Abstract
The nontoxic B subunit of Escherichia coli heat-labile enterotoxin (EtxB) is a potent immunomodulatory molecule that acts both as an adjuvant and to stimulate immune deviation processes, resulting in the suppression of Th1-associated inflammatory responses. The ability of EtxB to alter immune reactivity is dependent on its ability to modulate immune cell function through binding to cell surface molecules, the principal receptor of which is the ubiquitous GM1-ganglioside. EtxB activates B cells and antigen-presenting cells and induces the selective apoptosis of murine CD8+ T cells. We postulated that these effects are mediated by the induction of intracellular signaling pathways following EtxB-receptor interaction. We have previously shown that CD8+ T-cell apoptosis induced by EtxB results from the activation of the transcription factor NF-κB and caspases. Here we report that while caspase activity is required for apoptosis, additional features of cell death are caspase independent. EtxB induces a rapid loss of mitochondrial membrane potential and cell viability that are unaffected by caspase inhibitors. In addition, our data suggest that these processes are independent of the activity of Bax and Bcl-2 but are mediated by nitric oxide synthase.
Escherichia coli heat-labile enterotoxin (Etx) is composed of a single catalytically active A subunit (EtxA) and a pentameric B subunit (EtxB) that mediates receptor interaction and uptake of the toxin by target cells (34). In addition to the well-documented deleterious effects of the toxin, a wealth of data is accumulating suggesting that Etx can be used to modulate immune responses in a variety of beneficial ways (45). In this regard, Etx is a highly potent adjuvant and has been used in a large number of experimental, mucosal vaccines. Importantly, the toxicity of the holotoxin does not appear to be a prerequisite for this activity, and detoxified and B-subunit preparations of Etx retain the ability to act as adjuvants (8, 14, 29), at least when intranasal routes are used. Furthermore, recombinant preparations of EtxB act as immunomodulatory proteins capable of suppressing Th1-associated inflammatory processes in vivo (45). These findings have led to investigations of the ability of EtxB and the related cholera toxin B subunit, CtxB, to prevent and treat autoimmune processes in animal models of disease (23, 35, 36, 46).
In an attempt to understand the in vivo immunomodulatory properties of the cholera-like enterotoxins, a number of studies have documented the effects of Etx and Ctx and their subunits on immune cell function. Both CtxB and EtxB induce the activation of murine B cells in vitro, leading to enhancement of major histocompatibility complex class II and costimulatory molecule expression (12, 27). EtxB stimulates monocyte interleukin-10 (IL-10) production, while it inhibits IL-12 production (41); these effects are also mediated by Ctx but not by CtxB (3, 6). In contrast to these stimulatory properties, both EtxB and CtxB induce apoptosis of murine CD8+ T cells (25, 26, 32, 40, 48). Additional studies indicate that injection of CtxB into mice results in depletion of CD8+ T cells from Peyer's patches, suggesting that these effects may also occur in vivo (9).
The immunomodulatory activity of EtxB is dependent on binding to cell surface receptors, as evidenced by a failure of a non-receptor-binding mutant, EtxB(G33D), to induce any of the events described above. The principal receptor for both EtxB and CtxB is the ubiquitous ganglioside GM1 [Gal(β1-3)GalNAc(β1-4)NeuAc(α2-3)Gal(β1-4)Glc(β1-1)ceramide] (45). Both B subunits also bind ganglioside GD1b, while EtxB interacts with a number of additional receptors and galactoproteins for which CtxB has no affinity. Ganglioside GM1 is a principal constituent of glycolipid membrane rafts (4, 16, 21). We have postulated that receptor interaction with EtxB alters raft-associated intracellular signaling pathways and consequently leads to the activation, differentiation, or death of target cells. In this regard, it has recently been reported that EtxB-mediated apoptosis of CD8+ T cells results from receptor-dependent activation of NF-κB and c-Myc (31, 33). These events cause activation of caspases within the cells and proceed in a Fas- and p55 tumor necrosis factor receptor-independent pathway. Conversely, the activation of B cells by EtxB occurs as a result of phosphoinositide 3-kinase- and protein kinase C-dependent Erk mitogen-activated protein kinase activation (2).
Data from a number of groups have suggested that inhibition of caspase activity during apoptosis may be insufficient to prevent cell death. Inhibition of caspases following Fas signaling leads to the induction of necrotic, rather than apoptotic, cell death (24, 43). Similarly, tumor necrosis factor-induced necrosis of L929 cells is enhanced in the presence of caspase inhibitors (42). Furthermore, T-cell death induced by anti-CD2 antibodies or staurosporine does not require caspase activity (7). Data from previous studies indicated that caspase activity was required for the induction of DNA fragmentation during EtxB-induced apoptosis of CD8+ T cells (31, 32). However, it is not clear whether the activation of caspases via NF-κB is the only signal triggered by EtxB in CD8+ T cells and whether the fate of the cells is dependent upon this.
MATERIALS AND METHODS
Animals.
Female NIH mice were purchased from Harlan-Olac (Bicester, United Kingdom) and were maintained in the departmental animal facility. All mice were used when they were between 6 and 12 weeks old.
Preparation of recombinant EtxB.
Recombinant EtxB and Etx(G33D) were purified from culture supernatants of Vibrio sp. strain 60(pMMB68) and Vibrio sp. strain 60(TRH64) as previously described (29).
Isolation of murine CD8+ T lymphocytes.
Murine mesenteric lymph nodes were isolated as previously described (26). For purification of CD8+ T cells, cells were washed in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin and 5 mM EDTA prior to addition of specific antibodies conjugated with MACS colloidal superparamagnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Mesenteric lymph node cells were labeled with anti-CD4 and anti-CD45R microbeads, and cell suspensions were applied to VS selection columns (Miltenyi Biotec). The negative fractions, containing CD8+ T cells, were eluted. The purity of CD8+ T-cell populations was >90% as determined by flow cytometry.
Lymphocyte cultures.
