Abstract
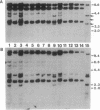
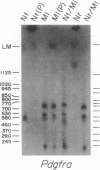
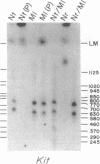
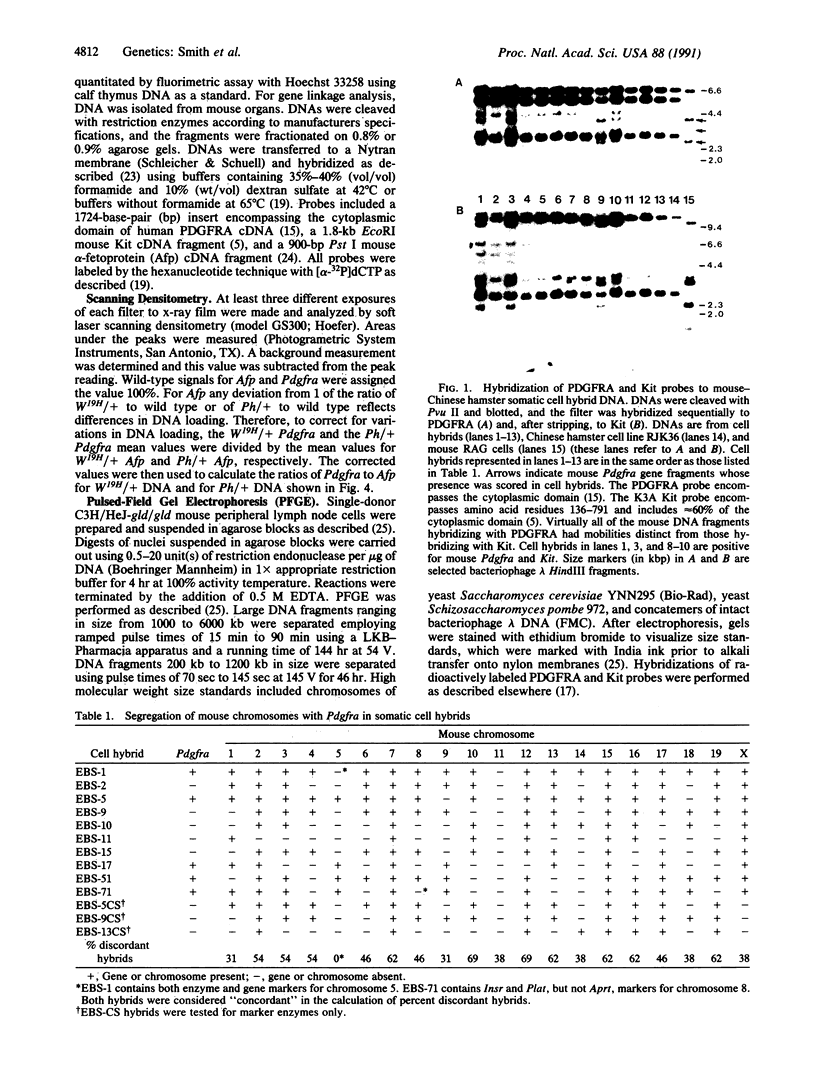
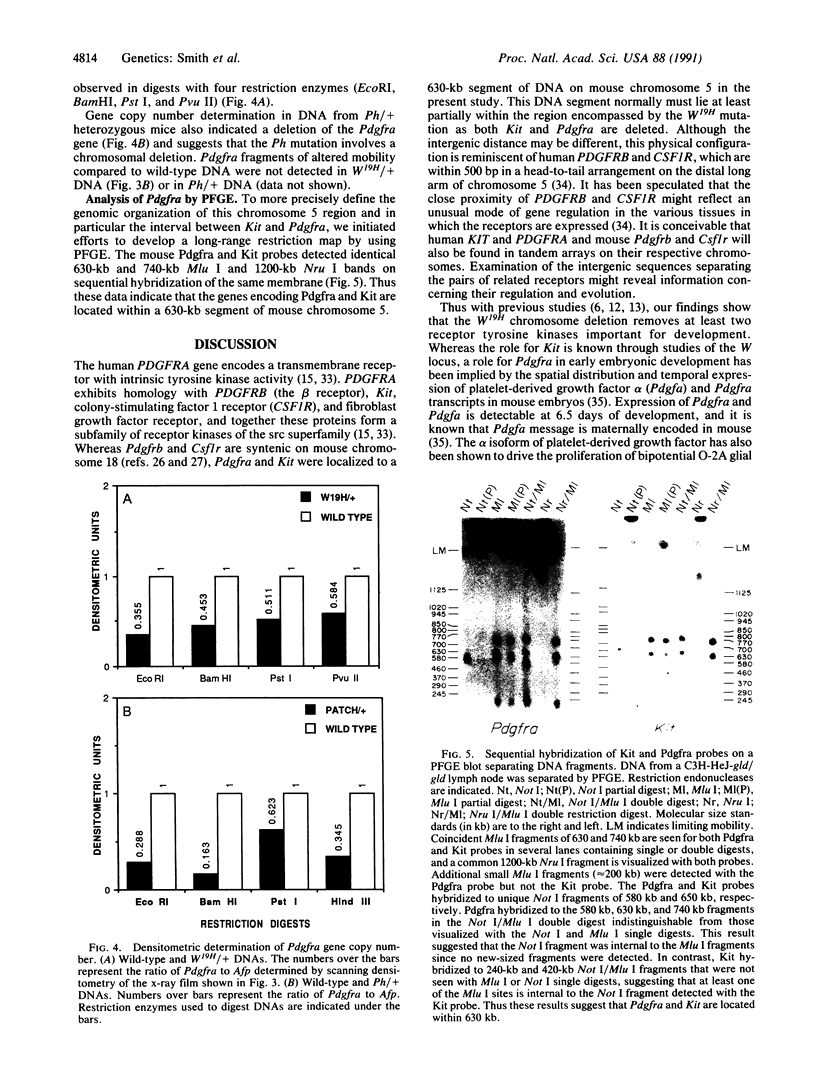
The mouse W19H mutation is an x-ray-induced deletion of more than 2 centimorgans on chromosome 5 encompassing the white spotting mutation W (encoded by the Kit protooncogene), patch (Ph), and recessive lethal (l) loci. The platelet-derived growth factor receptor alpha gene (PDGFRA) like Kit encodes a transmembrane receptor tyrosine kinase. By using mouse-Chinese hamster somatic cell hybrids and haplotype analysis in interspecific backcross mice, mouse Pdgfra was mapped to chromosome 5 in tight linkage with Kit. Hybridization of a PDGFRA probe to DNAs from W19H/ + heterozygous mice and patch heterozygous mice, and their wild-type littermates, demonstrated deletion of Pdgfra. Pulsed-field gel electrophoresis indicated that Kit and Pdgfra are linked on a 630-kilobase Mlu I DNA fragment. Thus the W19H deletion removes at least two receptor tyrosine kinases and the results suggest Pdgfra as a candidate for the Ph locus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. T. The information content of phase-known matings for ordering genetic loci. Genet Epidemiol. 1985;2(4):349–361. doi: 10.1002/gepi.1370020404. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot B., Stephenson D. A., Chapman V. M., Besmer P., Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988 Sep 1;335(6185):88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- Claesson-Welsh L., Eriksson A., Westermark B., Heldin C. H. cDNA cloning and expression of the human A-type platelet-derived growth factor (PDGF) receptor establishes structural similarity to the B-type PDGF receptor. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4917–4921. doi: 10.1073/pnas.86.13.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke U., Lalley P. A., Moss W., Ivy J., Minna J. D. Gene mapping in Mus musculus by interspecific cell hybridization: assignment of the genes for tripeptidase-1 to chromosome 10, dipeptidase-2 to chromosome 18, acid phosphatase-1 to chromosome 12, and adenylate kinase-1 to chromosome 2. Cytogenet Cell Genet. 1977;19(2-3):57–84. doi: 10.1159/000130799. [DOI] [PubMed] [Google Scholar]
