Abstract
Background
The acute respiratory distress syndrome (ARDS) is a life-threating disorder that contributes significantly to critical illness. No specific pharmacological interventions directed at lung injury itself, have proven effective in improving outcome of patients with ARDS. Platelet activation was identified as a key component in ARDS pathophysiology and may provide an opportunity for preventive and therapeutic strategies. We hypothesize that use of acetyl salicylic acid (ASA) may prevent and/or attenuate lung injury.
Methods
We conducted a systematic review of preclinical studies and meta-analysis of clinical studies investigating the efficacy of ASA in the setting of lung injury. MEDLINE, EMBASE AND COCHRANE databases were searched.
Results
The literature search yielded 1314 unique articles. Fifteen pre-clinical studies and eight clinical studies fulfilled the in- and exclusion criteria.
In the animal studies, the overall effect of ASA was positive, e.g. ASA improved survival and attenuated inflammation and pulmonary edema. Mechanisms of actions involved, among others, are interference with the neutrophil-platelets interaction, reduction of leukotrienes, neutrophil extracellular traps and prostaglandins. High dose ASA may be the drug of choice.
A meta-analysis of 3 clinical studies showed an association between ASA use and a reduced incidence of ARDS (OR 0.59, 95% CI 0.36–0.98), albeit with substantial between-study heterogeneity. All studies had their own shortcomings in methodological quality.
Conclusion
This systematic review of preclinical studies and meta-analysis of clinical studies suggests a beneficial role for ASA in ARDS prevention and treatment. However, the currently available data is insufficient to justify an indication for ASA in ARDS. The body of literature does support further studies in humans.
We suggest clinical trials in which the mechanisms of action of ASA in lung injury models is being evaluated to guide optimal timing and dose, before prospective randomized trials.
Keywords: Aspirin, Acute Lung injury, ARDS, Intensive Care, Platelets
Introduction
The acute respiratory distress syndrome (ARDS) is a life-threating disorder that contributes significantly to critical illness (1). ARDS places a heavy burden on public health resources (2, 3). Recently, the period prevalence of ARDS was 10% of ICU admissions and the mortality approaches 40 percent (4). ARDS leads to approximately 75,000 deaths each year in the U.S. (1). To date, no specific pharmacological treatment directed at lung injury itself has been consistently effective in ARDS.
ARDS is characterized by uncontrolled inflammation and coagulation. Key factors in the development of ARDS are damage to the microvascular system with an increased capillary permeability and alveolar instability (5), massive accumulation of inflammatory cells in different compartments of the lungs (6) and pulmonary microvascular coagulopathy (7, 8). There is a highly conserved coupling of the coagulation cascade and the immune response in the modulation of inflammatory states and evidence shows that the interplay between platelets, leukocytes and endothelial cells is critical in the pathogenesis (9, 10) and resolution (11) of ARDS. Traditionally, platelets were viewed as mediators of hemostasis. However, emerging data suggests a major role for platelets in immune modulation, including both inflammatory and anti-inflammatory responses, as well as antimicrobial host defense (12). In line with this, there is substantial evidence that platelets and platelet mediators have an important role in the development of inflammatory conditions, e.g. in multiple organ failure (MOF) in septic patients (13–15). Several studies evaluating acetylsalicylic acid (ASA) for the treatment of sepsis in animal models have suggested a potential benefit (16, 17).
An important role for platelets in ARDS has also been described (12). This pivotal role for platelet activation in ARDS pathogenesis represents an opportunity for a treatment strategy with ASA, and several studies, human and animal, have been undertaken testing the role of platelet inhibition in ARDS. We chose to study one specific antiplatelet agent in ARDS to produce a more homogeneous analysis. ASA is the most used and studied antiplatelet therapy, has known anti-inflammatory effects, is relatively safe, easy to administer, and readily available.
In order to investigate the potential mechanisms by which ASA is beneficial in ARDS, we first systematically searched the literature for preclinical studies to identify mechanisms of action. Secondly, we conducted a meta-analysis on the available clinical studies to determine the effect of ASA on important clinical outcomes in ARDS (e.g. prevention, ventilator-free days, survival).
Materials and Methods
Study selection, Data Extraction and Quality Assessment
A systemic search strategy was conducted to capture published preclinical and clinical studies of ASA as (pre)treatment for ARDS.
Pubmed, Embase, and Cochrane databases were searched to identify relevant studies. The search strategies were adapted to accommodate the unique searching feature of each database-specific vocabulary words. Key words were “Respiratory Distress Syndrome, Adult”, Acute Respiratory Distress Syndrome, “ARDS”,” acute lung injury”, “ALI”, “Aspirin”, “ acetylsalicylic acid”, transfusion-related lung injury (TRALI), TRALI, antiplatelet therapy (APT). Eligible trials were identified through a full search in June 2014. An update search was run in September 2015 through electronic searches. For references of identified trials, hand searching was used.
Key inclusion criteria for human studies were (1) adult patients; (2) lung injury defined as ARDS or TRALI as clinical diagnosis or (3) with ARDS or TRALI as primary outcome measure; (4) analyzed association between ASA and ARDS or TRALI. Randomized controlled trials (RCT), observational cohort studies (retrospective and prospective) and case-control studies were included. For preclinical studies, the inclusion criteria were (1) a model of lung injury in a laboratory animal; (2) intervention with ASA or an ASA-derived compound; (3) any outcome was allowed. Only studies written in English were included.
All trials that appeared to fit the inclusion criteria were identified for full review by two reviewers (BP and PRT). Differences were resolved during consensus meetings with other reviewers (HJG and AMES). Four reviewers (BP, AMAS, PRT and HJG) independently assessed the methodological quality of selected clinical studies. The methodological quality of the preclinical studies was assed using the SYRCLE format (18) by three reviewers (BP, ML and PRT). The Newcastle-Ottawa Quality Assessment Scale (19) for cohort and case-control studies was used, and for clinical randomized trials, the Jadad score (20) was used. The level of evidence for clinical studies was assessed according to the Oxford 2011 Levels of Evidence (21). The Preferred Reporting items for systematic Reviews and Meta-analysis (PRISMA) checklist for reporting systematic reviews was used.