Cells were cultured at 37°C in 5% CO2 in alpha-modified Eagle's medium supplemented with 4 mM l-glutamine, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, 5 × 10−5 M 2-mercaptoethanol, and 2% fetal bovine serum. Z-Val(OMe)-Arg-Asp(OMe)-fluoromethyl ketone (Z-VAD-FMK) and aminoguanidine (both obtained from Calbiochem) were added to cells 1 h prior to the addition of B subunits. In all experiments EtxB and EtxB(G33D) were used at a concentration of 40 μg/ml.
Flow cytometry.
Staining with propidium iodide was performed as previously described (31). For analysis of the mitochondrial membrane potential, cells were incubated with 50 nM 3,3′-dihexyloxacarbocyanine iodide [DiOC6(3)] (Calbiochem) for 20 min at 37°C and washed in PBS, and then fluorescence was measured on log scale FL1. Alternatively, tetramethylrhodamine ethyl ester (TMRE) (Molecular Probes, Eugene, Oreg.) was used to monitor mitochondrial dysfunction. Cells were incubated with 5 μM TMRE for 30 min at room temperature and washed in PBS, and the fluorescence was measured on log scale FL2. For determination of cell viability, immediately prior to analysis, 7-aminoactinomycin D (7-AAD) (Sigma) was added to a final concentration of 1 μg/ml, and viability was determined by examining dye exclusion by flow cytometry. For all flow cytometric analyses 10,000 events were collected and analyzed with a FACScan flow cytometer (Becton Dickinson, Oxford, United Kingdom).
Assessment of intracellular nitric oxide.
A total of 1 × 106 CD8+ T cells were cultured in the presence of EtxB or under control conditions for 3 or 6 h in phenol red-free minimal essential alpha medium (GibcoBRL) supplemented with 4 mM l-glutamine, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, 5 × 10−5 M 2-mercaptoethanol, and 2% fetal bovine serum. Cells were then washed and resuspended in serum-free medium containing 2 μM 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (Molecular Probes). Cells were incubated for 1 h at 37°C and then washed and resuspended in serum-free medium and incubated for a further 30 min. Finally, cells were washed and resuspended in PBS prior to analysis by flow cytometry.
Caspase activity assays.
Assays for caspase 3-like (DEVDase) activity were performed as previously described (31). Briefly, 5 × 106 CD8+ T cells were incubated in the presence of EtxB, EtxB(G33D), or PBS alone for 15 h, and then they were lysed in buffer A (50 mM EDTA [pH 8], 250 mM sucrose, 1% NP-40, 1 mM dithiothreitol, and protease inhibitors). The caspase 3-like activity of lysates was assessed by measuring specific cleavage of the substrate Ac-Asp-Glu-Val-Asp-aminomethyl coumarin (Biomol Research Laboratories, Plymouth Meeting, Pa.) at excitation and emission wavelengths of 380 and 460 nm, respectively.
Western blotting.
For preparation of whole-cell extracts 5 × 106 cells were lysed in lysis buffer A (10% [vol/vol] glycerol, 50 mM Tris [pH 7.7], 1% Triton X-100, 150 mM NaCl, 5 mM EDTA [pH 8.0], 1 mM Na3VO4, 10 mM NaF, 1 mM Na3MoO4, 1 mM phenylmethylsulfonyl fluoride, 5 μg of leupeptin per ml, 2 μg of aprotenin per ml, 1.4 μg of pepstatin per ml, 20 μg of soybean trypsin inhibitor per ml). Lysates were centrifuged at 14,000 × g for 30 s, and insoluble material was removed. Alternatively, cells were lysed in a solution containing 0.1% (wt/vol) saponin, 1 mM phenylmethylsulfonyl fluoride, 5 μg of leupeptin per ml, 2 μg of aprotenin per ml, 1.4 μg of pepstatin per ml, and 20 μg of soybean trypsin inhibitor per ml. Lysates were centrifuged at 14,000 × g for 15 min, and the resulting supernatant was considered the cytoplasmic fraction. Subsequently, the remaining membrane proteins were solubilized in lysis buffer A, as described above. Samples were resolved by sodium dodecyl sulfate-15% (vol/vol) polyacrylamide gel electrophoresis, and proteins were transferred to nitrocellulose membranes. To monitor protein loading, the membranes were stained with Ponceau S stain (Sigma). The membranes were blocked in 5% bovine serum albumin-0.05% Tween 20 (Sigma) and then probed with 0.6 μg of anti-PARP monoclonal antibody per ml, 0.5 μg of anti-Bax or anti-Bcl-2 monoclonal antibodies per ml, or 1 μg of anti-caspase 3 polyclonal antibody per ml (all antibodies were obtained from R&D Systems, Abingdon, United Kingdom). The membranes were washed and then probed with a 1:10,000 dilution of horseradish peroxidase-conjugated secondary antibodies (DAKO, Glostrup, Denmark). The membranes were washed, and the protein bands were visualized by ECL chemiluminescence (Amersham International, Little Chalfont, United Kingdom).
RESULTS
A broad-spectrum caspase inhibitor prevents EtxB-induced nuclear apoptosis but does not inhibit B-subunit-induced cell death.
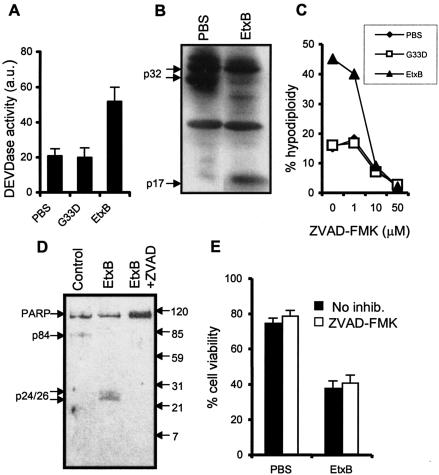
In order to investigate caspase-independent signaling in CD8+ T cells, it was important to determine the effects of blocking caspase activation on the cells. CD8+ T cells were purified and incubated in the presence of EtxB, EtxB(G33D), or PBS for 15 h, and caspase (DEVDase) activity was assessed. Cultures to which EtxB was added showed a marked increase in DEVDase activity (Fig. 1A). In contrast, the activity in cultures treated with EtxB(G33D) was equivalent to the control activity. In order to determine whether enhanced DEVDase activity was associated with activation of caspase 3, CD8+ T cells were treated with EtxB for 15 h, cell lysates were prepared, and Western blotting was performed. EtxB clearly induced cleavage of the p32 proform of caspase 3 into active p17 forms, which is consistent with a role for this enzyme in the apoptotic pathway (Fig. 1B). Preincubation of CD8+ T cells with the broad-spectrum caspase inhibitor Z-VAD-FMK completely abrogated EtxB-induced hypodiploidy in a dose-dependent manner (Fig. 1C). Background levels of DNA degradation were also inhibited in the presence of this inhibitor. The DEVDase activity of lysates prepared from EtxB-treated cells was also abrogated by preincubation of cells with Z-VAD-FMK (data not shown).