- Geissler E. N., Cheng S. V., Gusella J. F., Housman D. E. Genetic analysis of the dominant white-spotting (W) region on mouse chromosome 5: identification of cloned DNA markers near W. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9635–9639. doi: 10.1073/pnas.85.24.9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler E. N., McFarland E. C., Russell E. S. Analysis of pleiotropism at the dominant white-spotting (W) locus of the house mouse: a description of ten new W alleles. Genetics. 1981 Feb;97(2):337–361. doi: 10.1093/genetics/97.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler E. N., Ryan M. A., Housman D. E. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988 Oct 7;55(1):185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Bevins C. L., Cama A., Ojamaa K., Marcus-Samuels B., Kadowaki H., Beitz L., McKeon C., Taylor S. I. Two mutant alleles of the insulin receptor gene in a patient with extreme insulin resistance. Science. 1988 May 6;240(4853):787–790. doi: 10.1126/science.2834824. [DOI] [PubMed] [Google Scholar]
- Kingsmore S. F., Watson M. L., Howard T. A., Seldin M. F. A 6000 kb segment of chromosome 1 is conserved in human and mouse. EMBO J. 1989 Dec 20;8(13):4073–4080. doi: 10.1002/j.1460-2075.1989.tb08591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley P. A., Davison M. T., Graves J. A., O'Brien S. J., Womack J. E., Roderick T. H., Creau-Goldberg N., Hillyard A. L., Doolittle D. P., Rogers J. A. Report of the committee on comparative mapping. Cytogenet Cell Genet. 1989;51(1-4):503–532. doi: 10.1159/000132806. [DOI] [PubMed] [Google Scholar]
- Lyon M. F., Glenister P. H., Loutit J. F., Evans E. P., Peters J. A presumed deletion covering the W and Ph loci of the mouse. Genet Res. 1984 Oct;44(2):161–168. doi: 10.1017/s0016672300026367. [DOI] [PubMed] [Google Scholar]
- Matsui T., Heidaran M., Miki T., Popescu N., La Rochelle W., Kraus M., Pierce J., Aaronson S. Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes. Science. 1989 Feb 10;243(4892):800–804. doi: 10.1126/science.2536956. [DOI] [PubMed] [Google Scholar]
- Mercola M., Wang C. Y., Kelly J., Brownlee C., Jackson-Grusby L., Stiles C., Bowen-Pope D. Selective expression of PDGF A and its receptor during early mouse embryogenesis. Dev Biol. 1990 Mar;138(1):114–122. doi: 10.1016/0012-1606(90)90181-h. [DOI] [PubMed] [Google Scholar]
- Morrison-Graham K., Weston J. A. Mouse mutants provide new insights into the role of extracellular matrix in cell migration and differentiation. Trends Genet. 1989 Apr;5(4):116–121. doi: 10.1016/0168-9525(89)90042-5. [DOI] [PubMed] [Google Scholar]
- Moseley W. S., Seldin M. F. Definition of mouse chromosome 1 and 3 gene linkage groups that are conserved on human chromosome 1: evidence that a conserved linkage group spans the centromere of human chromosome 1. Genomics. 1989 Nov;5(4):899–905. doi: 10.1016/0888-7543(89)90132-8. [DOI] [PubMed] [Google Scholar]