Data Synthesis and Statistical Analysis of Clinical Studies
Studies with sufficient similarity in study population, intervention and outcome measure were quantitatively analyzed in a meta-analysis. Adjusted odds ratios (ORs) were used to compute a pooled OR. Heterogeneity between studies was quantified using the I2 statistic, which ranges from 0% to 100% and describes the proportion of variation in treatment effect estimates that is due to genuine variation rather than sampling error. Higgins et al suggest describing I2 values of 25%, 50%, and 75% as low, moderate, and high, respectively (22). A random effects model with Cochran (Hedges) estimator was used to estimate a pooled effect size. Studies were weighted by the inverse of the standard error of the estimated (adjusted) effect, as derived from the 95% confidence intervals. Sensitivity analysis was performed by recalculating the summary statistics after removing single studies or groups of studies from the analysis based on characteristics of the study design (23). Publication bias was assessed using the Egger test for funnel plot asymmetry (24). All quantitative data analyses were performed with the R software for statistical computing version 3.2.0 and the metafor package (25).
Results
Study Selection and Study Characteristics
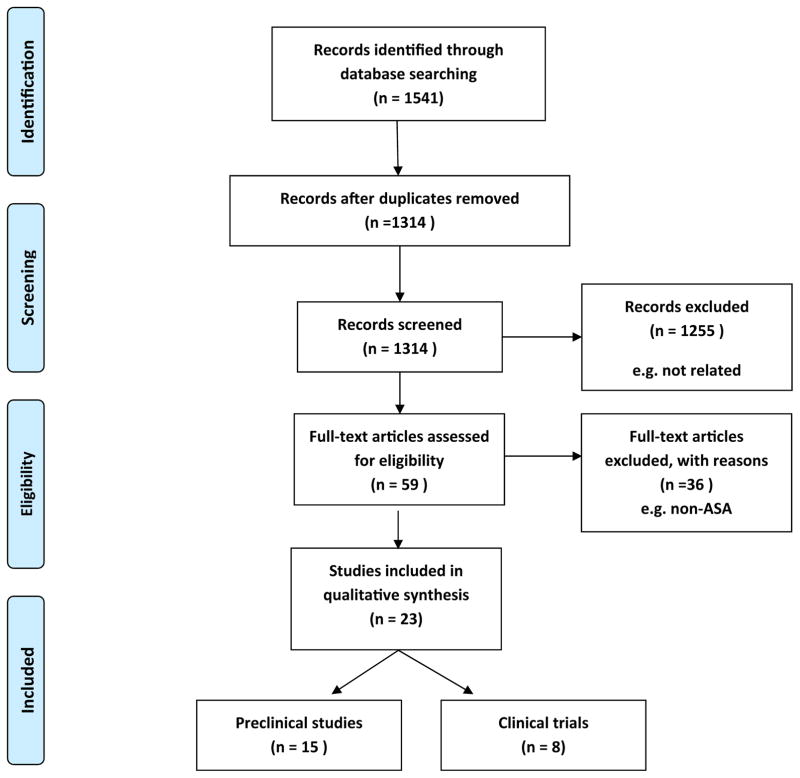
Figure 1 shows the procedure of our literature search and selection. Our initial query yielded 1541 potentially relevant records, of which 227 were excluded for duplicate records and another 1255 non-related studies were excluded according to the titles and abstracts. A total of 59 full-text studies were assed for eligibility after which 23 studies were included in this present meta-analysis.
Figure 1. Flow diagram showing the search strategy and selection process.
Preclinical studies
There were 15 preclinical studies (26–40) identified in this systematic review (Table 1). In 10 studies the antiplatelet drug was ASA (26–31, 35–38) in three studies ASA-triggered lipoxin (32, 33, 38), and in three studies ASA-triggered resolving D1 (34, 39, 40) were studied. In 12 preclinical studies (29–33, 35–40) a mouse model was used, in two preclinical studies a sheep model (27, 28) and in one study a dog model (26). Both direct and indirect models of acute lung injury were tested (table 1). In 8 studies (27–29, 31, 35–37, 39) a pretreatment model was used, in 4 studies (26, 32, 33, 40) a treatment model was used and 3 (30, 34, 38) studies consist of a combination of pretreatment and treatment for lung injury. All models induced lung injury, evidenced by an increase in lung edema and the appearance of neutrophils, proteins and inflammatory mediators in broncho-alveolar lavage fluid. In two studies respiratory mechanics (27, 28) and in three studies pulmonary and systemic hemodynamics were measured (26–28).
Table 1.
Pre-clinical studies investigating the role of acetylsalicylic acid in lung injury
| Reference | Lung injury model | Drug (dose; (pre)-treatment) | Animal (n/group) | Conclusion |
|---|---|---|---|---|
| Leeman et al[26] (1988) | Oleic acid iv | ASA (1 g iv; Treatment) | Dog (n=8 all groups) | ↑ PaO2, ↓venous admixture ↓ intra pulmonary shunt |
| Sigurdsson et al[27] (1989) | Ethanolamine oleate iv. | ASA (10 mg/kg, Pretreatment) | Sheep(n=7 vs 8 control) | prevent PHT prevent lung edema preserved PaO2 |
| Chelucci et al[28] (1992) | Oleic acid i.v. | ASA (10 mg/kg; Pretreatment) | Sheep (n=6 vs 8 control) | no morphological benefit no significant change of static compliance of respiratory system and on flow resistance of the airway at 3h |
| Gonçalves de Moraes et al[29] (1996) | LPS inhalation | ASA (50mg/kg; Pretreatment) | Mice (n=5 or 6) | ↑ inflammation |
| Fukunaga et al[30] (2005) | HCl intrabronchial | ASA (125mg/kg; Treatment and Pretreatment) | Mice (n=3–5) | ↓ inflammation |
| Zarbock et al[31] (2006) | HCL-intratracheal | ASA ( 1 g/kg; Pretreatment) | Mice (n=4–5) | ↓ inflammation ↑ PaO2/FiO2 (mmHg) |
| Jin et al[32] (2007) | LPS inhalation | AT-LX4 (0.7mg/kg; Treatment) | Mice (n=6) | ↓ lung edema ↓ microvascular permeability ↓ Inflammation ↑ survival ( at 72 h) |
| El Kebir et al[33] (2009) | Carrageenan + MPO intratracheal or E. coli intraperitoneal | AT-15 epiLXA4 (200 μg/kg; Treatment) | Mice (n=6–10) | ↓ inflammation |
| Eickmeier et al[34] (2013) | HCL intrabronchial | AT-RvD1 (0,5–5ug/kg; Treatment and Pretreatment) | Mice (n≥3–6) | ↓ lung edema ↓ resistance ↓ inflammation ↑restitution of lung- barrier function |
| Tuinman et al[35] (2013) | LPS intranasal | ASA(12.5 mg/kg) or 100 mg/kg; Pretreatment) | Mice (n=8 all groups) | ASA protects against ARDS. High dose ASA is superior to low-dose ASA. |
| Ortiz-Muñoz et al[38] (2014) | LPS intratracheal | ASA (100mg/kg Pretreatment with or without AT-15 epiLXA4 (100–5000ng; Pretreatment and Treatment) | Mice (n=3–10) | ↓ inflammation ↓ permeability |
| Two-event LPS-primed/MHC 1 mAb TRALI model | ASA (100mg/kg Pretreatment with or without AT-15 epiLXA4 (100–5000ng; Pretreatment and Treatment) | ↓inflammation lower dose: not effective |
||
| Tang et al[39] (2014) | Anti-BSA IgG intratracheal and iv. | AT-RvD1 (500 ng; Pretreatment) | Mice (n=3–5) | ↓ lung injury and mortality |
| Cox et al[40] ( 2015) | Hyperoxia | AT-RvD1 (100 ng; Treatment) | Mice (n=6–10) | ↓ inflammation ↓ permeability |
| Looney et al[36] (2009) | Two-event LPS-primed/MHC I mAb TRALI model | ASA (100mg/kg; Pretreatment) | Mice (n=4–10) | ↓ inflammation ↓ permeability ↓ mortality |
| Caudrillier et al[37] (2012) | Two-event LPS-primed/MHC I mAb TRALI model | ASA (100mg/kg; Pretreatment) | Mice (n=6–9) | ↓NETs (leading to ↓ lung injury) |