FIG. 1.
Role of caspases in EtxB-induced cell death. (A) DEVDase activity of lysates from CD8+ T cells treated with PBS, EtxB(G33D), or EtxB for 15 h. (B) Western blot for caspase 3 in lysates prepared from untreated and EtxB-treated CD8+ T cells. Arrows indicate the positions of native uncleaved 32-kDa caspase 3 and active cleaved 17-kDa caspase 3. (C) Dose-response effects of the pancaspase inhibitor Z-VAD-FMK on CD8+ T-cell hypodiploidy as measured after 24 h of culture. (D) Western blot analysis of PARP cleavage in cells treated with PBS, EtxB, or EtxB plus Z-VAD-FMK. (E) Effects of Z-VAD-FMK on EtxB-induced cell death. Cells were preincubated in the presence or absence of 50 μM Z-VAD-FMK and then were treated with EtxB or PBS for 24 h. Cell viability was assessed by determining the ability to exclude the dye 7-AAD. inhib., inhibitor. The data are means ± standard errors of the means (n = 3).
Poly(ADP-ribose) polymerase (PARP) is an enzyme that is thought to be involved in DNA repair mechanisms (22). Cleavage of PARP into 85- and 24-kDa fragments is a characteristic of apoptosis and is mediated primarily by caspases 3 and 7 (10, 39). Cleavage of PARP abrogates its enzymatic activity and may play an important role in nuclear destruction during apoptosis. Western blotting experiments indicated that incubation of CD8+ T cells with EtxB for 24 h resulted in cleavage of PARP into ∼24-kDa fragments (Fig. 1D). Importantly, preincubation of CD8+ T cells with 50 μM Z-VAD-FMK completely prevented PARP cleavage.
In order to ascertain whether inhibition of caspase activity was sufficient to inhibit cell death, CD8+ T cells were preincubated with 50 μM Z-VAD-FMK for 1 h and then treated with PBS or EtxB for 24 h. Viability was assessed by determining the ability of cells to exclude the vital dye 7-AAD. In contrast to the effects on hypodiploidy, preincubation of cells with the caspase inhibitor did not have an effect on the decrease in the viability of cells incubated in the presence of EtxB (Fig. 1E). Similar data were obtained when cells were pretreated with an additional pancaspase inhibitor, Boc-D-fluoromethyl ketone, or the caspase 3 selective inhibitor Z-DQMD-fluoromethyl ketone (data not shown).
Taken together, these data confirm that EtxB induces caspase activity and that this activity is essential for the cleavage of PARP and the induction of DNA degradation in the subsequent apoptotic pathway. However, inhibition of caspase activity does not prevent EtxB-induced cell death. Importantly, EtxB(G33D) did not induce any of these events, indicating that there was a requirement for receptor-binding activity (Fig. 1A and C and data not shown).
EtxB induces caspase-independent loss of mitochondrial membrane potential.
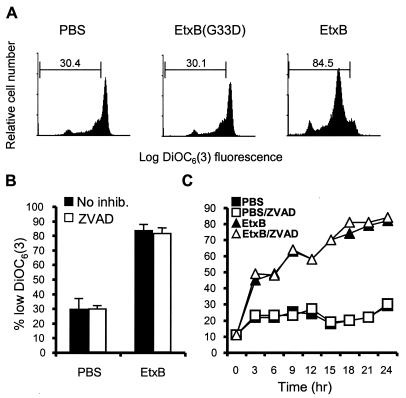
Loss of mitochondrial function is a feature of both apoptotic and necrotic cell death (19). Maintenance of an electrochemical gradient (Δψm) across the mitochondrial inner membrane is essential for driving ATP synthesis during respiration. Loss of Δψm can be measured by using the lipophilic cationic fluorochrome DiOC6(3); a decrease in the fluorescence intensity of DiOC6(3) is interpreted as an indication of Δψm dissipation (19). Incubation of CD8+ T cells with EtxB for 24 h, followed by DiOC6(3) staining and fluorescence-activated cell sorting (FACS) analysis, indicated that EtxB treatment induced the loss of Δψm. Approximately 85% of the cells incubated with EtxB exhibited a reduced level of DiOC6(3) staining, compared to only 30% of the control or EtxB(G33D)-treated cells (Fig. 2A). Importantly, this process was not inhibited by preincubation of cells with 50 μM Z-VAD-FMK (Fig. 2B), indicating that caspases were not required for the changes. Kinetic analyses showed that the loss of DiOC6(3) staining induced by EtxB was rapid, and differences between control and EtxB-treated cell cultures were apparent within 3 h (Fig. 2C). Previous studies indicated that activation of caspases in EtxB-treated cells does not occur until after approximately 12 h of culture, further suggesting that these events are separate events.
FIG. 2.
EtxB induces caspase-independent loss of mitochondrial membrane potential. (A) FACS blots of CD8+ T cells stained with DiOC6(3) following treatment with PBS, EtxB(G33D), or EtxB for 24 h. The values are the percentages of cells in the regions indicated. (B) Effect of Z-VAD-FMK (ZVAD) on EtxB-induced Δψm dissipation. CD8+ T cells were incubated in the presence of 50 μM caspase inhibitor plus EtxB or in the presence of EtxB alone for 24 h and then stained with DiOC6(3). The data are means ± standard errors of the means (n = 3). inhib., inhibitor. (C) Time course of EtxB-induced Δψm dissipation. The data are representative of the data obtained in two repeated experiments.
