Tarapore et al. 2013) that might also affect the network dynamics by changing the network effective functional connectivity. The documented posttraumatic network connectivity changes likely represent the action of homeostatic regulatory processes that aim to compensate for injury-induced changes in afferent/collateral input (Avramescu and Timofeev 2008; Dyhrfjeld-Johnsen et al. 2007; Santhakumar et al. 2004). The impact of homeostatic plasticity on chronic phase mTBI sequelae will be assessed in future computational models.
The role of conduction delays in spatiotemporal pattern formation in neuronal networks has been previously extensively explored in network simulations (Crook et al. 1997; Lubenov and Siapas 2008; Roxin et al. 2005; Wang et al. 2010). Crook et al. (1997) demonstrated, in a one-dimensional (1-D) network model, that axonal delays induce transitions from synchronous oscillations to waves, due to the introduction of phase shifts between neural oscillators. In 1-D networks of spiking neurons with ring topology, conduction delays induced a wealth of dynamical states with different spatiotemporal properties, such as traveling waves, standing waves, and aperiodic patterns (Roxin et al. 2005). Another simulation study demonstrated an important role of conduction delays in synchronizing/desynchronizing neuronal networks through Hebbian spike timing-dependent plasticity (Lubenov and Siapas 2008). In a small-world network of synaptically coupled Morris-Lecar neurons, conduction delays induced clustering anti-phase synchronization (Wang et al. 2010). The results of our study are consistent with the notion that conduction delays importantly determine network activity and show how axonal injury can affect network dynamics through alteration of axonal conduction delays.
Reaction time has been used as one of the few neurobehavioral indexes for quantifying mTBI sequelae (Bonnelle et al. 2011; Halterman et al. 2006; Malojcic et al. 2008; Scheibel et al. 2007). In our model, reaction time was approximated by the population response time, which was defined as the time since the onset of stimulation at which the network exhibited maximal response. Neural information flow between different model network regions depended on both ipsilateral and interhemispheric (callosal) connections and was affected in an injury-dose-dependent way, thus affecting the population response time (Fig. 6D). Our present results are in excellent qualitative agreement with the previously published clinical results of increased reaction time in mTBI patients performing different cognitive and behavioral tasks (Bonnelle et al. 2011, 2012; Halterman et al. 2006; Kinnunen et al. 2011; Smits et al. 2009), although we should note that in these clinical studies reaction time was assessed using behavioral measures (rather than being based on cortical activity). The dependence of the population response time on callosal injury severity in our model is consistent with clinical observations of longer reaction time for mTBI patients with injured corpus callosum (Dennis et al. 2015) and supports the proposal to view reaction time as an objective biomarker of mTBI (Norris et al. 2013; Talavage et al. 2014). Note that in the present study, reaction time was operationally defined as the time of maximal population response; this allowed us to avoid confounding the study conclusions with the notion of “reaction threshold.” However, from the model results presented in Fig. 6B, it is clear that a different reaction threshold would yield a different dependence of reaction time on the injury severity. In reality, reaction thresholds are likely to be individual specific; if so, this may help to explain intersubject variability in susceptibility to the same injury.
Network rhythms in the beta (12–30 Hz)- and gamma (30–80 Hz)-frequency bands have been suggested to be related to phenomena associated with processing of sensory information, such as perception, attention, and memory (Wang 2010). Furthermore, gamma rhythms were suggested to be related to interactions in local cortical networks in which the interacting neurons are in a relative spatial proximity and their associated axonal conduction delays are relatively short; on the other hand, beta rhythms are more likely to mediate longer range interareal interactions between different cortical areas, with longer associated axonal conduction delays (Donner and Siegel 2011; Kopell et al. 2000). Neocortical gamma rhythms are prominent in superficial cortical layers (layers 2/3), whereas beta rhythms primarily emerge in deeper cortical layers (layers 5/6) (Buffalo et al. 2004; Wang 2010). In addition, it has been suggested that top-down attention is associated with beta-band rhythms, whereas bottom-up attention is likely to be associated with gamma-band rhythms (Buschman and Miller 2007). In this study, we modeled interhemispheric communication via myelinated callosal axons, and Fig. 3C shows that the dominant rhythm generated by the model network during attention-like stimulation was in the beta-frequency band. Although, driven by the desire to keep the discussion general, we have not associated our model network with specific cortical layers, given the above it is reasonable to suggest that our model is better suited for describing the dynamics in large neural assemblies in deeper, rather than superficial, cortical layers.