- Nocka K., Majumder S., Chabot B., Ray P., Cervone M., Bernstein A., Besmer P. Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice--evidence for an impaired c-kit kinase in mutant mice. Genes Dev. 1989 Jun;3(6):816–826. doi: 10.1101/gad.3.6.816. [DOI] [PubMed] [Google Scholar]
- Pringle N., Collarini E. J., Mosley M. J., Heldin C. H., Westermark B., Richardson W. D. PDGF A chain homodimers drive proliferation of bipotential (O-2A) glial progenitor cells in the developing rat optic nerve. EMBO J. 1989 Apr;8(4):1049–1056. doi: 10.1002/j.1460-2075.1989.tb03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Lillien L. E., Richardson W. D., Burne J. F., Noble M. D. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988 Jun 9;333(6173):562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- Reith A. D., Rottapel R., Giddens E., Brady C., Forrester L., Bernstein A. W mutant mice with mild or severe developmental defects contain distinct point mutations in the kinase domain of the c-kit receptor. Genes Dev. 1990 Mar;4(3):390–400. doi: 10.1101/gad.4.3.390. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Pringle N., Mosley M. J., Westermark B., Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988 Apr 22;53(2):309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- Roberts W. M., Look A. T., Roussel M. F., Sherr C. J. Tandem linkage of human CSF-1 receptor (c-fms) and PDGF receptor genes. Cell. 1988 Nov 18;55(4):655–661. doi: 10.1016/0092-8674(88)90224-3. [DOI] [PubMed] [Google Scholar]
- Russell E. S. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- Sakaguchi A. Y., Lalley P. A., Choudhury G. G., Martinez L., Han E. S., Killary A. M., Naylor S. L., Wang L. M. Mouse melanoma growth stimulatory activity gene (Mgsa) is polymorphic and syntenic with the W, patch, rumpwhite, and recessive spotting loci on chromosome 5. Genomics. 1989 Oct;5(3):629–632. doi: 10.1016/0888-7543(89)90033-5. [DOI] [PubMed] [Google Scholar]
- Saunders A. M., Seldin M. F. The syntenic relationship of proximal mouse chromosome 7 and the myotonic dystrophy gene region on human chromosome 19q. Genomics. 1990 Feb;6(2):324–332. doi: 10.1016/0888-7543(90)90573-d. [DOI] [PubMed] [Google Scholar]
- Searle A. G., Truslove G. M. A gene triplet in the mouse. Genet Res. 1970 Apr;15(2):227–235. doi: 10.1017/s0016672300001555. [DOI] [PubMed] [Google Scholar]
- Seldin M. F., D'Hoostelaere L. A., Huppi K., Mock B. A., Steinberg A. D., Parnes J. R., Morse H. C., 3rd Mapping of the Ly-4 (L3T4) T-cell differentiation antigen on mouse chromosome 6 by the use of RFLPs in an interspecific cross. Immunogenetics. 1988;27(5):396–398. doi: 10.1007/BF00395138. [DOI] [PubMed] [Google Scholar]