ARDS: acute respiratory distress syndrome; ASA: acetylsalicylic acid;AT-LXA4: ASA-Triggered Lipoxin A4 ;AT-15 epiLXA4 : ASA-triggered 15-epi-lipoxin A4 ; AT-RvD1: ASA-triggered resolving D1; ; pAT-RvD1: 17R-hydroxy-19-para-fluorophenoxy-resolvin D1 methyl ester ; BALF-N: Broncho alveolar lavage fluid neutrophil; EVLW: extra vascular lung water; EVPE: lung vascular permeability; HCL: hydrochloric acid; IgG-IC: immune globulin G complex LPS: lipopolysaccharide; NET: neutrophil extravascular traps; Pap: pulmonary artery pressure; PHT: pulmonary hypertension; TRALI: transfusion-related cute lung injury; ↓: decrease; ↑: increase
Of the 15 preclinical trials, 13 reported a beneficial effect of antiplatelet drug on ARDS, (26, 27, 30–40) evidenced by improved oxygenation, diminished lung edema, inflammation and in some an increased survival (32, 36). No benefit was observed in one study (28) and in one study (29) worsening of inflammation was demonstrated.
Beneficial mechanisms of action of ASA in preclinical studies
Leeman et al reported a beneficial effect of ASA (26). The involved mechanism was further described in an subsequent study as a decrease of prostaglandin, leading to a reduced intrapulmonary shunt and increased oxygenation (41). Zarbock et al found that blocking of thromboxane (TX)-A2 by ASA pretreatment, attenuated platelet-neutrophil interactions, which resulted in significantly improved oxygenation and reduced neutrophil recruitment (31). Kebir et al found that ASA-triggered 15-epi-LXA4 (15-epi-LXA4) and its metabolic stable analog (ATL) reduced inflammation and attenuated edema formation (33). Furthermore, 15-epi-LXA4 accelerates the resolution of ARDS, which was the result of overriding MPO suppression of apoptosis of neutrophils by blocking a β2-integrin–mediated outside-in signaling. Fukunaga et al attributed the beneficial effects of ASA to the increased formation of 15-epi-LXA4 by ASA-acetylated cyclooxygenase (COX)-2 in injured lungs (30). Jin et al demonstrated that ATL inhibited tumor necrosis factor-α (TNF-α), intrapulmonary nitric oxide (NO) and malondialdehyde (MDA) production (32). In addition, these authors demonstrated that ATL markedly induced pulmonary heme oxygenase 1 (HO-1), which has been implicated in cytoprotective defense (42, 43). Ortiz-Muñoz et al (38) also demonstrated in both an LPS-ALI and a two-event model of TRALI that either ASA or 15-epi-LXA4 decreased neutrophil recruitment and activity in the airspace, decreased neutrophil-platelet aggregates in the lung and attenuated lung permeability (38). In this study it was shown that ASA not only decreased the production of the pro-inflammatory TXB2 but, as described earlier by Fukunaga et al (30), also increased the production of the anti-inflammatory 15-epi-LX A4. Here, it was also demonstrated that blocking of the lipoxin receptor, or using mice deficient in this receptor, mitigated the beneficial effect of ASA and 15-epi-LXA4. Eickmeier et al reported that aspirin-triggered resolving D1 (AT-RvD1), significantly reduced mucosal inflammation and promoted resolution, whether AT-RvD1 was administered as pretreatment or treatment approach (34). Decreased leukocyte recruitment and interaction between leukocytes and platelets was found. Furthermore, AT-RvD1 enhanced restitution of barrier function after ARDS. Tang et al also found a beneficial effect of AT-RvD1 and pAT-RvD1 (39). Here, it was also demonstrated that AT-RvD1 and pAT-RvD1 significantly reduced the pro-inflammatory peptide complement C5, inhibited the activities of the inflammatory transcription factors NF-κB and C/EBPβ in lung and alveolar macrophages, suppressed alveolar macrophage and neutrophil TNF-α and IL-6 production and also decreased macrophage inflammatory protein (MIP). When AT-RvD1 was administered after induction of lung injury, Cox et al found a decrease in lipid peroxidation, suggestive for resolution of oxidative stress (40). Mice treated with AT-RvD1 had significantly higher glutathione in lung tissue. Post-treatment with AT-RvD1 also improved lung mechanics evidenced by reduced lung resistance. Furthermore, AT-RvD1 enhanced the clearance of inflammatory cell infiltrate and reduced pulmonary edema and permeability. A decrease of the pro-inflammatory transcriptional regulator NF-κB activity was found, similar to the finding of Tang et al (39). Cox et al demonstrated that AT-RvD1 treatment resulted in an overall decrease in phosphorylation of Mitogen-activated protein kinases, which are critical mediators for the activation of transcription factors, such as NF-κB. Furthermore, a decrease of hyperoxia-induced apoptosis was indicated by the decreased apoptosis indicator Caspase-3 (40). Also, increased phosphorylation of the proapoptotic BH3-only proteins BAD in the presence of AT-RvD1 resulted in attenuated apoptosis. Tuinman et al found that high-dose ASA was superior to low-dose ASA, clopidogrel, and to a combination of clopidogrel and low-dose ASA in ameliorating LPS-induced lung injury, possibly through a combination of COX-1 and COX-2 inhibition (35). Looney et al demonstrated that treatment with ASA decreased plasma TXB2 levels and platelet sequestration in the lungs in a two-event TRALI model (36). In a subsequent study, Caudrillier et al reported that treatment with ASA, through inhibition of TXA2 signaling, decreased neutrophil extracellular traps (NETs) and subsequent lung injury (37). Sigurdsson et al demonstrated that ASA pretreatment prevented pulmonary hypertension and lung edema (27). The potential mechanism was not described. Table 2 summarizes potential beneficial mechanisms of ASA protection in relation to the known pathophysiology involved in ARDS.