The present model network captured several mTBI-related clinical observations, such as injury-induced slowing down of network rhythms and increased reaction time. Still, several modeling-related issues remain to be addressed. First, the characteristics of callosal axonal signal conduction in our model network did not incorporate possible heterogeneity related to nonuniformity of axonal properties across corpus callosum. Anatomical data (Innocenti et al. 2014) indicate that, on average, callosal axons mediating interhemispheric connections between motor, somatosensory, and visual areas are significantly thicker than those in prefrontal, premotor, and parietal areas. Accordingly, because axonal conduction speed depends on the axon caliber (Caminiti et al. 2013), callosal conduction delays are expected to be different for different cortical areas. Second, for the sake of clarity, we assumed all axons mediating intrahemispheric connections to be nonmyelinated. In reality, myelinated axons mediate long-range intrahemispheric connections (e.g., arcuate fasciculus and cingulum connections) (Ringo et al. 1994; Schmahmann et al. 2008); how such relatively fast intrahemispheric communication routes affect brain rhythms and postinjury dynamics remains to be investigated. In addition, motivated by DTI findings (Hulkower et al. 2013), in the present study we considered only the possible effects of callosal injury. Injury of other brain structures (e.g., the frontal lobes, internal capsules, and the cingulum) and its possible relation to mTBI sequelae remain to be explored in future studies that will need to account for potentially different injury sensitivity of these structures and different injury modes.
In conclusion, using the model, we explored the relation between axonal injury and clinical indexes of concussion. Our hope is that this modeling effort will help to better understand the role played by the injury of callosal myelinated axons in defining the neurobehavioral sequelae of mTBI.
GRANTS
This study was sponsored by the U.S. Army Medical Research and Materiel Command under contracts W81XWH-11-D-0011 and W81XWH-16-C-0042.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
DISCLAIMERS
This document is cleared for all audiences for OPSEC purposes. Cleared 04/29/2015. The opinions or assertions contained herein are private views of the authors, and are not to be construed as official or as reflecting views of the Department of the Army or the Department of Defense. PAO reviewed.
AUTHOR CONTRIBUTIONS
J.C., L.J.N., and V.V. conceived and designed research; J.C. and V.V. performed experiments; J.C. and V.V. analyzed data; J.C. and V.V. interpreted results of experiments; J.C., L.J.N., and V.V. drafted manuscript; J.C., L.J.N., and V.V. edited and revised manuscript; J.C., L.J.N., and V.V. approved final version of manuscript; V.V. prepared figures.
REFERENCES
- Aboitiz F, Montiel J. One hundred million years of interhemispheric communication: the history of the corpus callosum. Braz J Med Biol Res 36: 409–420, 2003. [DOI] [PubMed] [Google Scholar]
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res 598: 143–153, 1992. [DOI] [PubMed] [Google Scholar]
- Arciniegas DB. Clinical electrophysiologic assessments and mild traumatic brain injury: state-of-the-science and implications for clinical practice. Int J Psychophysiol 82: 41–52, 2011. [DOI] [PubMed] [Google Scholar]
- Avramescu S, Timofeev I. Synaptic strength modulation after cortical trauma: a role in epileptogenesis. J Neurosci 28: 6760–6772, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain AC, Raghupathi R, Meaney DF. Dynamic stretch correlates to both morphological abnormalities and electrophysiological impairment in a model of traumatic axonal injury. J Neurotrauma 18: 499–511, 2001. [DOI] [PubMed] [Google Scholar]
- Bazhenov M, Stopfer M, Rabinovich M, Huerta R, Abarbanel HD, Sejnowski TJ, Laurent G. Model of transient oscillatory synchronization in the locust antennal lobe. Neuron 30: 553–567, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C, Colonnier M. A laminar analysis of the number of round-asymmetrical and flat-symmetrical synapses on spines, dendritic trunks, and cell bodies in area 17 of the cat. J Comp Neurol 231: 180–189, 1985. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Colonnier M. The number of neurons in the different laminae of the binocular and monocular regions of area 17 in the cat, Canada. J Comp Neurol 217: 337–344, 1983. [DOI] [PubMed] [Google Scholar]
- Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol Rev 15: 59–71, 2005. [DOI] [PubMed] [Google Scholar]
- Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ. Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J Neurotrauma 12: 565–572, 1995. [DOI] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci USA 109: 4690–4695, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, De Boissezon X, Greenwood RJ, Sharp DJ. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci 31: 13442–13451, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgers C, Epstein S, Kopell NJ. Background gamma rhythmicity and attention in cortical local circuits: a computational study. Proc Natl Acad Sci USA 102: 7002–7007, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgers C, Epstein S, Kopell NJ. Gamma oscillations mediate stimulus competition and attentional selection in a cortical network model. Proc Natl Acad Sci USA 105: 18023–18028, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher PA, Joos B, Morris CE. Coupled left-shift of Nav channels: modeling the Na+-loading and dysfunctional excitability of damaged axons. J Comput Neurosci 33: 301–319, 2012. [DOI] [PubMed] [Google Scholar]
- Buffalo E, Fries P, Desimone R. Layer-specific attentional modulation in early visual areas. Program No. 717.6. 2004 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2004. Online. [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315: 1860–1862, 2007. [DOI] [PubMed] [Google Scholar]
- Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology 35: 278–300, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsàki G. Rhythms of the Brain. New York: Oxford University Press, 2006. [Google Scholar]
- Buzsàki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents–EEG, ECoG, LFP and spikes. Nat Rev Neurosci 13: 407–420, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminiti R, Carducci F, Piervincenzi C, Battaglia-Mayer A, Confalone G, Visco-Comandini F, Pantano P, Innocenti GM. Diameter, length, speed, and conduction delay of callosal axons in macaque monkeys and humans: comparing data from histology and magnetic resonance imaging diffusion tractography. J Neurosci 33: 14501–14511, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XP, Tao LY, Chen AC. Electroencephalogram and evoked potential parameters examined in Chinese mild head injury patients for forensic medicine. Neurosci Bull 22: 165–170, 2006. [PubMed] [Google Scholar]
- Crook SM, Ermentrout GB, Vanier MC, Bower JM. The role of axonal delay in the synchronization of networks of coupled cortical oscillators. J Comput Neurosci 4: 161–172, 1997. [DOI] [PubMed] [Google Scholar]
- Cui J, Ng LJ, Volman V. A computational model of cortical network for quantifying neurobehavioral sequelae of concussion linked to traumatic axonal injury. J Neurotrauma 32: A1–A152, 2015. [Google Scholar]
- Dennis EL, Ellis MU, Marion SD, Jin Y, Moran L, Olsen A, Kernan C, Babikian T, Mink R, Babbitt C, Johnson J, Giza CC, Thompson PM, Asarnow RF. Callosal Function in Pediatric Traumatic Brain Injury Linked to Disrupted White Matter Integrity. J Neurosci 35: 10202–10211, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Mainen ZF, Sejnowski TJ. Synthesis of models for excitable membranes, synaptic transmission and neuromodulation using a common kinetic formalism. J Comput Neurosci 1: 195–230, 1994. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Sejnowski TJ. Interactions between membrane conductances underlying thalamocortical slow-wave oscillations. Physiol Rev 83: 1401–1453, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron 18: 995–1008, 1997. [DOI] [PubMed] [Google Scholar]
- Donner TH, Siegel M. A framework for local cortical oscillation patterns. Trends Cogn Sci 15: 191–199, 2011. [DOI] [PubMed] [Google Scholar]
- Dow RS, Ulett G, Raaf J. Electroencephalographic studies immediately following head injury. Am J Psychiatry 101: 174–183, 1944. [Google Scholar]
- Dyhrfjeld-Johnsen J, Santhakumar V, Morgan RJ, Huerta R, Tsimring L, Soltesz I. Topological determinants of epileptogenesis in large-scale structural and functional models of the dentate gyrus derived from experimental data. J Neurophysiol 97: 1566–1587, 2007. [DOI] [PubMed] [Google Scholar]
