Abstract
The Golgi apparatus (GA) is a highly dynamic organelle, which is mainly involved in the post-translational processing and targeting of cellular proteins and which undergoes significant morphological changes in response to different physiological and pathological conditions. In the present study, we have analyzed the possible alterations of GA in neurons from the temporal neocortex and hippocampus of Alzheimer's disease (AD) patients, using double immunofluorescence techniques, confocal microscopy and 3D quantification techniques. We found that in AD patients, the percentage of temporal neocortical and CA1 hippocampal pyramidal neurons with a highly altered GA is much higher (approximately 65%) in neurons with neurofibrillary tangles (NFT) than in NFT-free neurons (approximately 6%). Quantitative analysis of the surface area and volume of GA elements in neurons revealed that, compared with NFT-free neurons, NFT-bearing neurons had a reduction of approximately one half in neocortical neurons and one third in CA1 neurons. In both regions, neurons with a pre-tangle stage of phospho-tau accumulation had surface area and GA volume values that were intermediate, that is, between those of NFT-free and NFT-bearing neurons. These findings support the idea that the progressive accumulation of phospho-tau is associated with structural alterations of the GA including fragmentation and a decrease in the surface area and volume of GA elements. These alterations likely impact the processing and trafficking of proteins, which might contribute to neuronal dysfunction in AD.
Keywords: Taupathy, Dementia, Human hippocampus, Human neocortex, Microtubules, Neurofibrillary tangles
Highlights
-
•
The Golgi apparatus of cortical neurons is affected in Alzheimer's disease patients.
-
•
Golgi apparatus alterations include fragmentation and a decrease in size.
-
•
Phospho-tau accumulation in neurons is correlated with Golgi apparatus alterations.
1. Introduction
The Golgi apparatus (GA) is an essential organelle in the processing and targeting of cellular proteins, and its disruption is related to neuronal dysfunctions and human diseases (Gonatas et al., 2006, Hu et al., 2007). It has been shown that the GA undergoes morphological plasticity processes that affect the GA shape and protein content under different physiological and pathological conditions (Anton-Fernandez et al., 2015, Fan et al., 2008, Glick, 2002, Levine et al., 1995). In different neurological diseases, including Alzheimer's disease (AD), the GA of certain populations of neurons becomes fragmented (Baloyannis, 2014, Dal Canto, 1996, Fan et al., 2008, Fujita et al., 2006, Fujita and Okamoto, 2005, Fujita et al., 2002, Gonatas et al., 1998b, Gonatas et al., 2006, Gonatas et al., 1992, Hu et al., 2007, Huse et al., 2002, Liazoghli et al., 2005, Mizuno et al., 2001, Rabouille and Haase, 2015, Sakurai et al., 2000, Stieber et al., 1996). Several studies carried out in animal models of AD have related this fragmentation to the accumulation of either amyloid β (Aβ) or hyperphosphorylated microtubule-associated protein tau—the two main pathological hallmarks of AD (see Jiang et al., 2014, Joshi and Wang, 2015, Liazoghli et al., 2005). However, the precise temporal sequence and the possible relationships between GA fragmentation and the accumulation of Aβ plaques or neurofibrillary tangles of hyperphosphorylated tau during the evolution of AD have not been fully characterized.
Proper functioning of the GA is necessary for Aβ production and for trafficking and maturation of amyloid precursor protein APP and its processing enzymes (Burgos et al., 2010, Choy et al., 2012, Greenfield et al., 1999, Huse et al., 2002, Joshi et al., 2015). In addition, studies using the APPswe/PS1ΔE9 animal model of the disease have reported that the accumulation of Aβ peptides leads to Golgi fragmentation, mediated by cdk5-dependent phosphorylation of Grasp 65, which in turn accelerates APP trafficking and Aβ production (Joshi et al., 2015, Joshi and Wang, 2015). Furthermore, the integrity of the microtubule (Burkhardt, 1998, Cole et al., 1996) and actin (Egea et al., 2006) cytoskeleton is necessary for the maintenance of the structural characteristics and the positioning of the GA. Various studies have suggested the existence of feedback mechanisms between tau hyperphosphorylation, microtubule organization and GA fragmentation. For instance, it has been proposed that the microtubule-interacting protein tau, which is localized on GA membranes along with many of the tau phosphorylation-related kinases participates in the association between Golgi membranes and microtubules (Farah et al., 2006). It has been suggested that tau pathology precedes GA fragmentation since, in animal models of AD, the overexpression of tau induced GA fragmentation (Liazoghli et al., 2005, Lin et al., 2003). However, other studies showing tau hyperphosphorylation after brefeldin A or nocodazole treatments of HEK293/tau cells led to the suggestion that tau hyperphosphorylation is a downstream event that is a consequence of the disorganization of the GA (Sutterlin et al., 2002, Jiang et al., 2014).
Pioneering studies in AD patients reported that the alterations (including fragmentation and decreases in size) of the GA of hippocampal neurons are unrelated to the content of intracellular NFT in CA1 hippocampal field (Salehi et al., 1995) or preferentially associated with neurons devoid of NTF in the subiculum and entorhinal cortex (Stieber et al., 1996). However, during the course of our research into the possible alterations of the GA in the hippocampus and neocortex of AD patients using different methodological approaches to those used by Salehi et al. (1995) and Stieber et al. (1996), we found that the progressive accumulation of phospho-tau may be associated with the fragmentation of the GA. Thus, in the present study, we have reexamined this issue using double immunofluorescence techniques, high-resolution confocal microscopy and 3D reconstruction and quantification methods to measure the volume and surface area of the GA in neurons from control cases and from AD patients with pre-tangle and tangle stages of hyperphosphorylated tau accumulation. To label the GA, we used antibodies that recognize MG160, an integral membrane Golgi protein commonly used as a GA marker (Anton-Fernandez et al., 2015, Burrus et al., 1992, Gonatas et al., 1998a, Gonatas et al., 1995, Stieber et al., 1995, Stieber et al., 1996, Yamaguchi et al., 2003, Zhou et al., 1997, Zuber et al., 1997).