Table 2.
beneficial mechanisms of ASA in pre-clinical studies investigating the role of acetylsalicylic acid in lung injury in relation to the pathophysiology in ARDS
| Study | Pathophysiology in ARDS | Beneficial mechanism of ASA in ARDS |
|---|---|---|
|
| ||
| Edema and Inflammation | ↓ edema and inflammation by: | |
| Eickmeier et al[34] (2013) | - improved epithelial and endothelial barrier integrity through ↓ leukocyte lung recruitment and regulation of vasoactive mediators | |
| Jin et al[32] (2007) | - ↑HO-1 protein (cytoprotective effects) | |
|
Zarbock et al[31] (2006) Caudrillier et al[37] (2012) |
- ↓ TXA2 | |
| Eickmeier et al[34] (2013) | - ↓ NETs through ↓ TXA2 | |
| Jin et al[32] (2007) | - inhibiting neutrophil – platelet heterotypic interactions by ↓ P-selectin and its ligand CD24; ↑ HO-1 protein it ↓ TNF-α,↓ NO, ↓ MDA leading to a mitigation of neutrophilic lung inflammation |
|
| Zarbock et al[31] (2006) | - ↓ neutrophil recruitment by ↓ TXA2 | |
| Tuinman et al[35] (2013) | - ↓ neutrophil activation by ↓ TXA2 | |
| Ortiz-Muñoz et al[38] (2014) | - ↓ influx neutrophils through inhibition of COX-1 and -2 | |
|
| ||
| Leeman et al[26] (1988) | Increased intrapulmonary shunt | ↓ intrapulmonary shunt by: - accentuating hypoxic pulmonary vasoconstriction; presumed mechanism are ↓ vasodilating prostacyclin or ↑ vasoconstricting leukotrienes |
|
| ||
| Not reported | Increased pulmonary dead space | - decrease in microvascular thrombi |
|
| ||
| Sigurdsson et al[27] (1989) | Pulmonary hypertension | through attenuating increased vascular tone |
|
| ||
| - | Microvascular thrombosis | Not reported by the included studies |
|
| ||
| Resolution of inflammation and fibrosis | ↑ resolution by: | |
| Fukunaga et al[30] (2005) | - ↑ COX-2 mediated anti-inflammatory LX | |
| Kebir et al[33] (2009) | - overriding antiapoptosis signal from MPO | |
| Eickmeier et al[34] (2013) | - through resolvin D1 | |
| Jin et al[32] (2007) | - through lipoxins generated HO-1 protein | |
HO-1 protein: Heme oxygenase-1 protein; TXA2: thromboxane2; NET: neutrophil extracellular traps; IL-1β: interleukin-1β; IL-6:interleukin-6; TNFα: tumor necrosis factor-α; NF-κB p65: nuclear factor-κB p65; NO:nitric oxide ; MDA: malondialdehyde; PGE2: prostaglandin E2; COX-2: cyclooxygenase-2; MPO: myeoloperoxidase; ↑: increase; ↓: reduction
No benefit
In the study of Chelucci et al, ASA pretreatment reduced early pulmonary vasoconstriction, airway resistance, and early pulmonary gas exchange (28). However, the late increase in pulmonary artery pressure and airway resistance was not inhibited by ASA and the late severe deterioration of gas exchange was even exaggerated compared to controls. In the study of de Moraes it was shown that pretreatment with ASA increased the neutrophil number in the bronchial alveolar fluid (BALF) (29).
Quality assessment of preclinical studies
Two studies (32, 35) reported random allocation of animals to an experimental group (to reduce confounding), no studies reported blinded assessment of outcome measures (to reduce detection bias), no studies reported a statement of sample size calculation (to provide reassurance that studies were adequately powered and that repeated testing of accumulating data was not performed), two studies (32, 35) reported animals that were excluded from the analysis (to guard against attrition bias and the ad hoc exclusion of data), and nine studies (30, 31, 34–40) reported a statement of whether or not a conflict of interest exists. All studies had methodological shortcomings.
Clinical studies
Eight clinical studies were included in our analysis (44–51). Erlich et al performed a retrospective population-based cohort study with a medical ICU admission (47). Patients with at least one major ARDS risk factor were included in the study, which resulted 161 evaluable patients out of 14479 ICU admissions (1.1%). Patients with documented APT (ASA, clopidogrel, ticlopidine, cilostazol, dipyridamole or anagrelide) had a lower incidence of ARDS than those not on APT, and this risk reduction persisted after adjustment for baseline severity of illness, baseline risk of ARDS and the propensity for APT (APT associated RR 0.34, 95% CI 0.13–0.88); level III evidence. The same research group performed a secondary analysis of a prospective multicenter international cohort study (48). The study included 3855 adult nonsurgical patients with at least one ARDS risk factor admitted to the ICUs of 20 US hospitals and 2 hospitals in Turkey. Of these patients 6.2% developed ARDS and propensity-stratified analysis showed that ASA use was associated with a non-significant reduction in ARDS incidence (OR 0.70, 95% CI 0.48–1.03); level III evidence. O’Neal et al performed a cross-sectional analysis of 575 ICU patients (>= 40 years old without an acute cardiac diagnosis) in a single tertiary-care hospital (44). Using a logistic regression model, prehospital statin use was found to be associated with lower risk of developing ARDS, while prehospital ASA use was not (OR 0.86, 95% CI 0.51–1.44); level III evidence. This study was extended into a larger cohort of 1149 patients by Chen et al, (45) which also included the cohort of 575 patients published before(44). In contrast to the earlier findings, prehospital ASA was now found to be associated with decreased risk of ARDS (OR 0.66, 95% CI 0.46–0.94) in a propensity adjusted analysis; level II evidence. The authors concluded that the smaller cohort was likely underpowered to detect an ASA effect or that the effect may have been confounded by statin use. Noteworthy in this extended cohort was the finding that concomitant statin use was not beneficial for the prevention of ARDS, which was in contrast to the finding in the smaller cohort of O’Neal et al (44) but in line with the recent published study of Boyle et al (46).