- Einevoll GT, Kayser C, Logothetis NK, Panzeri S. Modelling and analysis of local field potentials for studying the function of cortical circuits. Nat Rev Neurosci 14: 770–785, 2013. [DOI] [PubMed] [Google Scholar]
- Fame RM, MacDonald JL, Macklis JD. Development, specification, and diversity of callosal projection neurons. Trends Neurosci 34: 41–50, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain 123: 1293–1326, 2000. [DOI] [PubMed] [Google Scholar]
- Geddes DM, Cargill RS, LaPlaca MC. Mechanical stretch to neurons results in a strain rate and magnitude-dependent increase in plasma membrane permeability. J Neurotrauma 20: 1039–1049, 2003. [DOI] [PubMed] [Google Scholar]
- Gosselin N, Lassonde M, Petit D, Leclerc S, Mongrain V, Collie A, Montplaisir J. Sleep following sport-related concussions. Sleep Med 10: 35–46, 2009. [DOI] [PubMed] [Google Scholar]
- Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338: 334–337, 1989. [DOI] [PubMed] [Google Scholar]
- Gross J, Schmitz F, Schnitzler I, Kessler K, Shapiro K, Hommel B, Schnitzler A. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc Natl Acad Sci USA 101: 13050–13055, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halterman CI, Langan J, Drew A, Rodriguez E, Osternig LR, Chou LS, van Donkelaar P. Tracking the recovery of visuospatial attention deficits in mild traumatic brain injury. Brain 129: 747–753, 2006. [DOI] [PubMed] [Google Scholar]
- Ham TE, Sharp DJ. How can investigation of network function inform rehabilitation after traumatic brain injury? Curr Opin Neurol 25: 662–669, 2012. [DOI] [PubMed] [Google Scholar]
- Haneef Z, Levin HS, Frost JD Jr, Mizrahi EM. Electroencephalography and quantitative electroencephalography in mild traumatic brain injury. J Neurotrauma 30: 653–656, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited–comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 32: 989–994, 2006. [DOI] [PubMed] [Google Scholar]
- Houweling AR, Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Homeostatic synaptic plasticity can explain post-traumatic epileptogenesis in chronically isolated neocortex. Cereb Cortex 15: 834–845, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houzel JC, Carvalho ML, Lent R. Interhemispheric connections between primary visual areas: beyond the midline rule. Braz J Med Biol Res 35: 1441–1453, 2002. [DOI] [PubMed] [Google Scholar]
- Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol 34: 2064–2074, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti GM. General organization of callosal connections in the cerebral cortex. In: Cerebral Cortex. Sensory-Motor Areas and Aspects of Cortical Connectivity. New York: Plenum, 1986, vol. 5, p. 291–353. [Google Scholar]
- Innocenti GM. Some new trends in the study of the corpus callosum. Behav Brain Res 64: 1–8, 1994. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Vercelli A, Caminiti R. The diameter of cortical axons depends both on the area of origin and target. Cereb Cortex 24: 2178–2188, 2014. [DOI] [PubMed] [Google Scholar]
- Iwata A, Stys PK, Wolf JA, Chen XH, Taylor AG, Meaney DF, Smith DH. Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J Neurosci 24: 4605–4613, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbo K, Verstynen T, Schneider W. In vivo quantification of global connectivity in the human corpus callosum. Neuroimage 59: 1988–1996, 2012. [DOI] [PubMed] [Google Scholar]
- Kilinc D, Gallo G, Barbee KA. Mechanical membrane injury induces axonal beading through localized activation of calpain. Exp Neurol 219: 553–561, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen KM, Greenwood R, Powell JH, Leech R, Hawkins PC, Bonnelle V, Patel MC, Counsell SJ, Sharp DJ. White matter damage and cognitive impairment after traumatic brain injury. Brain 134: 449–463, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev 29: 169–195, 1999. [DOI] [PubMed] [Google Scholar]
- Kopell NJ, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA 97: 1867–1872, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn A, Golan H, Melamed I, Pascual-Marqui R, Friedman A. Focal cortical dysfunction and blood-brain barrier disruption in patients with postconcussion syndrome. J Clin Neurophysiol 22: 1–9, 2005. [DOI] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 130: 2508–2519, 2007. [DOI] [PubMed] [Google Scholar]