Importantly, differences in age (Jiang et al., 2014), fixation procedure and postmortem period prior to fixation might affect the structural characteristics of the GA. These factors vary between the control and the AD patient groups used in previous reports (Ellisman et al., 1987, Salehi et al., 1995, Stieber et al., 1996) and might also affect the morphology of the GA, as evaluated in the present study by the expression of MG160. Therefore, in order to evaluate the possible influence of normal aging and the postmortem delay period on the analysis of MG160 expression in the GA and discriminate the interference between these potential changes and those associated with the course of AD, we have analyzed the GA in brain sections from C57BL/6J mice with different ages and from mice with different postmortem times before brain fixation.
The results of the present study indicate that, in AD patients, the GA of numerous cortical and hippocampal neurons undergo specific and profound alterations including fragmentation and a decrease in the volume and surface area of the elements that are immunoreactive for MG160. These changes are likely to affect the protein processing, glycosylation and sorting (Wang et al., 2008, Xiang et al., 2013) and are more pronounced and more frequently found in neurons with a progressive accumulation of hyperphosphorylated tau. Therefore, the perturbation of the microtubule network by tau hyperphosphorylation could alter GA structure and the secretory pathway, impairing the highly regulated processes of protein sorting in neuronal compartments.
2. Materials and methods
Human brain tissue was obtained at autopsy from two sources: from seven patients with AD (aged 64–89) and from control human brain tissue from five individuals (aged 40–66) who died due to an accident or other cause and were free of any known neurological or psychiatric illness (Table 1). The AD brain and some control brain tissues were obtained from the Instituto de Neuropatología (Dr. I. Ferrer, Servicio de Anatomía Patológica, IDIBELL-Hospital Universitario de Bellvitge, Barcelona, Spain; IF4, IF10, IF12, IF13 and M16 cases), from the Banco de Tejidos Fundación CIEN (Dr. A. Rábano, Área de Neuropatología, Centro Alzheimer, Fundación Reina Sofia, Madrid, Spain; Vk11 case) and from the Neurological Tissue Bank (Biobanc-Hospital Clínic-IDIBAPS, Universidad de Barcelona, Spain; Bcn 5, Bcn7, Bcn8, Bcn13 cases). Control human brains were also obtained from Dr. Ricardo Insausti, Facultad de Medicina, Universidad UCLM (Albacete, Spain; AB1, AB2). Following neuropathological examination, the AD stages were defined according to the CERAD (Consortium to Establish a Registry for Alzheimer's Disease; (Mirra et al., 1991) and the Braak and Braak criteria (Braak and Braak, 1995); Table 1). Information regarding TDP43 inclusions was available for four of the seven patients used (Bcn5, Bcn7, Bcn8 and Bcn13). None of them showed TDP43 inclusions in CA1 or temporal neocortex, and only Bcn7 had TDP43 inclusions in neurons of the amigdala.
Table 1.
Summary of clinical and surgical data. Neurological diagnosis defined according to Braak and Braak criteria (Braak and Braak, 1995), defined by different stages (from I to VI) and also according to CERAD criteria (Consortium to Establish a Registry for Alzheimer's Disease; Mirra et al., 1991), which use a semi-quantitative score of the density of neuritic plaques in the most severely affected region of the isocortex (A = mild presence of plaques, B = moderate presence of plaques, C = severe presence of plaques).
| AD patients | Cortical area | Age (years) | Gender | Postmortem delay | Neurological diagnosis | Cause of death |
|---|---|---|---|---|---|---|
| Bcn5 | Area 21 | 83 | Female | 4 h | AD V/VI – C | Respiratory infection |
| Bcn7 | Area 21 | 89 | Female | 4/4.5 h | AD VI – C | – |
| Bcn8 | Area 21 | 85 | Female | 5/6 h | AD VI – C | – |
| P3 (IF4) | Area 22 | 80 | Female | 3 h | AD III – B | Pneumonia |
| P11 (IF13) | Area 20/21 | 75 | Male | 2 h | AD III – B | Lymphoproliferative disorder |
| P13 (Bcn13) | – | 83 | Male | 2.5 h | AD | – |
| P14 (Vk11) | Area 20/21 | 87 | Female | 1.5 h | AD III/IV – A | Respiratory infection |
| Non-demented cases | ||||||
| AB1 | Area 21 | 45 | Male | < 1 h | – | Pleural mesothelioma |
| AB2 | Area 21, 38 | 50 | Female | 4 h | – | Septic shock of pulmonary origin |
| M16 | Area 20 | 40 | Male | – | – | Traffic accident |
| C7 (IF10) | Area 20 | 66 | Male | 2 h | – | Bilateral pneumonia plus cardiac post-transplant |
| P10 (IF12) | Area 20/21 | 52 | Male | 2 h | – | Carcinoma – bronchopneumonia |
The postmortem delay between death and tissue processing ranged between 1.5 and 5.5 h (Table 1), and the brain samples were obtained following the guidelines of the Institutional Ethical Committees, which also granted approval. Tissue from some of these human brains has been used in previous studies (Blazquez-Llorca et al., 2010, Blazquez-Llorca et al., 2011).
Upon removal, the brains were immediately fixed in cold 4% paraformaldehyde in phosphate buffer (PB: 0.1 M, pH 7.4), and after 2 h, the tissue was cut into small blocks and post-fixed in the same fixative for 24–48 h at 4 °C. However, one human patient (AB1) was intraarterially perfused through the internal carotid arthery < 1 h after death with a saline solution followed by 4% paraformaldehyde in PB. The brain was then removed and post-fixed as mentioned above. After fixation, all the specimens were immersed in graded sucrose solutions and stored in a cryoprotectant solution at − 20 °C. Serial sections (50 μm) of the cortical tissue were obtained using a vibratome (St. Louis, MO, USA), and the sections from each region and case were batch-processed for immunohistochemical staining. The sections immediately adjacent to those stained immunohistochemically were Nissl-stained in order to identify the cortical areas and the laminar boundaries.
2.1. Immunofluorescence
For immunofluorescence experiments, free floating serial sections (50-μm thick) were first rinsed in PB and then pre-treated in 1.66% H2O2 for 30 min to inactivate the endogenous peroxidase activity and were then preincubated for 1 h in PB with 0.25% Triton-X100 and 3% normal serum of the species in which the secondary antibodies were raised (Vector Laboratories, Burlingame, CA, USA). The sections were then incubated for 48 h at 4 °C in the same stock solution containing the following primary antibodies in the combinations indicated: rabbit anti-MG160 (Abcam, 1:100), mouse anti-NeuN (Chemicon, 1:2000), mouse phospho-PHF-tau pSer202 + Thr205 antibody (AT8, 1:2000, Pierce Endogen).
