Abstract
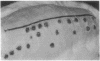
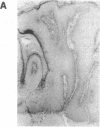
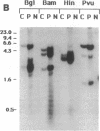
A simple inoculation method to induce papillomas efficiently with cottontail rabbit papillomavirus (CRPV) DNA is described. Using a jet injector, recombinant CRPV DNA is easily delivered to 100 or more sites per rabbit and induces typical epithelial papillomas in approximately 50% of those sites. Papillomas begin to form by 3 weeks and continue to develop for up to 7 weeks, a pattern similar to that reported following infection with intact virus. This system readily lends itself to investigation of viral gene function by delivering mutant viral genomes into an immunologically intact host. Two mutations in the E7 open reading frame were introduced into the complete CRPV genome and analyzed by this method. One was a frameshift mutation encoding just nine amino-terminal amino acids of the E7 protein; the other was an in-frame insertion mutation at position 9. Both E7 mutations were in a region of homology to the 300-kDa protein binding domain of adenovirus E1A protein. Neither mutant construct was able to induce papillomas, thereby demonstrating that the E7 gene participates in this biologic function. Exploitation of this approach, which demonstrates that a papillomavirus E7 gene is involved in the induction of papillomas in vivo, should permit detailed studies into molecular mechanisms involved in papilloma induction, malignant conversion, and host immune response. The high efficiency of papilloma induction with recombinant CRPV DNA suggests that the jet injector can also be used to study the biologic effects of other genetic elements in rabbits or in other species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbosa M. S., Vass W. C., Lowy D. R., Schiller J. T. In vitro biological activities of the E6 and E7 genes vary among human papillomaviruses of different oncogenic potential. J Virol. 1991 Jan;65(1):292–298. doi: 10.1128/jvi.65.1.292-298.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell M. A., Jones K. H., Grossman S. R., Laimins L. A. Identification of human papillomavirus type 18 transforming genes in immortalized and primary cells. J Virol. 1989 Mar;63(3):1247–1255. doi: 10.1128/jvi.63.3.1247-1255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandsma J. L., Abramson A. L. Association of papillomavirus with cancers of the head and neck. Arch Otolaryngol Head Neck Surg. 1989 May;115(5):621–625. doi: 10.1001/archotol.1989.01860290079018. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Billeter M. A., Sheppard R. D., Udem S. A. Multiple viral mutations rather than host factors cause defective measles virus gene expression in a subacute sclerosing panencephalitis cell line. J Virol. 1988 Apr;62(4):1388–1397. doi: 10.1128/jvi.62.4.1388-1397.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan C., Jelsma T. N., Howe J. A., Bayley S. T., Ferguson B., Branton P. E. Mapping of cellular protein-binding sites on the products of early-region 1A of human adenovirus type 5. Mol Cell Biol. 1988 Sep;8(9):3955–3959. doi: 10.1128/mcb.8.9.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C. A., Rashad A. L. Virus content of Shope papillomas of cottontail rabbits. Cancer Res. 1967 Jun;27(6):1011–1015. [PubMed] [Google Scholar]
- Giri I., Danos O., Yaniv M. Genomic structure of the cottontail rabbit (Shope) papillomavirus. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1580–1584. doi: 10.1073/pnas.82.6.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley-Nelson P., Vousden K. H., Hubbert N. L., Lowy D. R., Schiller J. T. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989 Dec 1;8(12):3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J. B., Bedell M. A., McCance D. J., Laiminis L. A. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol. 1990 Feb;64(2):519–526. doi: 10.1128/jvi.64.2.519-526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T., Furuno A., Yoshiike K. Human papillomavirus type 16 open reading frame E7 encodes a transforming gene for rat 3Y1 cells. J Virol. 1988 Feb;62(2):610–613. doi: 10.1128/jvi.62.2.610-613.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller L. D., Olson C. Attempted transmission of warts from man, cattle, and horses and of deer fibroma, to selected hosts. J Invest Dermatol. 1972 Jun;58(6):366–368. doi: 10.1111/1523-1747.ep12540579. [DOI] [PubMed] [Google Scholar]