- Laughlin SB, Sejnowski TJ. Communication in neuronal networks. Science 301: 1870–1874, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima B, Singer W, Chen NH, Neuenschwander S. Synchronization dynamics in response to plaid stimuli in monkey V1. Cereb Cortex 20: 1556–1573, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XC, Hartings JA, Si Y, Balbir A, Cao Y, Tortella FC. Electrocortical pathology in a rat model of penetrating ballistic-like brain injury. J Neurotrauma 28: 71–83, 2011. [DOI] [PubMed] [Google Scholar]
- Lubenov EV, Siapas AG. Decoupling through synchrony in neuronal circuits with propagation delays. Neuron 58: 118–131, 2008. [DOI] [PubMed] [Google Scholar]
- MacFlynn G, Montgomery EA, Fenton GW, Rutherford W. Measurement of reaction time following minor head injury. J Neurol Neurosurg Psychiatry 47: 1326–1331, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malojcic B, Mubrin Z, Coric B, Susnic M, Spilich GJ. Consequences of mild traumatic brain injury on information processing assessed with attention and short-term memory tasks. J Neurotrauma 25: 30–37, 2008. [DOI] [PubMed] [Google Scholar]
- Mann EO, Kohl MM, Paulsen O. Distinct roles of GABAA and GABAB receptors in balancing and terminating persistent cortical activity. J Neurosci 29: 7513–7518, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell WL. Histopathological changes at central nodes of Ranvier after stretch-injury. Microsc Res Tech 34: 522–535, 1996. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Linden H, Cuntz H, Lansner A, Panzeri S, Einevoll GT. Computing the local field potential (LFP) from integrate-and-fire network models. PLoS Comput Biol 11: e1004584, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HS, Wimmer VC, Oberlaender M, de Kock CP, Sakmann B, Helmstaedter M. Number and laminar distribution of neurons in a thalamocortical projection column of rat vibrissal cortex. Cereb Cortex 20: 2277–2286, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WJ, Leventhal I, Scarsella D, Haydon PG, Janmey P, Meaney DF. Mechanically induced reactive gliosis causes ATP-mediated alterations in astrocyte stiffness. J Neurotrauma 26: 789–797, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller JJ, Tu B, Bazil CW. Quantitative and qualitative analysis of ambulatory electroencephalography during mild traumatic brain injury. Arch Neurol 68: 1595–1598, 2011. [DOI] [PubMed] [Google Scholar]
- Morris C, Lecar H. Voltage oscillations in the barnacle giant muscle fiber. Biophys J 35: 193–213, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison B, Elkin BS, Dolle JP, Yarmush ML. In vitro models of traumatic brain injury. Annu Rev Biomed Eng 13: 91–126, 2011. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson C, Kolster RA, Sarkar R, Lee H, Meeker M, Zimmerman RD, Manley GT, McCandliss BD. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol 29: 967–973, 2008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson CE, Kolster R, Lee H, Suh M, Zimmerman RD, Manley GT, McCandliss BD. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain 131: 3209–3221, 2008b. [DOI] [PubMed] [Google Scholar]
- Norris JN, Carr W, Herzig T, Labrie DW, Sams R. ANAM4 TBI reaction time-based tests have prognostic utility for acute concussion. Mil Med 178: 767–774, 2013. [DOI] [PubMed] [Google Scholar]
- Perez AM, Adler J, Kulkarni N, Strain JF, Womack KB, Diaz-Arrastia R, Marquez de la Plata CD. Longitudinal white matter changes after traumatic axonal injury. J Neurotrauma 31: 1478–1485, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, Ratte S, De Koninck Y, Sejnowski TJ. Pyramidal neurons switch from integrators in vitro to resonators under in vivo-like conditions. J Neurophysiol 100: 3030–3042, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PE, Keyser DO, Albano A, Hernandez R, Gibson DB, Zambon RA, Hairston WD, Hughes JD, Krystal A, Nichols AS. Traumatic brain injury detection using electrophysiological methods. Front Hum Neurosci 9: 11, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves TM, Greer JE, Vanderveer AS, Phillips LL. Proteolysis of submembrane cytoskeletal proteins ankyrin-G and αII-spectrin following diffuse brain injury: a role in white matter vulnerability at nodes of Ranvier. Brain Pathol 20: 1055–1068, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickett T, Connell S, Bastijanic J, Shi R. Functional and mechanical evaluation of nerve stretch injury. J Med Syst 35: 787–793, 2011. [DOI] [PubMed] [Google Scholar]
- Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb Cortex 4: 331–343, 1994. [DOI] [PubMed] [Google Scholar]
- Rosenberg LJ, Teng YD, Wrathall JR. Effects of the sodium channel blocker tetrodotoxin on acute white matter pathology after experimental contusive spinal cord injury. J Neurosci 19: 6122–6133, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxin A, Brunel N, Hansel D. Role of delays in shaping spatiotemporal dynamics of neuronal activity in large networks. Phys Rev Lett 94: 238103, 2005. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Creed J, Raghupathi R. Calpain as a therapeutic target in traumatic brain injury. Neurotherapeutics 7: 31–42, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci 2: 539–550, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Aradi I, Soltesz I. Role of mossy fiber sprouting and mossy cells loss in hyperexcitability: a network model of the dentate gyrus incorporating cell types and axonal topography. J Neurophysiol 93: 437–453, 2004. [DOI] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Steinberg JL, Pearson DA, Rauch RA, Mao H, Troyanskaya M, Sharma RG, Levin HS. Altered brain activation during cognitive control in patients with moderate to severe traumatic brain injury. Neurorehabil Neural Repair 21: 36–45, 2007. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann NY Acad Sci 1142: 266–309, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R, Blight AR. Compression injury of mammalian spinal cord in vitro and the dynamics of action potential conduction failure. J Neurophysiol 76: 1572–1580, 1996. [DOI] [PubMed] [Google Scholar]
- Shi R, Pryor JD. Pathological changes of isolated spinal cord axons in response to mechanical stretch. Neuroscience 110: 765–777, 2002. [DOI] [PubMed] [Google Scholar]
- Singh A, Kallakuri S, Chen S, Cavanagh JB. Structural and functional changes in nerve roots due to tension at various strains and strain rates: an in-vivo study. J Neurotrauma 26: 627–640, 2009. [DOI] [PubMed] [Google Scholar]
- Smith DH, Meaney DF. Axonal damage in traumatic brain injury. Neuroscientist 6: 483–495, 2000. [Google Scholar]
- Smits M, Dippel DW, Houston GC, Wielopolski PA, Koudstaal PJ, Hunink MG, van der Lugt A. Postconcussion syndrome after minor head injury: brain activation of working memory and attention. Hum Brain Mapp 30: 2789–2803, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez R, Gobius I, Richards LJ. Evolution and development of interhemispheric connections in the vertebrate forebrain. Front Hum Neurosci 8: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Fu Y, Shi Y, Cheng JX, Cao P, Shi R. Paranodal myelin damage after acute stretch in guinea pig spinal cord. J Neurotrauma 29: 611–619, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA. Efferent neurons and suspected interneurons in S-1 forelimb representation of the awake rabbit: receptive fields and axonal properties. J Neurophysiol 63: 1477–1498, 1990. [DOI] [PubMed] [Google Scholar]
- Talavage TM, Nauman EA, Breedlove EL, Yoruk U, Dye AE, Morigaki KE, Feuer H, Leverenz LJ. Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J Neurotrauma 31: 327–338, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Tomioka R. Long-range GABAergic connections distributed throughout the neocortex and their possible function. Front Neurosci 4: 202, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarapore PE, Findlay AM, Lahue SC, Lee H, Homma SM, Mizuiri D, Luks TL, Manley GT, Nagarajan SS, Mukherjee P. Resting state magnetoencephalography functional connectivity in traumatic brain injury. J Neurosurg 118: 1306–1316, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavalin SJ, Ellis EF, Satin LS. Mechanical perturbation of cultured cortical neurons reveals a stretch-induced delayed depolarization. J Neurophysiol 74: 2767–2773, 1995. [DOI] [PubMed] [Google Scholar]
- Tebano MT, Cameroni M, Gallozzi G, Loizzo A, Palazzino G, Pezzini G, Ricci GF. EEG spectral analysis after minor head injury in man. Electroencephalogr Clin Neurophysiol 70: 185–189, 1988. [DOI] [PubMed] [Google Scholar]
- Thatcher RW. Electroencephalography and Mild Traumatic Brain Injury. In: Foundations of Sport-Related Brain Injuries, edited by Slobounov S and Sebastianelli W. New York: Springer, 2006, p. 241–265. [Google Scholar]