After rinsing in PB, the sections were incubated for 2 h at room temperature in the appropriate combinations of Alexa 488- or Alexa 594-conjugated goat anti-mouse or goat anti-rabbit antibodies (1:2000; Molecular Probes, Eugene, OR, USA). Sections were also stained with a nuclear stain DAPI (4,6-diamidino-2-phenylindole; Sigma, St. Louis, MO, U.S.A.). After rinsing in PB, the sections were treated with Autofluorescence Eliminator Reagent (Chemicon) to reduce autofluorescence, mounted in antifade mounting medium (ProlongGold, Invitrogen) and studied by confocal microscopy (Zeiss, 710).
From the temporal neocortex and the CA1 region of the hippocampus, we obtained image stacks recorded at 0.35 μm intervals through separate channels with a 63x oil-immersion lens (NA, 1.40, refraction index, 1.45). The number of optical planes in the confocal stacks ranged from 40 to 142 (mean = 84.5) in the neocortex and 69 to 183 (mean = 108.3) in the hippocampus. ZEN 2012 software (Zeiss) was used to construct composite images from each optical series by combining the images recorded through the different channels, and the same software was used to obtain Z projection images (image resolution: 1024 × 1024 pixels; pixel size: 0.11 μm). Adobe Photoshop (CS4) software was used to compose figures.
2.2. Image analysis and statistics
Fiji software (3D object counter tool) was used to analyze the volume and surface area of the elements immunostained for the different GA markers in image stacks according to a previous study (Anton-Fernandez et al., 2015). A limitation of the present study is that unfortunately the combinations of antibodies used, known to work satisfactorily in human brain tissue, did not allow the simultaneous visualization of the GA (MG160), the neuronal somata (NeuN) and phospho-PHF-tau (AT8). Despite this, we cropped 3D substacks from the original confocal stacks taken from AT8-MG160 double-stained sections counterstained with DAPI, trying to limit the analysis to the complete GA corresponding to the somata of single neurons. These cells included neurons without hyperphosphorylated tau (AT8 −) and neurons with hyperphosphorylated tau (AT8 +) at different stages (non-tangle-bearing neurons and tangle-bearing neurons; see Table 2 for the number of neurons of each type analyzed for all of the brain regions and cases studied). To make sure that we analyzed complete cell bodies, the substacks were centered on the location of those single DAPI-stained neuronal nuclei that were separated in all dimensions from the edges of the stack by at least 10 μm. From these positions, we analyzed the whole thickness of the confocal stacks and we defined the limits of every image substack according to the ‘reach’ of the MG160-ir elements surrounding the selected neuronal nuclei. The number of optical planes in the cropped confocal substacks ranged from 10 to 62 (mean = 52) in the neocortex and 15 to 89 (mean = 40) in the hippocampus. We selected isolated neurons that were separated from other neurons by neuropil, in order to avoid the inclusion of MG160-ir elements belonging to neighboring neurons. For this purpose, in AT8 + neurons, the presence of AT8 immunostaining helped to identify the limits of the neuronal somata. In AT8 − neurons, background MG160 staining also helped to distinguish neuronal somata from neuropil. To determine differences between values obtained in AT8 − neurons and AT8 + non-tangle-bearing and tangle-bearing neurons, paired Friedman analysis of variance tests were performed using SPSS software (version 22). When the Friedman test was significant, a Wilcoxon matched pair test was performed between groups.
Table 2.
Table showing the percentages and total number (in parenthesis) of pyramidal neurons analyzed, from the CA1 and temporal neocortex of control cases and Alzheimer disease patients, showing the relationship between the appearance of Golgi apparatus (GA) (NF, non-fragmented; F, fragmented; HA, highly altered) with the different patterns of immunostaining for PHF-tauAT8 (AT8 −, type I and II).
| Ctx |
CA1 |
|||||||
|---|---|---|---|---|---|---|---|---|
| NF | F | HA | NF | F | HA | |||
| Control | 91.6 (296) | 7.4 (24) | 1 (3) | 75.78 (144) | 22.6 (43) | 1.6 (3) | ||
| Patients | All cell types | 58.7 (430) | 24 (176) | 17.3 (127) | 33.09 (236) | 43.19 (308) | 23.7 (169) | |
| AT8 − | 71.8 (412) | 21.9 (126) | 6.3 (36) | 47.9 (201) | 45.7 (192) | 6.4 (27) | ||
| AT8 + | Type I | 25.4 (14) | 40 (22) | 34.5 (19) | 16.6 (28) | 46.7 (79) | 36.7 (62) | |
| Type II | 3.9 (4) | 26.9 (28) | 69.2 (72) | 5.7 (7) | 29.8 (37) | 64.5 (80) | ||
2.3. Aging and postmortem autolysis effects on Golgi apparatus
The human tissue used in the present study came from patients who died at different ages and with different postmortem delay periods until brain fixation—up to 6 h in some cases (Table 1). Thus, in order to test the possible involvement of aging and postmortem autolysis effects on the structure of the neuronal GA, and to discriminate between these effects and those related to AD, in the present study we analyzed GA immunostaining in two groups of mice. These experiments were performed in accordance with the guidelines established by the European Union regarding the use and care of laboratory animals (86/609/EEC) and the experimental procedures were approved by our institutional animal use and care committee. Special care was taken to minimize animal suffering and to reduce the number of animals used to the minimum required for statistical accuracy.
In order to test possible effects of aging on the GA of pyramidal neurons, we analyzed C57BL/6J mice (Charles River Laboratories, Wilmington, MA) at different ages: 8, 32, 60 and 80 weeks (three animals per age group). Animals were sacrificed by a lethal intraperitoneal injection of sodium pentobarbital (200 mg/kg b.w.) and were then perfused intracardially with a saline solution followed by 4% paraformaldehyde in PB. The brain of each animal was removed, post-fixed by immersion in the same fixative for 24 h at 4 °C, cryoprotected in 30% sucrose and serial coronal sections (50-μm thick) were obtained with a freezing sliding microtome (Microm HM 450, Microm International, Germany).