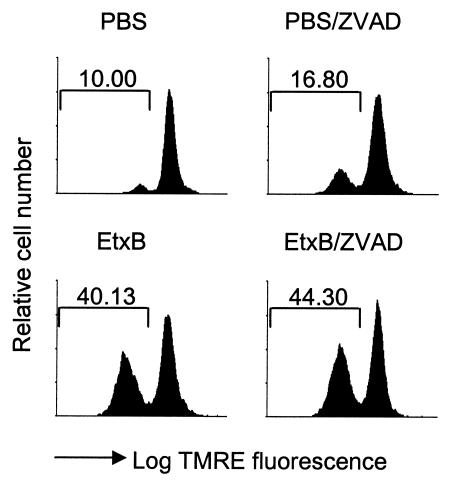
Recent findings have suggested that DiOC6(3) can itself inhibit cell respiration, lowering the Δψm, and that decreased levels of fluorescence under some circumstances are an indicator of changes in plasma membrane permeability which allow dye leakage rather than an indicator of mitochondrial depolarization (30). Therefore, we carried out experiments to confirm the results obtained with DiOC6(3) using an alternative dye, TMRE. TMRE has not been reported to have any toxic effects on mitochondrial activity and is considered to be less affected by plasma membrane changes (28). Cells were preincubated with a caspase inhibitor, treated with EtxB or PBS alone for 6 h, and then were loaded with TMRE in order to assess Δψm. These experiments also indicated that EtxB induced a loss of mitochondrial membrane potential in the presence of caspase inhibitors, as shown by the loss of TMRE fluorescence (TMRElow (Fig. 3). By contrast, EtxB(G33D) did not inhibit the loss of TMRE fluorescence (data not shown). The slightly higher number of TMRElow cells in the presence of Z-VAD-FMK was not a consistent observation in replicate experiments.
FIG. 3.
TMRE staining of CD8+ T cells. Cells were preincubated in the presence or absence of Z-VAD-FMK (ZVAD) and then were treated with EtxB or PBS for 5 h. Following TMRE staining, cells were analyzed by flow cytometry.
EtxB-induced Δψm dissipation is not associated with changes in the expression or subcellular localization of Bax or Bcl-2.
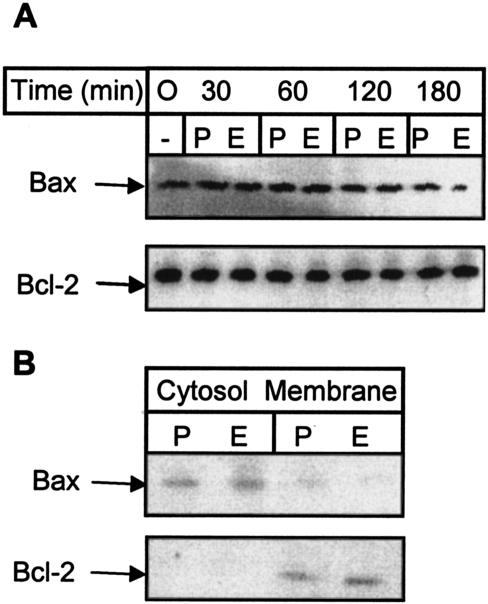
We also tried to define the mechanisms leading to the loss mitochondrial integrity. The Bcl-2 family member Bax induces cell death by disrupting the mitochondrial membrane (17). Disruption of the mitochondrial membrane potential by Bax is caspase independent and is inhibited by Bcl-2 (47). Induction of cell death by Bcl-2 family members may depend on the relative levels of expression of pro- and antiapoptotic proteins (1). Therefore, we assessed the levels of Bax and Bcl-2 expression in CD8+ T cells by Western blotting. Our analyses indicated that the expression of both Bax and Bcl-2 was unaffected by incubation with EtxB over a 3-h period (Fig. 4A). This time interval was sufficient for EtxB to induce dissipation of Δψm (Fig. 2C), suggesting that this event was not triggered by changes in the absolute levels of expression of Bax or Bcl-2. However, the data indicate that the cellular localization of Bax controls its prodeath activities. Thus, while Bax is generally a cytoplasmic protein, following induction of cell death the protein is redistributed to mitochondria (15). Bcl-2 is localized to mitochondria in both viable and apoptotic cells (15). We prepared cytoplasmic and membrane extracts from cells that had been incubated for 3 h in the presence or absence of EtxB and carried out Western blotting for Bcl-2 and Bax as described above. Bax was localized primarily in the cytosolic fractions, although a minor portion was present in membrane fractions (Fig. 4B). Importantly, there was no difference in the distribution of Bax in control and EtxB-treated cells. As expected, Bcl-2 was localized solely to membranes in both control and EtxB-treated cells (Fig. 4B). Together, these data indicate that the early dissipation of Δψm induced by EtxB is not associated with changes in the expression or subcellular localization of either Bax or Bcl-2 and is therefore likely to be independent of the action of either protein.
FIG. 4.
Lack of effect of EtxB on Bax and Bcl-2 expression or localization. (A) Western blot analysis of Bax and Bcl-2 expression in whole-cell lysates of CD8+ T cells incubated with PBS (P) or EtxB (E) for 0, 30, 60, 120, or 180 min. Membranes were loaded with equal amounts of protein, as assessed with Ponceau S stain. The same membrane was probed with both anti-Bcl-2 and Bax monoclonal antibodies. (B) Bax and Bcl-2 localization. Membrane and cytosolic fractions were prepared from cells treated with EtxB or PBS for 3 h. The data are representative of the data obtained in three experiments.
Role of nitric oxide synthase in EtxB-induced Δψm dissipation.