In a retrospective cohort study, Mazzeffi et al (51) evaluated the association between preoperative aspirin use and the risk of ARDS in 375 adult patients after aortic valve replacement surgery. A propensity-adjusted analysis showed a non-significant reduction in ARDS risk among ASA users (OR 0.46, 95% CI 0.12–1.73); level III evidence. Boyle analyzed the association between ASA use and mortality in a convenience sample of 202 prospectively identified ARDS patients (46). Multivariate logistic regression indicated reduced mortality in patients with a history of ASA use (OR 0.38, 95% CI 0.15–0.96); level II evidence. Harr et al. identified a significant interaction between the number of transfused RBC units and APT in a cohort of 839 severely injured trauma patients (49). The interaction effect is difficult to quantify because only p-values of the relevant coefficients are reported (p=0.01 for lung dysfunction, p=0.03 for MOF); level III evidence. Tuinman et al evaluated the association between ASA and TRALI in a nested case-control study (50). After propensity adjustment, no association was found between ASA and the risk of TRALI after transfusion of platelets, plasma or red blood cells (aspirin associated OR for TRALI 0.91, 95% CI 0.49–1.69); level III evidence.
The only RCT on the effect of antiplatelet therapy (APT) for the prevention of ARDS was performed by Vincent et al in 1985 (52). In this small (N=33) double-blinded pilot RCT, patients with circulatory shock received either ASA plus dipyridamole or ASA plus placebo, the active comparator thus being the addition of dipyridamole, and was therefore excluded from our analysis. Of note, no benefits were found.
All observational studies scored equally well on the Newcastle-Ottowa Quality Assessment scale: 4 out of 4 on the selection scale, 2 out of 2 on the comparability scale, and 2 out of 3 on the outcome scale.
Meta-analysis of clinical studies
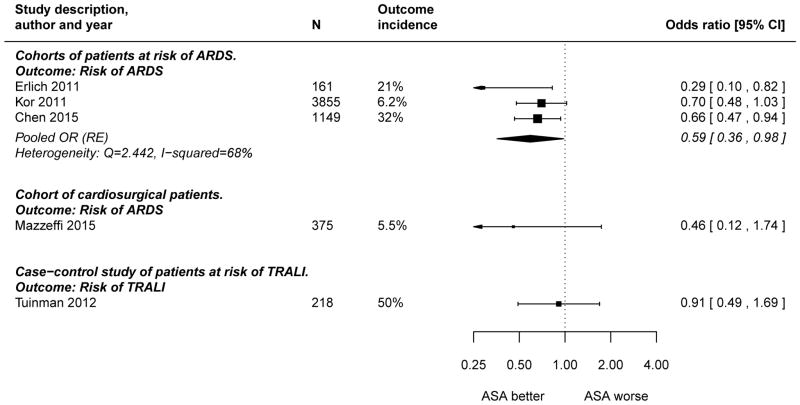
Figure 2 shows a Forest plot of the seven independent studies that can be quantified on the odds ratio scale. A pooled OR was only calculated for the three independent cohort studies that estimated the ASA effect on the risk of developing ARDS in mixed critically ill patients. The pooled OR was 0.59 (95% CI 0.36–0.98), with significant between-study heterogeneity (Q=2.44, I2 =68%). The Egger test for funnel plot asymmetry yielded a p-value of 0.12, but this test has very lower power with only three studies (data not shown). The study by Erlich et al. has the highest SE and the largest effect size and did not differentiate between ASA and other antiplatelet therapy (47). Eliminating this study from the meta-analysis did not fundamentally alter the results (pooled OR 0.67, 95% CI 0.52–0.87).
Figure 2. Forest plot of estimated associations between aspirin (ASA) use and adverse clinical outcomes in patients at risk of Acute Respiratory Distress Syndrome (ARDS) or Transfusion-Associated Acute Lung Injury (TRALI) or with established ARDS.
The cohort analyzed by O’Neal et al (44) was also included in a later and larger study by Chen et al (45) and was therefore excluded from the meta-analysis. Erlich et al reported an adjusted risk ratio (47), which was converted to OR using the study’s incidence rate of 0.20 (33/161), so that the RR of 0.34 (95% CI 0.13 to 0.88) converted to an OR of 0.29 (95% CI 0.10 to 0.82) (53). Harr et al only reported the p-value of the interaction coefficient of APT and the number of packed red blood cell transfusions on the lowered risk of lung dysfunction (p=0.0116), which was unusable for this meta-analysis (49).
Discussion
The meta-analysis of clinical studies is suggestive of a beneficial association between ASA and ARDS prevention. The systematic review of preclinical studies reveals several potential mechanisms of action of ASA as a preventive and therapeutic strategy in different lung injury models, indicating a solid pathophysiological base for the beneficial effect observed in the clinical studies. The strengths of this review are that we used a robust search strategy, we assessed the quality of included studies with validated tools for both preclinical and clinical studies, and the fact that we combine clinical data with preclinical data. In addition, we are the first to report a meta-analysis of clinical studies demonstrating an association between ASA and diminished risk of ARDS in critically-ill patients at risk for acute lung injury. Although our results are in line with data from studies with ASA in other inflammatory conditions, (54–56) the results of this meta-analysis should be interpreted with caution. General limitations of the clinical studies include lack of information regarding ASA dose, lack of control of prehospital vs. in hospital ASA use and the bias or confounding inherent to observational studies.