To assess post-mortem time-induced effects on the structure of the GA, two-month-old male C57BL/6J mice (n = 3, per group) (Charles River Laboratories) were used. Mice were deeply anaesthetized with a pentobarbital lethal injection (200 mg/kg b.w, Vetoquinol, Madrid, Spain). The cadavers were subjected to different post-mortem times and temperature conditions. Two groups of mice were maintained at room temperature for 0.5 and 2 h, respectively. A third group was kept at room temperature for 2 h followed by 3 h at 4 °C in a fridge. The brains were then removed from the skull and fixed by immersion with 4% paraformaldehyde for 20 h at 4 °C. After fixation, brains were cryoprotected in 30% sucrose and then flash-frozen in isopentane (2-methylbutane, Merck, Billerica, MA), cooled in a 70% ethanol dry ice bath, and stored at − 80° until cutting. Coronal sections (30 μm) were cut with a freezing sliding microtome (Microm HM450). Sections from all animal groups were immunocytochemically stained as described above using anti-MG160 antibodies. Confocal image stacks were taken from the temporal neocortex and the hippocampal formation and were analyzed in the same way as described for the human tissue (see above). To determine differences between values obtained in animals with different postmortem delay times as well as in mice groups with different ages, Kruskal-Wallis one-way analysis of variance was performed followed by Bonferroni-corrected Mann-Whitney U test, using SPSS software.
3. Results
3.1. Distribution of MG160 in the GA of control human cortical neurons
To characterize alterations in the Golgi apparatus (GA) of AD patients, we first studied its normal morphological characteristics in neocortical and hippocampal pyramidal neurons from human control autopsy cases. We studied sections stained with DAPI and double-immunostained with antibodies that recognize the neuronal marker NeuN and MG160, a sialoglycoprotein that is frequently used as a GA marker and is mainly localized in the medial cisternae of the GA (Fig. 1). The identification of pyramidal neurons relied on the presence of an apical dendrite. MG160-ir GA elements were located in the neuronal cytoplasm, as defined by the NeuN immunostaining, usually in a perinuclear position. We found a significant variability in the morphological characteristics of GA elements that were immunoreactive (ir) for MG160, which we qualitatively subdivided into three different morphological types (Fig. 2, Table 2):
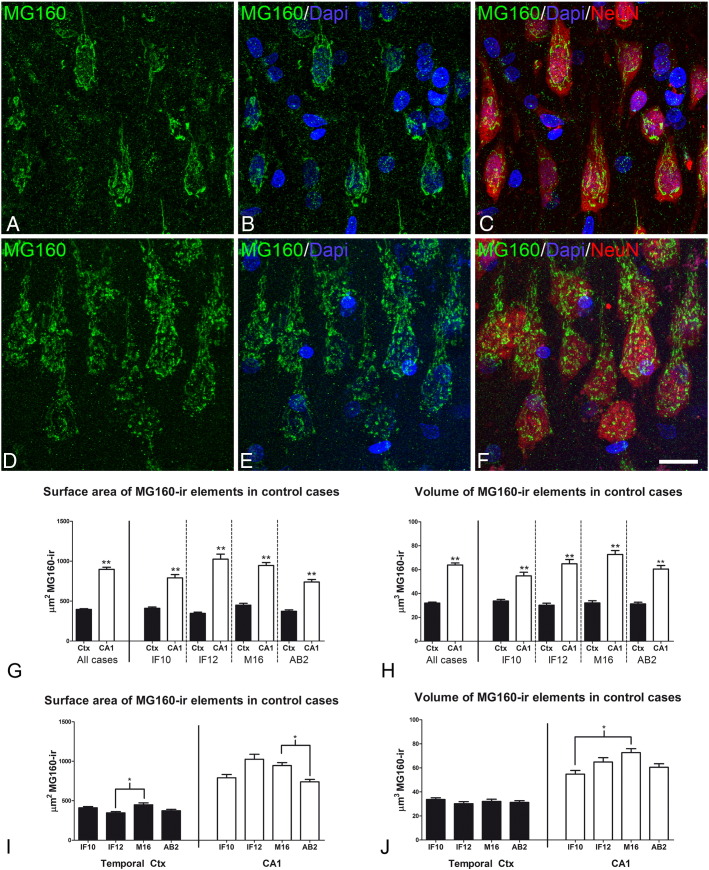
Fig. 1.
Distribution of MG160 in the GA of human pyramidal neurons
A–F: Trios of confocal stack projection images taken from the temporal neocortex (A–C) and the CA1 hippocampal region (D–F) of sections double-immunostained for MG160/NeuN and counterstained with DAPI showing the distribution of MG160 in the GA. Scale bar in F indicates 18 μm in A–C and 16 μm in D–F. G–J: Histograms showing surface area (G, I) and volume (H, J) values (mean ± SE) of GA elements immunoreactive for MG160, obtained from cropped confocal image stacks including complete single pyramidal neurons from temporal neocortex (black bars; total n = 323) and CA1 (white bars; total n = 189). G and H show the statistical comparisons of mean values (surface area and volume, respectively) between neocortical and hippocampal neurons. I and J show the comparisons of the values obtained across the different cases in each region (Friedman test, *p ≤ 0.01; **p ≤ 0.001). Note that in all cases the surface area and volume values of MG160-ir elements in CA1 are higher than in neocortical pyramidal cells (G, H) despite the inter-individual differences (I, J).
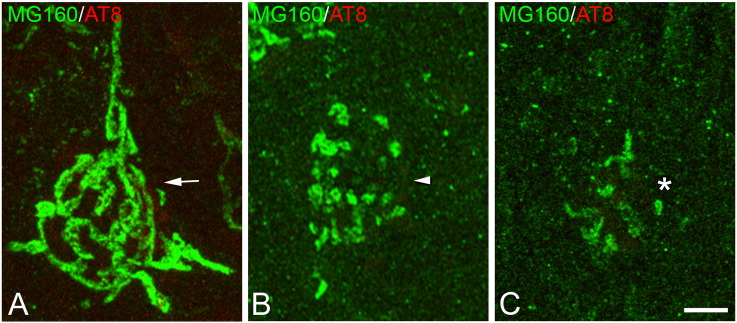
Fig. 2.