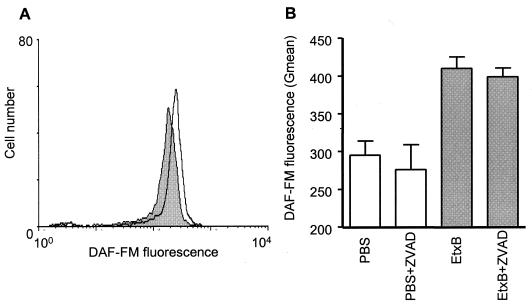
The formation of free radicals is known to play an important role in cell death in a number of cell types (20, 38). We sought to investigate the role of nitric oxide (NO) in EtxB-induced cell death using a flow cytometric assay for intracellular NO levels. CD8+ T cells that had been treated with EtxB or PBS for 3 h were assayed for intracellular NO by using the dye DAF-FM. The data indicated that EtxB induced an increase in the level of intracellular NO, as shown by a clear shift in DAF-FM fluorescence (Fig. 5A). By contrast, EtxB(G33D) had no effect on intracellular NO (data not shown). Furthermore, pretreatment of cells with Z-VAD-FMK did not prevent upregulation of NO production (Fig. 5B), indicating that this effect of EtxB did not require caspase activity.
FIG. 5.
EtxB enhances NO production. (A) Representative FACS plot of DAF-FM-stained CD8+ T cells treated with PBS alone (shaded histogram) or EtxB (open histogram) for 3 h. (B) Effects of Z-VAD-FMK (ZVAD) on EtxB-induced NO production. The bars indicate the geometric means (Gmean) for DAF-FM fluorescence of cells treated as indicated, and the error bars indicate standard errors of the means. The data are representative of the data obtained in four similar experiments.
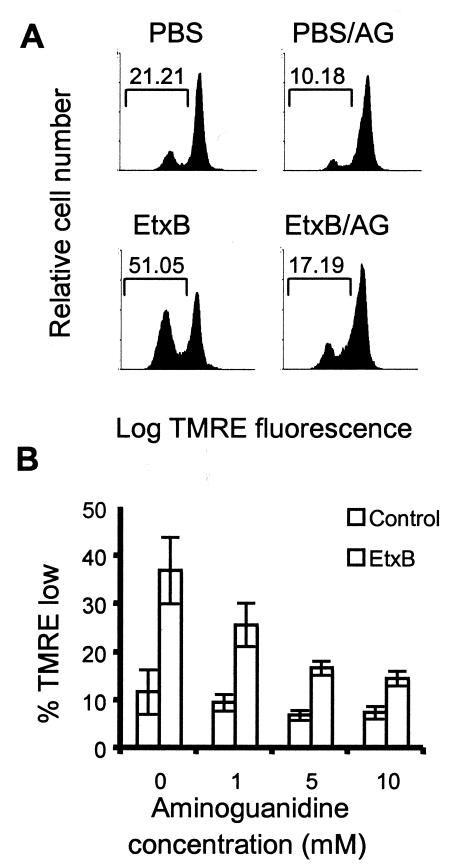
In order to determine the role of NO in EtxB-induced signaling, we used a potent inhibitor of nitric oxide synthase, aminoguanidine. Thus, CD8+ T cells were preincubated with 50 μM Z-VAD-FMK in the presence or absence of a range of doses of aminoguanidine, and they were then treated with PBS alone or EtxB for 5 h. Loss of Δψm was assessed following staining of cells with TMRE as described above. The data indicated that in the presence of caspase inhibition, aminoguanidine inhibited Δψm dissipation in a dose-dependent fashion (Fig. 6). Following preincubation of cells with 10 mM aminoguanidine, the level of TMRElow-stained cells in an EtxB-treated culture was similar to background levels. Aminoguanidine also inhibited the loss of TMRE fluorescence in control cultures. Flow cytometry revealed that preincubation of cells with aminoguanidine did not affect the levels of EtxB binding (data not shown). Therefore, the data suggest that EtxB-induced Δψm dissipation is mediated via activation of nitric oxide synthase (NOS) in CD8+ T cells.
FIG. 6.
Aminoguanidine inhibits EtxB-induced Δψm dissipation. (A) Representative FACS plots of TMRE-stained CD8+ T cells preincubated with Z-VAD-FMK in the presence or absence or 10 mM aminoguanidine (AG) and then treated with PBS or EtxB for 6 h. (B) Dose response of the inhibition of EtxB-induced Δψm dissipation mediated by aminoguanidine. The data are means ± standard errors of the means (n = 4).
DISCUSSION
These studies showed that receptor cross-linking by EtxB triggers multiple processes within CD8+ T cells that lead to cell death. In addition to the cascade that was previously reported to lead to caspase-dependent apoptosis of the cells, EtxB causes loss of mitochondrial integrity in a NOS-dependent manner.
EtxB induces degradation of DNA and PARP in CD8+ T cells following receptor interaction. The broad-spectrum caspase inhibitor Z-VAD-FMK inhibited both of these processes. However, this inhibitor did not prevent cell death or loss of Δψm. In parallel experiments, we tested the effects of additional broad-spectrum and enzyme-selective caspase inhibitors on EtxB-induced cell death and obtained very similar results (data not shown). While it is formally possible that EtxB treatment of CD8+ T cells induces activation of a caspase that is not inhibited by Z-VAD-FMK, the results which we obtained with additional, structurally distinct inhibitors strongly support our view that caspase activity is responsible for the induction of nuclear apoptosis but not all features of cell death.
The loss of mitochondrial membrane potential in CD8+ T cells, as measured by DiOC6(3) or TMRE staining and FACS analysis, was very rapid, and kinetic studies showed that Δψm dissipation was induced within 3 h. This is much more rapid than the activation of caspases during EtxB-induced apoptosis (31). We reasoned that additional caspase-independent signals must be responsible for the effects of EtxB on mitochondria. Our data indicate that the early loss of Δψm induced by EtxB is not associated with changes in expression or localization of Bax and Bcl-2, suggesting that these proteins are not involved in this process. By contrast, treatment of cells with EtxB induced increased NO production, and additional experiments indicated that aminoguanidine abrogated EtxB-induced dissipation of Δψm, implicating NOS in this event. It is important that we also assessed the levels of cell death and loss of mitochondrial membrane potential at later times and that the data indicate that after 48 h of incubation, 100% of CD8+ T cells underwent cell death in response to EtxB (data not shown).