- Thatcher RW, North DM, Curtin RT, Walker RA, Biver CJ, Gomez JF, Salazar AM. An EEG severity index of traumatic brain injury. J Neuropsychiatry Clin Neurosci 13: 77–87, 2001. [DOI] [PubMed] [Google Scholar]
- Tokoro K, Sato H, Yamamoto M, Nagai Y. [Thalamus and Attention]. Brain Nerve 67: 1471–1480, 2015. [DOI] [PubMed] [Google Scholar]
- Toth Z, Hollrigel GS, Gorcs T, Soltesz I. Instantaneous perturbation of dentate interneuronal networks by a pressure wave-transient delivered to the neocortex. J Neurosci 17: 8106–8117, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsodyks MV, Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci USA 94: 719–723, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci 22: 221–227, 1999. [DOI] [PubMed] [Google Scholar]
- Ursino M, La Cara GE. Travelling waves and EEG patterns during epileptic seizure: analysis with an integrate-and-fire neural network. J Theor Biol 242: 171–187, 2006. [DOI] [PubMed] [Google Scholar]
- van der Knaap LJ, van der Ham IJ. How does the corpus callosum mediate interhemispheric transfer? A review. Behav Brain Res 223: 211–221, 2011. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P, Langan J, Rodriguez E, Drew A, Halterman C, Osternig LR, Chou LS. Attentional deficits in concussion. Brain Inj 19: 1031–1039, 2005. [DOI] [PubMed] [Google Scholar]
- Viano DC, Casson IR, Pellman EJ, Zhang L, King AI, Yang KH. Concussion in professional football: brain responses by finite element analysis: part 9. Neurosurgery 57: 891–916; discussion 891–916, 2005. [DOI] [PubMed] [Google Scholar]
- Volman V, Ng LJ. Computer modeling of mild axonal injury: implications for axonal signal transmission. Neural Comput 25: 2646–2681, 2013. [DOI] [PubMed] [Google Scholar]
- Volman V, Ng LJ. Perinodal glial swelling mitigates axonal degradation in a model of axonal injury. J Neurophysiol 115: 1003–1017, 2016. [DOI] [PubMed] [Google Scholar]
- Volman V, Ng LJ. Primary paranode demyelination modulates slowly developing axonal depolarization in a model of axonal injury. J Comput Neurosci 37: 439–457, 2014. [DOI] [PubMed] [Google Scholar]
- von Stein A, Rappelsberger P, Sarnthein J, Petsche H. Synchronization between temporal and parietal cortex during multimodal object processing in man. Cereb Cortex 9: 137–150, 1999. [DOI] [PubMed] [Google Scholar]
- Wang JA, Lin W, Morris T, Banderali U, Juranka PF, Morris CE. Membrane trauma and Na+ leak from Nav1.6 channels. Am J Physiol Cell Physiol 297: C823–C834, 2009. [DOI] [PubMed] [Google Scholar]
- Wang Q, Perc M, Duan Z, Chen G. Impact of delays and rewiring on the dynamics of small-world neuronal networks with two types of coupling. Physica A 389: 3299–3306, 2010. [Google Scholar]
- Wang SS, Shultz JR, Burish MJ, Harrison KH, Hof PR, Towns LC, Wagers MW, Wyatt KD. Functional trade-offs in white matter axonal scaling. J Neurosci 28: 4047–4056, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev 90: 1195–1268, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Buzsàki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci 16: 6402–6413, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MR, Fenton GW, McClelland RJ, Lumsden J, Headley M, Rutherford WH. The post-concussional state: neurophysiological aspects. Br J Psychiatry 167: 514–521, 1995. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve 3: 141–150, 1980. [DOI] [PubMed] [Google Scholar]
- West M, Parkinson D, Havlicek V. Spectral analysis of the electroencephalographic response to experimental concussion in the rat. Electroencephalogr Clin Neurophysiol 53: 192–200, 1982. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373: 612–615, 1995. [DOI] [PubMed] [Google Scholar]
- Wolf JA, Stys PK, Lusardi T, Meaney DF, Smith DH. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J Neurosci 21: 1923–1930, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P. The role of neuronal synchronization in selective attention. Curr Opin Neurobiol 17: 154–160, 2007. [DOI] [PubMed] [Google Scholar]
- Wróbel A. Beta activity: a carrier for visual attention. Acta Neurobiol Exp (Wars) 60: 247–260, 2000. [DOI] [PubMed] [Google Scholar]
- Yuen TJ, Browne KD, Iwata A, Smith DH. Sodium channelopathy induced by mild axonal trauma worsens outcome after a repeat injury. J Neurosci Res 87: 3620–3625, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]