- Kreider J. W., Bartlett G. L. The Shope papilloma-carcinoma complex of rabbits: a model system of neoplastic progression and spontaneous regression. Adv Cancer Res. 1981;35:81–110. doi: 10.1016/s0065-230x(08)60909-4. [DOI] [PubMed] [Google Scholar]
- Kreider J. W., Howett M. K., Wolfe S. A., Bartlett G. L., Zaino R. J., Sedlacek T., Mortel R. Morphological transformation in vivo of human uterine cervix with papillomavirus from condylomata acuminata. Nature. 1985 Oct 17;317(6038):639–641. doi: 10.1038/317639a0. [DOI] [PubMed] [Google Scholar]
- Lacey M., Alpert S., Hanahan D. Bovine papillomavirus genome elicits skin tumours in transgenic mice. Nature. 1986 Aug 14;322(6080):609–612. doi: 10.1038/322609a0. [DOI] [PubMed] [Google Scholar]
- Matlashewski G., Schneider J., Banks L., Jones N., Murray A., Crawford L. Human papillomavirus type 16 DNA cooperates with activated ras in transforming primary cells. EMBO J. 1987 Jun;6(6):1741–1746. doi: 10.1002/j.1460-2075.1987.tb02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue J. P., Stevens D. J., Kermon V. L., Ashe M. C., Heim L. R. Platelet lesion of collection. Scand J Haematol. 1980 Oct;25(4):301–307. doi: 10.1111/j.1600-0609.1981.tb01407.x. [DOI] [PubMed] [Google Scholar]
- Nasseri M., Wettstein F. O. A variant of CRPV DNA preferentially maintained as a plasmid in NIH 3T3 cells and characterization of its transcripts in nude mouse tumors. Virology. 1987 Dec;161(2):541–548. doi: 10.1016/0042-6822(87)90149-8. [DOI] [PubMed] [Google Scholar]
- Phelps W. C., Yee C. L., Münger K., Howley P. M. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988 May 20;53(4):539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Sato H., Furuno A., Yoshiike K. Expression of human papillomavirus type 16 E7 gene induces DNA synthesis of rat 3Y1 cells. Virology. 1989 Jan;168(1):195–199. doi: 10.1016/0042-6822(89)90423-6. [DOI] [PubMed] [Google Scholar]
- Stein R. W., Corrigan M., Yaciuk P., Whelan J., Moran E. Analysis of E1A-mediated growth regulation functions: binding of the 300-kilodalton cellular product correlates with E1A enhancer repression function and DNA synthesis-inducing activity. J Virol. 1990 Sep;64(9):4421–4427. doi: 10.1128/jvi.64.9.4421-4427.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey A., Pim D., Murray A., Osborn K., Banks L., Crawford L. Comparison of the in vitro transforming activities of human papillomavirus types. EMBO J. 1988 Jun;7(6):1815–1820. doi: 10.1002/j.1460-2075.1988.tb03013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Noda T., Yajima H., Hatanaka M., Ito Y. Identification of a transforming gene of human papillomavirus type 16. J Virol. 1989 Mar;63(3):1465–1469. doi: 10.1128/jvi.63.3.1465-1469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden K. H., Doniger J., DiPaolo J. A., Lowy D. R. The E7 open reading frame of human papillomavirus type 16 encodes a transforming gene. Oncogene Res. 1988 Sep;3(2):167–175. [PubMed] [Google Scholar]
- Watanabe S., Kanda T., Yoshiike K. Human papillomavirus type 16 transformation of primary human embryonic fibroblasts requires expression of open reading frames E6 and E7. J Virol. 1989 Feb;63(2):965–969. doi: 10.1128/jvi.63.2.965-969.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K. Transformation of rat 3Y1 cells by human papillomavirus type-18 DNA. Int J Cancer. 1988 Jun 15;41(6):896–900. doi: 10.1002/ijc.2910410622. [DOI] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]