Nitric oxide is a key mediator of physiological and pathological processes. The role of NO in cell death is particularly complicated, and this signaling molecule is known to have both pro- and antiapoptotic properties, depending on the cellular context, the concentration, and the source from which it is derived (38). A low level of NO can be critical in maintaining cell viability, while high levels are directly toxic to normal mitochondrial function. In this regard, NO directly binds to and inhibits cytochrome oxidase, and NO derivatives, such as peroxynitrite, irreversibly damage mitochondrial complexes I, II, IV, and V (5). Constitutive low levels of NO are generated by NOS I and NOS III, whereas the high levels that function as a cytostatic or cytotoxic molecule are usually the result of the activity of inducible NOS (iNOS) (NOS II). Our data indicated that a NOS inhibitor potently inhibited Δψm dissipation, as measured after 6 h of culture in the presence of EtxB. The NOS inhibitor that we used in our studies, aminoguanidine, has been reported to be a selective iNOS inhibitor. The 50% inhibitory concentrations of aminoguanidine indicate that it is between 20- and 30-fold more effective at inhibiting iNOS than at inhibiting constitutive NOS depending on the substrate used in cell-free systems. However, the precise doses that inhibit iNOS and constitutive NOS are different in whole-cell assays and are likely to vary depending on the cell type studied. As a result, it is not possible to conclude whether the NO that triggered cell death in our experiments resulted from the enhanced activity of constitutive NOS or resulted from the generation of iNOS. At 6 h there were no differences in the levels of cell death between EtxB- and PBS-treated cell cultures. Unfortunately, culture of CD8+ T cells overnight with NOS inhibitors triggered cell death, reflecting the critical role of NO in maintaining viability (data not shown). As a result, it was not possible to directly demonstrate that NOS was the cause of cell death following receptor interaction with EtxB. In this regard, while compromising mitochondrial function is a classical trigger of cell death, there is evidence that cells can recover from a transient loss of mitochondrial membrane potential (37). Despite this, it is highly likely that in our system, compromised mitochondrial function as a result of NOS activity was the reason that the cells died. Following exposure to EtxB the mitochondrial membrane potential dropped rapidly and remained low throughout the period over which cell viability was lost.
How might EtxB trigger enhanced NOS activity within CD8+ T cells? It is clear that regulation of both the expression and the activity of the three NOS isoforms is extremely complicated. Expression of NOS I is thought to be restricted to neuronal tissue. NOS III is classically referred to as endothelial NOS, but it is clearly also expressed by other cell types, including T cells (44). While NOS I and NOS III are considered to be constitutively expressed proteins, data indicate that these isoforms are also regulated at the level of protein expression (11). Furthermore, expression of iNOS can be differentially regulated by the same signal. In this regard, data indicate that cyclic AMP can have opposite effects on iNOS expression depending on the stimulus and cell type studied (13). In addition, sites for multiple transcription factors, including NF-κB, CREB and C/EBP, are known to present in the promoter regions of the iNOS gene (13). Despite these complications, data indicate that activation of NOS I and NOS III is regulated by calcium signals (5). EtxB does not trigger calcium flux in CD8+ T cells within 20 min, suggesting that it is not involved in the processes observed here. However, it is conceivable that low levels of Ca2+ or late changes in the Ca2+ level could be involved. Unfortunately, treatment with the calcium chelating agent 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetate is directly toxic to T cells, causing changes in mitochondrial membrane potential (R. Pitman, S. Fraser, R. Brownlie, and N. Williams, unpublished data). In addition, while we have reported that EtxB causes translocation of NF-κB to the nucleus and upregulates its transcriptional activity, a potent inhibitor of NF-κB activation, SN50, did not block mitochondrial membrane changes or cell death (data not shown). This suggests that if cell death results from upregulation of iNOS, then this occurs in an NF-κB-independent manner. As a result of these findings, the mechanism by which EtxB triggers NO production remains a focus of investigation. For this purpose it would be interesting to study the effects of EtxB on CD8+ T cells derived from mice genetically modified to be deficient in specific NOS isoforms. Similarly, in future experiments the requirement for specific caspases in EtxB-induced nuclear apoptosis could be analyzed by using caspase knockout mice.
Although NF-κB may not be involved in regulating alterations to NOS activity in EtxB-treated CD8+ T cells, previous studies demonstrated that NF-κB is an upstream mediator of caspase activation in EtxB-induced apoptosis (31). Thus, it was shown that a peptide inhibitor of NF-κB inhibited DEVDase activation and hypodiploidy in EtxB-treated cells, while more definitively, EtxB-induced apoptosis was abrogated in cells from transgenic mice expressing a dominant negative version of the I-κB protein. This indicates that following receptor binding, EtxB triggers two distinct signaling pathways: (i) an NF-κB/caspase-dependent pathway that results in nuclear degradation and apoptosis, and (ii) a caspase-independent pathway, mediated via the activation of NOS, that induces mitochondrial dysfunction.
In conclusion, the data presented in this paper demonstrate that receptor binding by EtxB induces caspase-dependent and -independent events that contribute to CD8+ T-cell death. Furthermore, our findings provide additional evidence for the emerging paradigm indicating that inhibition of caspases following a proapoptotic insult may be insufficient to prevent cell death. The finding that EtxB triggers upregulated NOS activity is of interest in the context of the ability of this molecule to modulate immune responses. NO is a potent inhibitor of IL-2 and gamma interferon production and has been shown to enhance IL-4 expression (18). It is interesting that administration of EtxB in vivo potently suppresses gamma interferon production in autoimmune disease models (23, 46). Furthermore, the adjuvant activity of EtxB is associated with downregulation of Th1-associated antibodies and upregulation of Th2-dependent immunoglobulin G subclasses (29). Studies which investigate the role of NO in mediating the immune modulation by EtxB would therefore be of considerable interest.
Acknowledgments
This work was supported by a grant from the Biotechnology and Biological Sciences Research Council. N.A.W. is a Wellcome Trust Research Leave Fellow.
We thank Sylvia Fraser for her assistance in carrying out the measurements of NO levels.