There was moderate between-study heterogeneity and publication bias cannot be ruled out. All studies were observational in design. Although these studies reported effects that were statistically adjusted for baseline risk and propensity of ASA use, there remains an important inherent risk of bias and confounding by indication. The external validity of the study by Erlich et al is limited by the small number of included patients as compared to all screened ICU admissions (inclusion rate 1.1%) and the homogenous population base (47). The study by Chen et al (45) is the largest study with a significant association between ASA and ARDS, but the cohort in this study has a much higher incidence of ARDS compared to the other large observational study by Kor et al (48) (32% vs. 6.2%). This sample enrichment results in an improved power to detect an ASA effect, but it may also limit the generalizability and external applicability of the findings. The positive results of the meta-analysis are supported by ample preclinical work in which both ASA pretreatment and treatment were effective approaches. The benefits include a reduction in intrapulmonary shunt (41), prevention of pulmonary hypertension (27), and reduced pulmonary edema and inflammation. In the pathogenesis of ARDS there is an induction of inflammatory mediators, inflammatory cells, expression of adhesion molecules, and enzymes, including TNF-α, IL-8, COX and lipoxygenase products, and reactive oxygen species (57). ASA attenuates these inflammatory mediators. The role of these inflammatory mediators in human ARDS has also been described (1). Another important benefit of ASA is the increased restitution of pulmonary barrier function and promotion of resolution of injury through lipoxins (58). Lipoxins are eicosanoids, generated during inflammation, that elicit distinct anti-inflammatory and pro-resolution bioactions (59, 60). ASA is the only nonsteroidal anti-inflammatory drug capable of triggering lipoxin formation. Tang et al provide evidence of the mechanism by which resolvins exert their beneficial effects (39). In TRALI, an important mechanism resulting in injury is the formation of NETs (37). The pro-inflammatory activity of NETs has also been described in humans (61). Inhibition of TXA2 signaling with ASA reduced NET formation. Interestingly, blockage of P-selectin, glycoprotein IIb/IIIa receptors (36, 62), or the P2Y12-receptor (63) on platelets does not confer the same benefit, indicating that outcomes are not simply related to platelet-neutrophil interactions, but that aspirin itself confers additional benefits. This is in line with the mechanisms described above. Notably, this review shows that ASA was not always beneficial. In the study of Chelluci et al. a biphasic behavior of ASA on gas exchange was observed, with a benefit in the early phase followed by a worsening of gas exchange (28). The study of Gonçalves et al observed an increase in inflammation with ASA treatment (29).
The impact of sources of bias, including publication bias, selection bias, and measurements bias was difficult to quantify. All preclinical studies had methodological shortcomings. However, our systematic review of the preclinical studies is important, so that unnecessary duplication of animal studies with ASA can be avoided. Gaps in the preclinical data include the determination of which drug is superior in the class of ASA compounds and optimal timing and duration of treatment. For example, a potential explanation for the difference in preclinical and clinical trial results, since the clinical studies were not universally positive, could be related to the ASA dose, timing, duration or the difference between species. The difference in effective ASA dose used in the animals models compared to the ASA dose in humans was pointed out by Tuinman et al (35). Whereas a low dose of ASA mainly blocks COX-1, ASA in a high dose also blocks COX-2. COX-1 inhibition results in anti-thrombotic effects through inhibiting TXA2 and platelet aggregation, whereas COX-2 inhibits inflammation (64). In the clinical studies, low-dose ASA was tested. In contrast, in all the animal studies, a high dose is used. This review can be used for hypothesis generation to inform the design and conduct of future studies with ASA. Although there appears to be an association between ASA use and the risk of lung injury from observational studies that are supported by ample preclinical work, the applicability of these findings is unclear. The highly enriched samples in some of the clinical studies are useful to detect an effect of ASA use but limit the external validity of the findings. Ultimately, the timing of ASA administration will be a critical factor in the design of future clinical studies that focus on prevention. We like to point out that there is a treatment paradigm shift to employ pharmacological interventions to patients at risk of ARDS (65). Thereby pretreatment with aspirin could be clinically relevant in patients who are at increased risk of developing ARDS. However, there is likely only a small window of opportunity to intervene with patients who are at high-risk for the development of ARDS. Furthermore, the pathophysiologic cascade of lung injury is likely well-advanced even in patients who do not meet the clinical definition of ARDS (66).
This systematic review points to a possible beneficial role for ASA in ARDS prevention and treatment. Future trials (67–69) that endeavour to more clearly define the role of ASA in the management of ARDS are justified. We also suggest that the mechanisms of ASA protection in lung injury be evaluated in “dose-finding “studies in humans to optimize the design of future randomized controlled trials.
Conclusion
The evidence for ASA protection in acute lung injury in preclinical studies is compelling and observational studies in aggregate also suggest promising results. However, the currently available data is insufficient to justify an indication for ASA in ARDS. The body of literature does support further mechanistic studies in humans and randomized controlled trials for clinical efficacy.
References
- 1.Ware LB, Matthay MA. The Acute Respiratory Distress Syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand J-A, Villar J, Thorsteinsson A, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30(1):51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 4.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 5.Murray JF. Mechanism of acute respiratory failure. NHLBI Conference report. Am Rev Respir Dis. 1977;115:1071–1078. doi: 10.1164/arrd.1977.115.6.1071. [DOI] [PubMed] [Google Scholar]
- 6.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364(20):1976–1977. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saldeen T. Trends in microvascular research. The microembolism syndrome. Microvasc Res. 1976;11(2):227–59. doi: 10.1016/0026-2862(76)90054-6. [DOI] [PubMed] [Google Scholar]
- 8.Greene R, Zapol WM, Snider MT, Reid L, Snow R, O’Connell RS, Novelline RA. Early bedside detection of pulmonary vascular occlusion during acute respiratory failure. Am Rev Respir Dis. 1981;124(5):593–601. doi: 10.1164/arrd.1981.124.5.593. [DOI] [PubMed] [Google Scholar]
- 9.Zarbock A, Ley K. The role of platelets in acute lung injury (ALI) Front Biosci (Landmark Ed. 2009;14:150–8. doi: 10.2741/3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarbock A, Polanowska-Grabowska RK, Ley K. Platelet-neutrophil-interactions: Linking hemostasis and inflammation. Blood Rev. 2007;21(2):99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Maderna P, Godson C. Lipoxins: resolutionary road. Br J Pharmacol. 2009;158(4):947–59. doi: 10.1111/j.1476-5381.2009.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadav HKD. Platelets in the pathogenesis of acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2015;309(9):915–923. doi: 10.1152/ajplung.00266.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaguchi A, Lobo FLM, Vincent J-L, Pradier O. Platelet function in sepsis. J Thromb Haemost. 2004;2(12):2096–102. doi: 10.1111/j.1538-7836.2004.01009.x. [DOI] [PubMed] [Google Scholar]
- 14.Katz JN, Kolappa KP, Becker RC. Beyond thrombosis: The versatile platelet in critical illness. Chest. 2011;139(3):658–668. doi: 10.1378/chest.10-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russwurm S, Vickers J, Meier-Hellmann A, Spangenberg P, Bredle D, Reinhart K, Lösche W. Platelet and leukocyte activation correlate with the severity of septic organ dysfunction. Shock. 2002;17(4):263–268. doi: 10.1097/00024382-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Hinshaw LB, Solomon LA, Erdös EG, Reins DA, Gunter BJ. Effects of acetylsalicylic acid on the canine response to endotoxin. J Pharmacol Exp Ther. 1967;157(3):665–71. [PubMed] [Google Scholar]
- 17.Halushka PV, Wise WC, Cook JA. Protective effects of aspirin in endotoxic shock. J Pharmacol Exp Ther. 1981;218(2):464–9. [PubMed] [Google Scholar]
- 18.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14(1):43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet] [cited 2015 Sep 1] Available from: http://www.ohri.ca/Programs/clinical_epidemiology/nosgen.pdf.