- Seldin M. F., Howard T. A., D'Eustachio P. Comparison of linkage maps of mouse chromosome 12 derived from laboratory strain intraspecific and Mus spretus interspecific backcrosses. Genomics. 1989 Jul;5(1):24–28. doi: 10.1016/0888-7543(89)90082-7. [DOI] [PubMed] [Google Scholar]
- Seldin M. F., Martinez L., Howard T. A., Naylor S. L., Sakaguchi A. Y. Localization of mouse melanoma growth stimulatory activity gene (Mgsa) between Afp and Gus on chromosome 5 using interspecific backcross mice. Cytogenet Cell Genet. 1990;54(1-2):68–70. doi: 10.1159/000132959. [DOI] [PubMed] [Google Scholar]
- Seldin M. F., Morse H. C., 3rd, Reeves J. P., Scribner C. L., LeBoeuf R. C., Steinberg A. D. Genetic analysis of autoimmune gld mice. I. Identification of a restriction fragment length polymorphism closely linked to the gld mutation within a conserved linkage group. J Exp Med. 1988 Feb 1;167(2):688–693. doi: 10.1084/jem.167.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan S., Francke U. Genes for beta 2-adrenergic receptor and platelet-derived growth factor receptor map to mouse chromosome 18. Somat Cell Mol Genet. 1989 Jul;15(4):367–371. doi: 10.1007/BF01534975. [DOI] [PubMed] [Google Scholar]
- Tan J. C., Nocka K., Ray P., Traktman P., Besmer P. The dominant W42 spotting phenotype results from a missense mutation in the c-kit receptor kinase. Science. 1990 Jan 12;247(4939):209–212. doi: 10.1126/science.1688471. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Kioussis D., Gorin M. B., Ruiz J. P., Ingram R. S. The presence of intervening sequences in the alpha-fetoprotein gene of the mouse. J Biol Chem. 1979 Aug 10;254(15):7393–7399. [PubMed] [Google Scholar]
- Wang L. M., Killary A. M., Fang X. E., Parriott S. K., Lalley P. A., Bell G. I., Sakaguchi A. Y. Chromosome assignment of mouse insulin, colony stimulating factor 1, and low-density lipoprotein receptors. Genomics. 1988 Aug;3(2):172–176. doi: 10.1016/0888-7543(88)90150-4. [DOI] [PubMed] [Google Scholar]
- Williams D. E., Eisenman J., Baird A., Rauch C., Van Ness K., March C. J., Park L. S., Martin U., Mochizuki D. Y., Boswell H. S. Identification of a ligand for the c-kit proto-oncogene. Cell. 1990 Oct 5;63(1):167–174. doi: 10.1016/0092-8674(90)90297-r. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Kuang W. J., Yang-Feng T., Coussens L., Munemitsu S., Dull T. J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987 Nov;6(11):3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsebo K. M., Williams D. A., Geissler E. N., Broudy V. C., Martin F. H., Atkins H. L., Hsu R. Y., Birkett N. C., Okino K. H., Murdock D. C. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990 Oct 5;63(1):213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]