Editor: J. D. Clements
REFERENCES
- 1.Akbar, A. N., N. J. Borthwick, R. G. Wickremasinghe, P. Panaliotidis, D. Pilling, M. Bofill, S. Krajewski, J. C. Reed, and M. Salmon. 1996. Interleukin-2 receptor common γ-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of antiapoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur. J. Immunol. 26:294-299. [DOI] [PubMed] [Google Scholar]
- 2.Bone, H., S. Eckoldt, and N. A. Williams. 2002. Modulation of B lymphocyte signaling by the B subunit of Escherichia coli heat-labile enterotoxin. Int. Immunol. 14:647-658. [DOI] [PubMed] [Google Scholar]
- 3.Braun, M. C., J. He, C.-Y. Wu, and B. L. Kelsall. 1999. Cholera toxin suppresses interleukin (IL)-12 production and IL-12 receptor βand βchain expression. J. Exp. Med. 189:541-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng, P. C., M. L. Dykstram R.N. Mitchell, and S. K. Pierce. 1999. A role for lipid rafts in B cell receptor signaling and antigen targeting. J. Exp. Med. 190:1549-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman, J. W. 2001. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 1:1397-1406. [DOI] [PubMed] [Google Scholar]
- 6.Cong, Y., A. O. Oliver, and C. O. Elson. 2001. Effects of cholera toxin on macrophage production of co-stimulatory cytokines. Eur. J. Immunol. 31:64-71. [DOI] [PubMed] [Google Scholar]
- 7.Deas, O., C. Dumont, M. MacFarlane, M. Rouleau, C. Hebib, F. Harper, F. Hirsch, B. Charpentier, G. M. Cohen, and A. Senik. 1998. Caspase-independent cell death induced by anti-CD2 or staurosporine in activated human peripheral T lymphocytes. J. Immunol. 161:3375-3383. [PubMed] [Google Scholar]
- 8.Douce, G., C. Turcotte, I. Cropley, M. Pizza, M. Domenghini, R. Rappuoli, and G. Dougan. 1995. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc. Natl. Acad. Sci. USA 92:1644-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elson, C. O., S. P. Holland, M. T. Dertzbaugh, C. F. Cuff, and A. O. Anderson. 1995. Morphologic and functional alterations of mucosal T cells by cholera toxin and its B subunit. J. Immunol. 154:1032-1040. [PubMed] [Google Scholar]
- 10.Fernandes-Alnemri, T., A. Takahashi, R. C. Armstrong, J. Krebs, L. C. Fritz, K. J. Tomaselli, L. Wang, Z. Lu, C. M. Croce, G. Salvesen, and E. S. Alnemri. 1995. Mch3, a novel human apoptotic cysteine protease highly related to CPP32. Cancer Res. 55:6045-6052. [PubMed] [Google Scholar]
- 11.Forstermann, U., J. P. Boissel, and H. Kleinert. 1998. Expressional control of the ′constitutive' isoforms of nitric oxide synthase (NOS I and NOS III). FASEB J. 12:773-790. [PubMed] [Google Scholar]
- 12.Francis, M. L., J. Ryan, M. G. Jobling, R. K. Holmes, J. Moss, and J. J. Mond. 1992. Cyclic AMP-independent effects of cholera toxin on B cell activation. II. Binding of ganglioside GM1 induces B cell activation. J. Immunol. 148:1999-2005. [PubMed] [Google Scholar]
- 13.Galea, E., and D. L. Feinstein. 1999. Regulation of the expression of the inflammatory nitric oxide synthase (NOS2) by cyclic AMP. FASEB J. 13:2125-2137. [DOI] [PubMed] [Google Scholar]
- 14.Giuliani, M. M., G. Del Giudice, V. Giannelli, G. Dougan, G. Douce, R. Rappuoli, and M. Pizza. 1998. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J. Exp. Med. 187:1123-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu, Y.-T., K. G. Wolter, and R. J. Youle. 1997. Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Proc. Natl. Acad. Sci. USA 94:3668-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janes, P. W., S. C. Ley, and A. I. Magee. 1999. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 147:447-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jurgensmeier, J. M., Z. Xie, Q. Deveraux, L. Ellerby, D. Bredesen, and J. C. Reed. 1998. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 95:4997-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolb, H., and V. Kolb-Bachofen. 1998. Nitric oxide in autoimmune disease: cytotoxic or regulatory mediator? Immunol. Today 19:556-561. [DOI] [PubMed] [Google Scholar]
- 19.Kroemer, G., and J. C. Reed. 2000. Mitochondrial control of cell death. Nat. Med. 6:513-516. [DOI] [PubMed] [Google Scholar]
- 20.Kumar, S., A. Bharti, N. C. Mishra, D. Raina, S. Kharbanda, S. Saxena, and D. Kufe. 2001. Targeting of the c-Abl tyrosine kinase to mitochondria in the necrotic cell death response to oxidative stress. J. Biol. Chem. 276:17281-17285. [DOI] [PubMed] [Google Scholar]
- 21.Langlet, C., A.-M. Bernard, P. Drevot, and H.-T. He. 2000. Membrane rafts and signaling by the multichain immune recognition receptors. Curr. Opin. Immunol. 12:250-255. [DOI] [PubMed] [Google Scholar]
- 22.Lazebnik, Y. A., S. H. Kaufmann, S. Desnoyers, G. G. Poirier, and W. C. Earnshaw. 1994. Reconstitution of the apoptotic cascade in vitro: Pivotal role for prICE, a protease resembling interleukin-1β converting enzyme, and demonstration that poly(ADP ribose) polymerase is a substrate for this enzyme during apoptosis. Nature 371:346-347. [DOI] [PubMed] [Google Scholar]
- 23.Luross, J. A., T. Heaton, T. R. Hirst, M. J. Day, and N. A. Williams. 2002. Escherichia coli heat-labile enterotoxin B subunit prevents autoimmune arthritis through induction of regulatory CD4+ T cells. Arthritis Rheum. 46:1671-1682. [DOI] [PubMed] [Google Scholar]
- 24.Matsumura, H., Y. Shimizu, Y. Ohsawa, A. Kawahara, Y. Uchiyama, and S. Nagata. 2000. Necrotic death pathway in Fas receptor signaling. J. Cell Biol. 151:1247-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nashar, T. O., N. A. Williams, and T. R. Hirst. 1996. Cross-linking of cell surface ganglioside GM1 induces the selective apoptosis of mature CD8+ T lymphocytes. Int. Immunol. 8:731-736. [DOI] [PubMed] [Google Scholar]