- 20.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence. Oxford Centre for Evidence-Based Medicine; http://www.cebm.net/index.aspx?o=5653. [Google Scholar]
- 22.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 2010;36(3) [Google Scholar]
- 26.Leeman M, Lejeune P, Hallemans R, Melot C, Naeije R. Effects of increased pulmonary vascular tone on gas exchange in canine oleic acid pulmonary edema. J Appl Physiol. 1988;65(2):662–668. doi: 10.1152/jappl.1988.65.2.662. [DOI] [PubMed] [Google Scholar]
- 27.Sigurdsson GH, Vallgren S, Christenson JT. Influence of aspirin and steroids on acute lung injury after i.v. Injection of a sclerosing agent. Acta Chir Scand. 1989;155(3):163–170. [PubMed] [Google Scholar]
- 28.Chelucci GL, Boncinelli S, Marsili M, Lorenzi P, Allegra A, Linden M, Chelucci A, Merciai V, Cresci F, Rostagno C. Aspirin effect on early and late changes in acute lung injury in sheep. Intensive Care Med. 1993;19(1):13–21. doi: 10.1007/BF01709272. [DOI] [PubMed] [Google Scholar]
- 29.Goncalves de Moraes VL, Boris Vargaftig B, Lefort J, Meager A, Chignard M. Effect of cyclo-oxygenase inhibitors and modulators of cyclic AMP formation on lipopolysaccharide-induced neutrophil infiltration in mouse lung. Br J Pharmacol. 1996;117(8):1792–6. doi: 10.1111/j.1476-5381.1996.tb15356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol. 2005;174(8):5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 31.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116(12):3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin S-W, Zhang L, Lian Q-Q, Liu D, Wu P, Yao S-L, Ye D-Y. Posttreatment with aspirin-triggered lipoxin A4 analog attenuates lipopolysaccharide-induced acute lung injury in mice: the role of heme oxygenase-1. Anesth Analg. 2007;104(2):369–77. doi: 10.1213/01.ane.0000252414.00363.c4. [DOI] [PubMed] [Google Scholar]
- 33.El Kebir D, József L, Pan W, Wang L, Petasis Na, Serhan CN, Filep JG. 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am J Respir Crit Care Med. 2009;180(4):311–9. doi: 10.1164/rccm.200810-1601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eickmeier O, Seki H, Haworth O, Hilberath JN, Gao F, Uddin M, Croze RH, Carlo T, Pfeffer Ma, Levy BD. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol. 2013;6(2):256–66. doi: 10.1038/mi.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuinman PR, Müller MC, Jongsma G, Hegeman Ma, Juffermans NP. High-dose acetylsalicylic acid is superior to low-dose as well as to clopidogrel in preventing lipopolysaccharide-induced lung injury in mice. Shock. 2013;40(4):334–8. doi: 10.1097/SHK.0b013e3182a384f0. [DOI] [PubMed] [Google Scholar]
- 36.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest. 2009;119(11):3450–61. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z, Looney MR. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122(7):2661–71. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortiz-Muñoz G, Mallavia B, Bins A, Headley M, Krummel MF, Looney MR. Aspirin-triggered 15-epi-lipoxin A4 regulates neutrophil-platelet aggregation and attenuates acute lung injury in mice. Blood. 2014;124(17):2625–34. doi: 10.1182/blood-2014-03-562876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang H, Liu Y, Yan C, Petasis NA, Serhan CN, Gao H. Protective actions of aspirin-triggered (17R) resolvin D1 and its analogue, 17R-hydroxy-19-para-fluorophenoxy-resolvin D1 methyl ester, in C5a-dependent IgG immune complex-induced inflammation and lung injury. J Immunol. 2014;193(7):3769–78. doi: 10.4049/jimmunol.1400942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cox R, Phillips O, Fukumoto J, Fukumoto I, Parthasarathy PT, Arias S, Cho Y, Lockey RF, Kolliputi N. Enhanced Resolution of Hyperoxic Acute Lung Injury as a result of Aspirin Triggered Resolvin D1 Treatment. Am J Respir Cell Mol Biol. 2015;53(3):422–35. doi: 10.1165/rcmb.2014-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leeman M, Delcroix M, Vachiéry JL, Mélot C, Naeije R. Blunted hypoxic vasoconstriction in oleic acid lung injury: effect of cyclooxygenase inhibitors. J Appl Physiol. 1992;72(1):251–8. doi: 10.1152/jappl.1992.72.1.251. [DOI] [PubMed] [Google Scholar]
- 42.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell Ra, Choi aM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6(4):422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 43.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: Unleashing the protective properties of heme. Trends Immunol. 2003;24(8):449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 44.O’Neal HR, Koyama T, Koehler EAS, Siew E, Curtis BR, Fremont RD, May AK, Bernard GR, Ware LB. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39(6):1343–50. doi: 10.1097/CCM.0b013e3182120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen W, Janz DR, Bastarache JA, May AK, O’Neal HR, Bernard GR, Ware LB. Prehospital Aspirin Use Is Associated With Reduced Risk of Acute Respiratory Distress Syndrome in Critically Ill Patients. Crit Care Med. 2015;1 doi: 10.1097/CCM.0000000000000789. (c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyle AJ, Di Gangi S, Hamid UI, Mottram L-J, McNamee L, White G, Cross LJM, McNamee JJ, O’Kane CM, McAuley DF. Aspirin therapy in patients with acute respiratory distress syndrome (ARDS) is associated with reduced intensive care unit mortality: a prospective analysis. Crit Care. 2015;19(1):109. doi: 10.1186/s13054-015-0846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erlich JM, Talmor DS, Cartin-Ceba R, Gajic O, Kor DJ. Prehospitalization antiplatelet therapy is associated with a reduced incidence of acute lung injury: A population-based cohort study. Chest. 