Morphological types of Golgi apparatus
Confocal stack projection mages taken from the temporal neocortex of sections from control cases, double-immunostained for MG160/AT8 showing examples of neurons with a non-fragmented (A), fragmented (B) or highly altered (C) GA. Scale bar indicates 4,8 μm.
1) neurons with a GA that showed a normal non-fragmented (NF in Table 2, arrows in Fig. 2A) appearance, consisting of a network of twisted and convoluted cisternae and tubular structures with a ribbon-like appearance that was distributed throughout the cell body and partially extended to the apical dendrite.
2) neurons with a fragmented (F in Table 2, arrowheads in Fig. 2B) GA—without ribbon-like appearance and often showing a variable number of independent elements with globular appearance dispersed throughout the soma—that were more abundant in CA1 than in the neocortex.
3) neurons in which the GA, as identified by MG160 immunostaining, was very scarce or had a highly altered (HA) morphology (HA in Table 2, asterisks in Fig. 2C).
The percentage of neurons with a non-fragmented GA was higher in the temporal neocortex than in the CA1 hippocampal field (Table 2). In addition, GA of neocortical neurons (Fig. 1A–C) showed a less complex appearance, that is, with a lower degree of cisternae extension and convolution than in the case of hippocampal neurons (Fig. 1D–F).
To further characterize the GA in control neocortical and hippocampal CA1 pyramidal neurons, we used the 3D object counter tool in the Fiji software package to quantify the volume and surface area of MG160-ir GA elements of the different control human brains (Figs. 1G–J). We found a significantly larger volume and surface area of MG160-ir elements in CA1 hippocampal neurons than in neurons from temporal neocortex (Fig. 1G, H). We found the same trend when data from different control cases were analyzed separately (Fig. 1G, H). We also compared the mean values for the surface and volume of the MG160-ir GA elements between the different cases. Both in neocortical and CA1 pyramidal cells, these values were very homogeneous across cases with only few inter-individual comparisons yielding statistically significant differences (Fig. 1I, J).
3.2. Alterations in MG-160 distribution in the GA of neurons from Alzheimer disease patients
According to previous studies (Salehi et al., 1995, Stieber et al., 1996), the GA in neurons from AD patients is altered, usually showing a fragmented appearance in some cortical neurons. In the present study we found that the fragmentation of the GA in some neurons, both in neocortex and CA1 pyramidal neurons, was apparently associated with a reduction in the size of the GA (Fig. 4A, B). The surface area and volume of the GA was reduced in fragmented neurons (only in temporal neocortex), and especially in highly altered neurons, as compared with non-fragmented neurons (Fig. 4A, B).
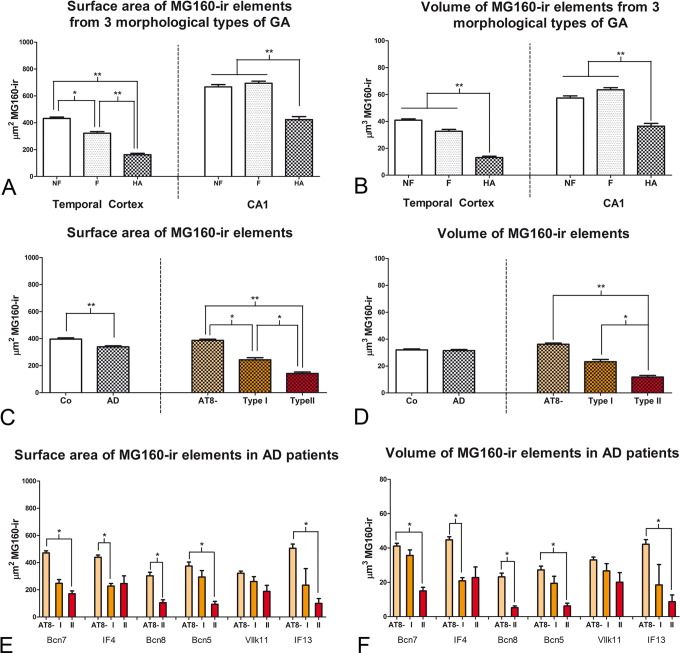
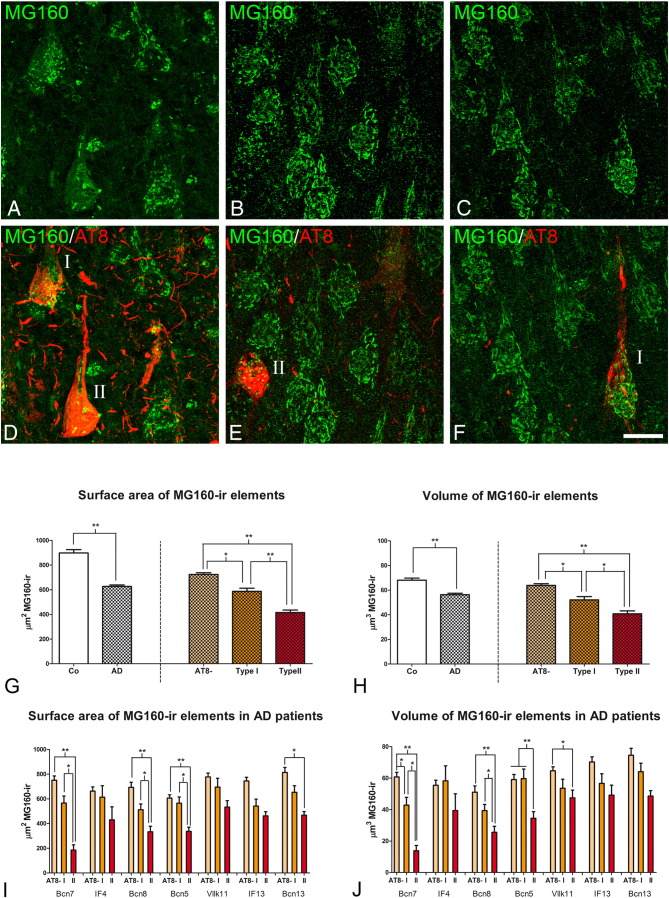
Fig. 4.