- 26.Nashar, T. O., H. M. Webb, S. Eaglestone, N. A. Williams, and T. R. Hirst. 1996. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc. Natl. Acad. Sci. USA 93:226-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nashar, T. O., T. R. Hirst, and N. A. Williams. 1997. Modulation of B-cell activation by the B-subunit of Escherichia coli heat-labile enterotoxin: receptor interaction upregulates MHC class II, B7, CD40, CD25 and ICAM-1. Immunology 91:572-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholls, D. G., and S. L. Budd. 2000. Mitochondria and neuronal survival. Physiol. Rev. 80:315-360. [DOI] [PubMed] [Google Scholar]
- 29.Richards, C. M., A. T. Aman, T. R. Hirst, T. J. Hill, and N. A. Williams. 2001. Protective mucosal immunity to ocular herpes simplex virus type 1 infection in mice by using Escherichia coli heat-labile enterotoxin B subunit as an adjuvant. J. Virol. 75:1664-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rottenberg, H., and S. Wu. 1998. Quantitative assay by flow cytometry of the mitochondrial membrane potential in intact cells. Biochim. Biophys. Acta 1404:393-404. [DOI] [PubMed] [Google Scholar]
- 31.Salmond, R. J., R. S. Pitman, E. Jimi, M. Soriani, T. R. Hirst, S. Ghosh, M. Rincon, and N. A. Williams. 2002. CD8+ T cell apoptosis induced by Escherichia coli heat-labile enterotoxin occurs via a novel pathway involving NF-kappaB-dependent caspase activation. Eur. J. Immunol. 32:1737-1747. [DOI] [PubMed] [Google Scholar]
- 32.Salmond, R. J., R. Williams, T. R. Hirst, and N. A. Williams. 2003. Selective induction of CD8+ CD4− thymocyte apoptosis by the B-subunit of Escherichia coli heat-labile enterotoxin. Immunol. Lett. 88:43-46. [DOI] [PubMed] [Google Scholar]
- 33.Soriani, M., N. A. Williams, and T. R. Hirst. 2001. Escherichia coli heat-labile enterotoxin B subunit triggers apoptosis of CD8+ T cells by activating transcription factor c-Myc. Infect. Immun. 69:4923-4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spangler, B. D. 1992. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol. Rev. 56:622-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun, J.-B., J. Holmgren, and C. Czerkinsky. 1994. Cholera toxin B subunit: an efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc. Natl. Acad. Sci. USA 91:10795-10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun, J.-B., C. Rask, T. Olsson, J. Holmgren, and C. Czerkinsky. 1996. Treatment of experimental autoimmune encephalomyelitis by feeding myelin basic protein conjugated to cholera toxin B subunit. Proc. Natl. Acad. Sci. USA 93:7196-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szalai, G., R. Krischnamurthy, and G. Hajnoczky. 1999. Apoptosis driven by IP3-linked mitochondrial calcium signals. EMBO J. 18:6349-6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor, E. L., I. L. Megson, C. Haslett, and A. G. Rossi. 2003. Nitric oxide: a key regulator of myeloid inflammatory cell apoptosis. Cell Death Differ. 10:418-430. [DOI] [PubMed] [Google Scholar]
- 39.Tewari, M., L. T. Quan, K. O'Rourke, S. Desnoyers, Z. Zeng, D. R. Beidler, G. G. Poirier, G. Salvesen, and V. M. Dixit. 1995. Yama/CPP32β, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell 81:801-810. [DOI] [PubMed] [Google Scholar]
- 40.Truitt, R. L., C. Hanke, J. Radke, R. Mueller, and J. T. Barbieri. 1998. Glycosphingolipids as novel targets for T-cell suppression by the B subunit of recombinant heat-labile enterotoxin. Infect. Immun. 66:1299-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turcanu, V., T. R. Hirst, and N. A. Williams. 2002. Modulation of human monocytes by Escherichia coli heat-labile enterotoxin B-subunit; altered cytokine production and its functional consequences. Immunology 106:316-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vercammen, D., R. Beyaert, G. Denecker, V. Goossens, G. Van Loo, W. Declerq, J. Grooten, W. Fiers, and P. Vandenabeele. 1998. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187:1477-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vercammen, D., G. Brouckaert, G. Denecker, M. van de Craen, W. Declerq, W. Fiers, and P. Vandenabeele. 1998. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J. Exp. Med. 188:919-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams, M. S., S. Noguchi, P. A. Henkart, and Y. Osawa. 1998. Nitric oxide synthase plays a signaling role in TCR-triggered apoptotic death. J. Immunol. 161:6526-6531. [PubMed] [Google Scholar]
- 45.Williams, N. A., T. R. Hirst, and T. O. Nashar. 1999. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol. Today 20:95-101. [DOI] [PubMed] [Google Scholar]
- 46.Williams, N. A., L. M. Stasiuk, T. O. Nashar, C. M. Richards, A. K. Lang, M. J. Day, and T. R. Hirst. 1997. Prevention of autoimmune disease due to lymphocyte modulation by the B-subunit of Escherichia coli heat-labile enterotoxin. Proc. Natl. Acad. Sci. USA 94:5290-5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiang, J., D. T. Chao, and S. J. Korsmeyer. 1996. BAX-induced cell death may not require interleukin 1β-converting enzyme-like proteases. Proc. Natl. Acad. Sci. USA 93:14559-14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yankelevich, B., V. A. Soldatenov, J. Hodgson, A. J. Polotsky, K. Cresswell, and A. Mazumder. 1996. Differential induction of programmed cell death in CD8+ and CD4+ T cells by the B subunit of cholera toxin. Cell. Immunol. 168:229-235. [DOI] [PubMed] [Google Scholar]