2011;139(2):289–295. doi: 10.1378/chest.10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kor DJ, Erlich J, Gong MN, Malinchoc M, Carter RE, Gajic O, Talmor DS. Association of prehospitalization aspirin therapy and acute lung injury: Results of a multicenter international observational study of at-risk patients*. Crit Care Med. 2011;39(11):2393–2400. doi: 10.1097/CCM.0b013e318225757f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harr JN, Moore EE, Johnson J, Chin TL, Wohlauer MV, Maier R, Cuschieri J, Sperry J, Banerjee A, Silliman CC, et al. Antiplatelet Therapy Is Associated With Decreased Transfusion-Associated Risk of Lung Dysfunction, Multiple Organ Failure, and Mortality in Trauma Patients*. Crit Care Med. 2013;41(2):399–404. doi: 10.1097/CCM.0b013e31826ab38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuinman PR, Vlaar AP, Binnenkade JM, Juffermans NP. The effect of aspirin in transfusion-related acute lung injury in critically ill patients. Anaesthesia. 2012;67(6):594–9. doi: 10.1111/j.1365-2044.2011.07054.x. [DOI] [PubMed] [Google Scholar]
- 51.Mazzeffi M, Kassa W, Gammie J, Tanaka K, Roman P, Zhan M, Griffith B, Rock P. Preoperative Aspirin Use and Lung Injury After Aortic Valve Replacement Surgery: A Retrospective Cohort Study. Anesth Analg. 2015;121(2):271–7. doi: 10.1213/ANE.0000000000000793. [DOI] [PubMed] [Google Scholar]
- 52.Vincent JL, Brimioulle S, Berre J, Kahn RJ. Prevention of the adult respiratory distress syndrome with dipyridamole. Crit Care Med. 1985;13(10):783–5. doi: 10.1097/00003246-198510000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ. 2014 Jan 24;348(1):f7450. doi: 10.1136/bmj.f7450. [DOI] [PubMed] [Google Scholar]
- 54.Sossdorf M, Otto GP, Boettel J, Winning J, Lösche W. Benefit of low-dose aspirin and non-steroidal anti-inflammatory drugs in septic patients. Crit Care. 2013;17(1):402. doi: 10.1186/cc11886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winning J, Neumann J, Kohl M, Claus Ra, Reinhart K, Bauer M, Lösche W. Antiplatelet drugs and outcome in mixed admissions to an intensive care unit. Crit Care Med. 2010;38(1):32–37. doi: 10.1097/CCM.0b013e3181b4275c. [DOI] [PubMed] [Google Scholar]
- 56.Lösche W, Boettel J, Kabisch B, Winning J, Claus Ra, Bauer M. Do Aspirin and Other Antiplatelet Drugs Reduce the Mortality in Critically Ill Patients? Thrombosis. 2012;2012:1–8. doi: 10.1155/2012/720254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–71. doi: 10.1056/NEJM200103013440908. [DOI] [PubMed] [Google Scholar]
- 58.Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92(21):9475–9. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiang N, Arita M, Serhan CN. Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids. 2005;73(3–4):163–77. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Serhan CN. Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim Biophys Acta. 1994;1212(1):1–25. doi: 10.1016/0005-2760(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 61.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 62.Grommes J, Alard JE, Drechsler M, Wantha S, Mörgelin M, Kuebler WM, Jacobs M, Von Hundelshausen P, Markart P, Wygrecka M, et al. Disruption of platelet-derived chemokine heteromers prevents neutrophil extravasation in acute lung injury. Am J Respir Crit Care Med. 2012;185(6):628–636. doi: 10.1164/rccm.201108-1533OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tuinman PR, Müller MC, Jongsma G, Hegeman Ma, Juffermans NP. High-Dose Acetylsalicylic Acid Is Superior to Low-Dose as Well as to Clopidogrel in Preventing Lipopolysaccharide-Induced Lung Injury in Mice. Shock. 2013;40(4):334–338. doi: 10.1097/SHK.0b013e3182a384f0. [DOI] [PubMed] [Google Scholar]
- 64.Schrör K. Aspirin and platelets: the antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis. Semin Thromb Hemost. 1997;23(4):349–56. doi: 10.1055/s-2007-996108. [DOI] [PubMed] [Google Scholar]
- 65.Cornet AD, Oudemans-van Straaten HM, Schultz MJ, Juffermans NP, Tuinman PR. Anticoagulants for ARDS: facts and future. Netherlands J Crit Care. 2014;18(6):3–8. [Google Scholar]
- 66.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 67.McAuley D. The Effect of Aspirin on REducing iNflammation in Human in Vivo Model of Acute Lung Injury (ARENA) ( NCT01659307) [Internet] Clin Regist. [cited 2015 Oct 1] Available from: https://clinicaltrials.gov/ct2/show/NCT01659307.
- 68.McAuley D. ASpirin as a Treatment for ARDS (STAR): a Phase 2 Randomised Control Trial ( NCT02326350) [Internet] Clin Regist. [cited 2015 Oct 1] Available from: https://clinicaltrials.gov/ct2/show/NCT01659307.
- 69.Kor DJ, Talmor DS, Banner-Goodspeed VM, Carter RE, Hinds R, Park PK, Gajic O, Gong MN. Lung Injury Prevention with Aspirin (LIPS-A): a protocol for a multicentre randomised clinical trial in medical patients at high risk of acute lung injury. BMJ Open. 2012;2(5):e001606–e001606. doi: 10.1136/bmjopen-2012-001606. [DOI] [PMC free article] [PubMed] [Google Scholar]