Measurements of the GA in neocortical pyramidal neurons from AD patients
A and B: histograms show comparisons in surface area (A) and volume (B) values of MG160-ir elements between neurons with non-fragmented, fragmented or highly altered GA in AD cases (Friedman test, *p ≤ 0.01; **p ≤ 0.001). C–D: histograms showing, on the left side, surface area (C) and volume (D) values (mean ± SE) of MG160-ir GA elements, in control (white bars, n = 323) and AD (patterned bars, n = 733) pyramidal neurons from temporal neocortex. Note that Golgi elements had a higher surface area (but not volume) in control than in neurons from AD patients. Also note (in the histograms on the right) that for AD neurons (taking all cases together) both surface area and volume values were reduced in AT8 + (especially in type II) neurons, compared to AT8 − neurons. Note that in E and F there was a tendency for this reduction in all AD patients analyzed, with statistically significant differences between the different cell types of each patient in five out of the six cases analyzed (Friedman test, *p ≤ 0.01; **p ≤ 0.001).
The possible relationship between GA fragmentation and the progressive neuronal accumulation of hyperphosphorylated tau protein has not been explored thoroughly in previous studies. Here, we have analyzed this relationship both in pyramidal neurons from temporal neocortex and CA1 of AD patients, in confocal stacks taken from sections with double immunofluorescence staining using antibodies directed to MG160, and AT8 antibodies which recognize phospho Ser202 and phospho Thr205 sites in microtubule-associated tau protein (Fig. 3, Fig. 4, Fig. 5), and counterstained with Dapi. According to one of our previous studies (Blazquez-Llorca et al., 2010), it is possible to distinguish between AT8-negative neurons and two types of tau AT8-ir neurons showing different patterns of immunostaining for paired helical filaments (PHF): pattern I, which have diffuse cytoplasmic staining with no fibrillar aggregates (pre-tangle stage) and pattern II, which typically form NTF. Regarding GA fragmentation—considering all cell types analyzed in AD patients—we found that, as in control cases, the percentage of neurons with a fragmented and highly altered GA was higher in CA1 than in temporal neocortex (Table 2). Considering neurons with different patterns of PHF-tauAT8 immunostaining separately, we found that the percentage of neurons with a normal, non-fragmented GA appearance decreased in AD patients, even in AT8-negative neurons, as compared to controls, indicating a generalized alteration of GA in AD (Table 2). In addition, we observed that, compared to AT8-negative neurons, the percentage of neocortical and hippocampal neurons showing a highly altered GA was higher in those neurons with hyperphosphorylated tau (both in type I and more markedly in type II neurons) (Table 2, Fig. 3, Fig. 5).
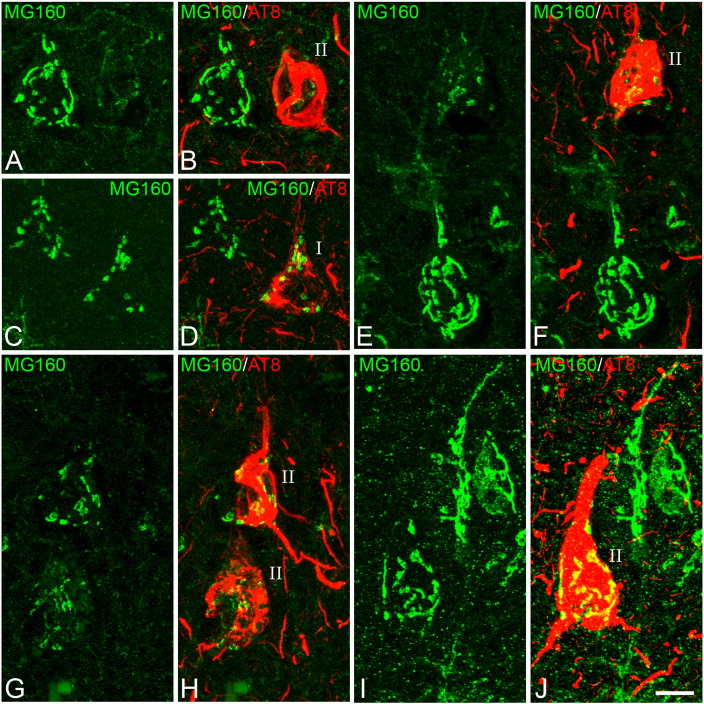
Fig. 3.
Distribution of MG160 in the GA of AT8 − and AT8 + human neocortical pyramidal neurons
A, B; C, D; E, F; G, H; I, J: pairs of confocal stack projection images taken from the temporal neocortex of sections double-immunostained for MG160/AT8 showing the distribution of MG160 in the GA of AT8 −, and AT8 + (type I and type II) pyramidal neurons. Scale bar shown in J indicates 6.4 μm in A and B, 9.1 μm in C and D, 8.1 μm in E and F, 9.6 μm in G and H and 8.8 μm in I and J.
Fig. 5.
Distribution of MG160 in the GA of AT8 − and AT8 + human CA1 pyramidal neurons
A, D; B, E; C, F; Pairs of confocal stack projection images taken from sections from the hippocampal CA1 region double-immunostained for MG160/AT8 showing the distribution of MG160 in the GA of AT8 −, and AT8 + (type I and type II) pyramidal neurons. Scale bar in F indicates 20 μm in A–D and C–F, and 19 μm in B–E. Histograms showing, on the left side, higher surface area (G) and volume (H) values (mean ± SE) of MG160-ir GA elements, in control (white bars, n = 189) compared with AD (patterned bars, n = 713) CA1 pyramidal neurons. Note on the right side that for AD neurons (taking all cases together) both surface area and volume values were reduced in AT8 + (especially in type II) neurons, compared to AT8 − neurons. Note that in I and J there was a tendency for this reduction in all AD patients analyzed, with statistically significant differences between the different cell types of each patient in four out of the seven cases analyzed.
We also found a general drop in surface area and volume of MG160-ir GA elements in neocortical and hippocampal neurons from AD patients compared to neurons from control cases (Figs. 4C–D, 5G–H). In order to determine if there was a specific deleterious effect of the accumulation of hyperphosphorylated tau on the GA in AD patients, we analyzed these data distinguishing between cells in pre-tangle stage (type I, Fig. 3, Fig. 5D) and in tangle stage (type II, Fig. 3, Fig. 5). According to the above qualitative analysis, the results showed a progressive decline in the mean surface area and mean volume of MG160-ir GA elements from AT8 − to type I and, more markedly, type II AT8-positive neurons—both in the temporal neocortex (Fig. 4C,D) and in CA1 (Fig. 5G,H). To evaluate the possible influences of inter-individual variability on these results, we analyzed separately data obtained from the different AD patients. The results showed a similar trend in all patients, with the decrease in surface area and volume of GA reaching statistical significance in most (but not all) of the patients (Figs. 4E–F, 5I–J).
