Abstract
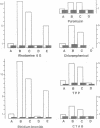
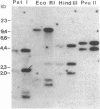
Bacillus subtilis cells selected for their resistance to rhodamine 6G demonstrated a multidrug-resistance (MDR) phenotype resembling that of mammalian MDR cells. Like MDR in mammalian cells, MDR in bacteria was mediated by the efflux of the drugs from the cells. The bacterial multidrug efflux system transported similar drugs and was sensitive to similar inhibitors as the mammalian multidrug transporter, P-glycoprotein. The gene coding for the bacterial multidrug transporter, like the P-glycoprotein gene in mammalian MDR cells, was amplified in the resistant bacteria. On the other hand, the bacterial multidrug transporter showed no sequence similarity to P-glycoprotein but exhibited an obvious homology to tetracycline efflux pumps and carbohydrate-ion symporters. These results show that the transport of structurally unrelated molecules can be mediated by members of different families of membrane transporters.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck W. T., Cirtain M. C., Glover C. J., Felsted R. L., Safa A. R. Effects of indole alkaloids on multidrug resistance and labeling of P-glycoprotein by a photoaffinity analog of vinblastine. Biochem Biophys Res Commun. 1988 Jun 30;153(3):959–966. doi: 10.1016/s0006-291x(88)81321-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Gros P., Raymond M., Bell J., Housman D. Cloning and characterization of a second member of the mouse mdr gene family. Mol Cell Biol. 1988 Jul;8(7):2770–2778. doi: 10.1128/mcb.8.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannière L., Niaudet B., Pierre E., Ehrlich S. D. Stable gene amplification in the chromosome of Bacillus subtilis. Gene. 1985;40(1):47–55. doi: 10.1016/0378-1119(85)90023-x. [DOI] [PubMed] [Google Scholar]
- Juranka P. F., Zastawny R. L., Ling V. P-glycoprotein: multidrug-resistance and a superfamily of membrane-associated transport proteins. FASEB J. 1989 Dec;3(14):2583–2592. doi: 10.1096/fasebj.3.14.2574119. [DOI] [PubMed] [Google Scholar]
- Kaneko M., Yamaguchi A., Sawai T. Energetics of tetracycline efflux system encoded by Tn10 in Escherichia coli. FEBS Lett. 1985 Dec 2;193(2):194–198. doi: 10.1016/0014-5793(85)80149-6. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Varshavsky A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature. 1989 Aug 3;340(6232):400–404. doi: 10.1038/340400a0. [DOI] [PubMed] [Google Scholar]
- Midgley M. The phosphonium ion efflux system of Escherichia coli: relationship to the ethidium efflux system and energetic studies. J Gen Microbiol. 1986 Nov;132(11):3187–3193. doi: 10.1099/00221287-132-11-3187. [DOI] [PubMed] [Google Scholar]
- Sullivan M. A., Yasbin R. E., Young F. E. New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene. 1984 Jul-Aug;29(1-2):21–26. doi: 10.1016/0378-1119(84)90161-6. [DOI] [PubMed] [Google Scholar]
- Tennent J. M., Lyon B. R., Midgley M., Jones I. G., Purewal A. S., Skurray R. A. Physical and biochemical characterization of the qacA gene encoding antiseptic and disinfectant resistance in Staphylococcus aureus. J Gen Microbiol. 1989 Jan;135(1):1–10. doi: 10.1099/00221287-135-1-1. [DOI] [PubMed] [Google Scholar]
- Vandeyar M. A., Zahler S. A. Chromosomal insertions of Tn917 in Bacillus subtilis. J Bacteriol. 1986 Aug;167(2):530–534. doi: 10.1128/jb.167.2.530-534.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. R., Morgan A. E. Chromosomal-DNA amplification in Bacillus subtilis. J Bacteriol. 1985 Aug;163(2):445–453. doi: 10.1128/jb.163.2.445-453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. Gene amplification in Bacillus subtilis. J Gen Microbiol. 1984 Jul;130(7):1613–1621. doi: 10.1099/00221287-130-7-1613. [DOI] [PubMed] [Google Scholar]
- Yusa K., Tsuruo T. Reversal mechanism of multidrug resistance by verapamil: direct binding of verapamil to P-glycoprotein on specific sites and transport of verapamil outward across the plasma membrane of K562/ADM cells. Cancer Res. 1989 Sep 15;49(18):5002–5006. [PubMed] [Google Scholar]