3.3. Alterations of the Golgi apparatus in neurons with hyperphosphorylated tau in a non-demented case
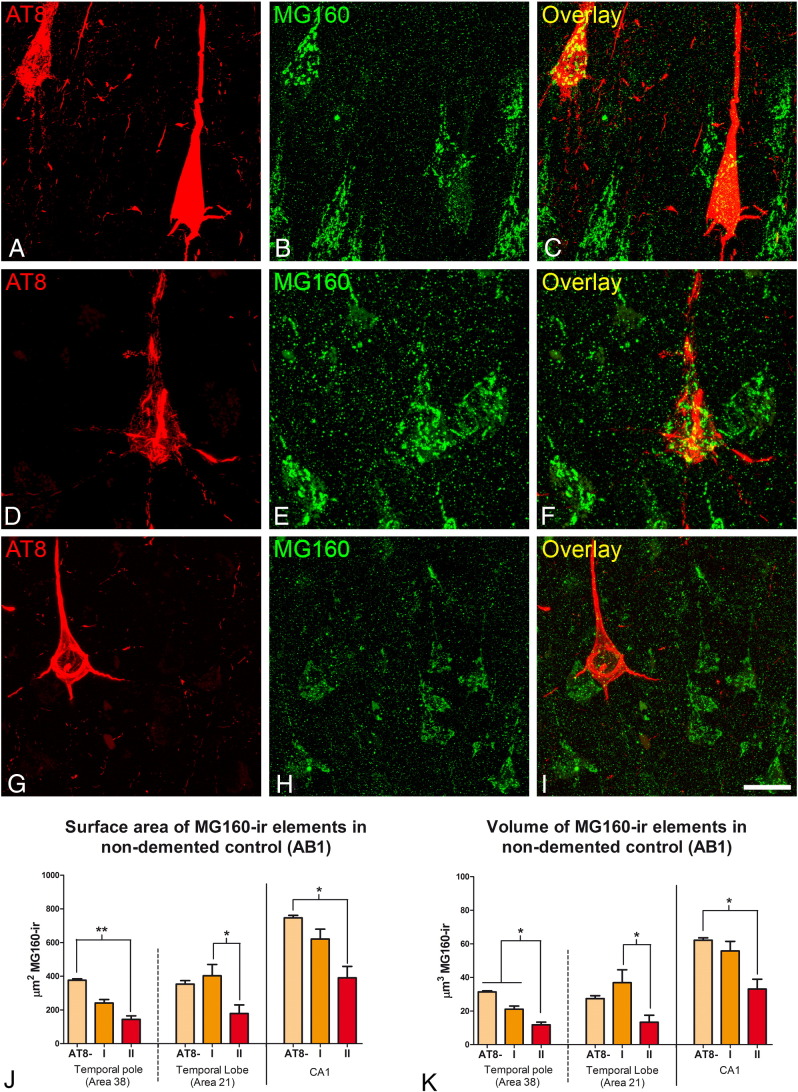
To test in human tissue the effects induced by the sole presence of the aggregation of hyperphosphorylated tau (presumably without any other possible pathologic factors present in the AD such as the presence of β-amyloid plaques), we studied the morphological features of neuronal GA in a human brain from a 45-year-old male adult who was free of any neurological or psychiatric disease (not diagnosed with AD) and had died from a lung tumor (AB1, see Table 1). In the brain of this patient, we found a relatively large number of neurons with intracellular aggregation of hyperphosphorylated tau protein in temporal (Brodmann areas 20, 21, 22 and 38), prefrontal (9, 10 12) and frontal (areas 44, 45, 46 and 47) cortical areas while they were not detected in other cortical regions such as the primary motor (area 4), primary somatosensory (area 3b), primary visual (area 17) cortical areas. No β-amyloid plaques were found in any of these regions. In confocal stacks taken from sections double-immunostained for MG160 and hyperphosphorylated tau (AT8 antibodies), we obtained surface area and volume values of MG160-ir GA elements (Fig. 6J–K) in neurons with phospho-tau (in pre-tangle (I) and tangle (II) stages) and without phospho-tau, in CA1 (Fig. 6A–C). Values were also obtained in the temporal neocortex, in area 22 (Fig. 6D–F) and in area 38 (Fig. 6G–I), where tau-ir neurons were more abundant. The results in the three regions analyzed showed a general reduction in surface area and volume measurements of MG160-ir in AT8 + neurons, which reached statistical significance only in the type II pattern of AT8 immunostaining, that is, in neurofibrillary tangle-bearing neurons (Fig. 5J–K).
Fig. 6.
Distribution of MG160 in the GA of AT8 − and AT8 + human CA1 and neocortical pyramidal neurons in a non-demented control case
Pairs of confocal stack projection images taken from CA1 (A–C) and areas 21 (D–F) and 38 (G–I) of the temporal neocortex from sections double-immunostained for MG160/AT8 showing the distribution of MG160 in the GA of AT8 −, and AT8 + (type I and type II) pyramidal neurons from a human in a non-demented control case. Scale bar in I indicates 24 μm in A–C, 14 μm in D–F and 22,65 in G–I. Histograms show surface area (J) and volume (K) values (mean ± SE) of MG160-ir GA elements, in AT8 − neurons (area 38 n = 397; area 21 n = 133; CA1 n = 185) and AT8 + neurons (patterns I and II; area 38, n = 49; area 21, n = 12; CA1, n = 47). Note that both surface area and volume values were significantly reduced in type II AT8 + neurons, compared to AT8 − neurons (Friedman test, *p ≤ 0.01; **p ≤ 0.001).
Consequently, these results further support the idea that the aggregation of hyperphosphorylated tau may exert a specific deleterious effect on the morphological features of the GA in cortical neurons, which might contribute to neuronal dysfunction and degeneration in AD.
3.4. Effects of aging and postmortem delay on the morphological features of the neuronal GA
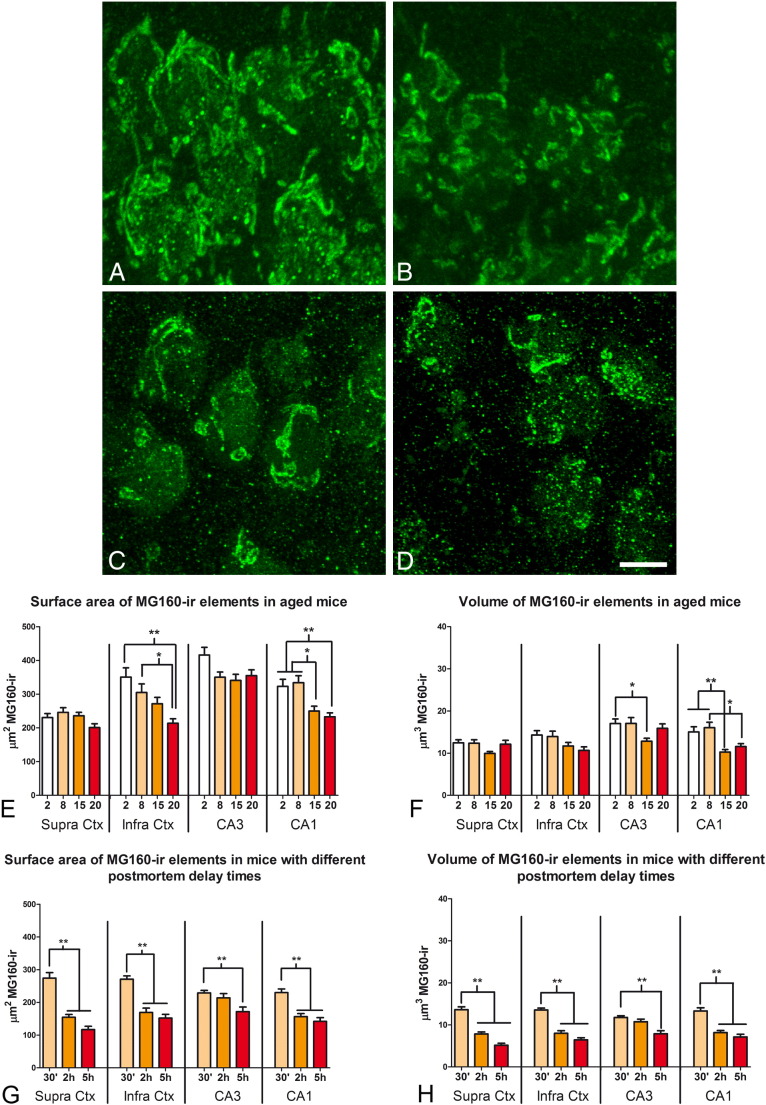
The differences in the size and fragmentation of the GA found between control and AD cases, and even the inter-individual differences found within each group, could be partially due to the differences in age of the patients and/or postmortem delay before fixation procedures. Fixation by immersion might also contribute to the differences in the GA between neocortical and CA1 hippocampal neurons (Table 2) as a consequence of the deeper localization of CA1 compared to the neocortex. Therefore, to evaluate the potential contribution of these factors to the observed differences and to help elucidate those alterations that were due to the course of the disease, we next used C57BL/6J mice with different ages (2, 8, 15 and 20 months, Fig. 7A, B, E, F) and young adult mice brains, which were extracted and fixed by immersion after different postmortem delays (Fig. 7C, D, G, H). We measured the surface area and volume of the MG160-ir GA elements in CA1 and CA3 hippocampal neurons and supra- and infra-granular pyramidal neurons from somatosensory cortex in confocal stacks taken from sections double-immunostained for MG160 and hyperphosphorylated tau (AT8 antibody).
Fig. 7.
Effects of aging and the postmortem delay period on the distribution of MG160 in the GA of pyramidal neurons
A and B: confocal stack projection mages showing examples of MG160 immunostaining of the GA of CA1 pyramidal neurons from mice aged 2 (A) and 20 (B) months. C and D show the MG160 immunostaining of the GA of layer II neocortical pyramidal neurons from mice fixed after postmortem periods of 30 min (C) or 5 h (D). Scale bar in D indicates 5,3 μm. Histograms show the reductions in surface area (E and G) and volume (F and H) values (mean ± SE) of MG160-ir GA elements, in pyramidal neurons from different areas in mice with different ages (E and F) or mice fixed after different postmortem times G and H). (Kruskal Wallis test, *p ≤ 0.01; **p ≤ 0.001).
As expected, in all regions and ages analyzed we found no AT8 immunostaining above background levels. That is, all neurons analyzed were AT8 negative. In addition, the GA of neurons in all regions and age groups had a normal, non-fragmented appearance (Fig. 7A, B). The quantitative analysis revealed no differences between the different age groups in terms of the surface area and volume of MG160-ir elements of supragranular pyramidal neurons (Fig. 7E, F). However, a tendency for a reduction with age in the size of the GA, as judged by MG160 immunostaining, was found in pyramidal neurons from infragranular neocortical layers and the CA3 region. This reduction was more marked in pyramidal neurons from CA1 (Fig. 7E, F). Finally, we found that the surface area and volume of MG160-ir GA elements of pyramidal neurons were sensitive to the post-mortem delay period, as they were significantly reduced, in all regions examined, in animals with postmortem periods of 2 h and 5 h as compared with those with a delay of 30 min before fixation (Fig. 7G, H).
4. Discussion
The present results show that MG160 is widely expressed in the GA of human and mouse neocortical and hippocampal neurons. Using this protein as a GA marker, we showed by immunofluorescence, confocal microscopy and 3D quantification that, in both species, both the volume and surface area of the GA in CA1 hippocampal neurons are larger than in neocortical neurons, which is in line with previous studies in the cerebral cortex of Syrian hamsters (Anton-Fernandez et al., 2015). The present study also shows that the GA in mouse neocortical neurons is sensitive to aging and delay time prior to fixation—a sensitivity which is even more marked in the case of hippocampal neurons. These factors should therefore be taken in consideration when analyzing the differences in the size of the GA between human control neurons and those from AD patients (see below) or when comparing animal models of AD with patients, as the brain from animal models is commonly fixed by perfusion with no postmortem period.
In human control cases we also found that the GA of < 10% of the neocortical neurons analyzed displayed a fragmented or highly altered appearance with a concomitant reduction in volume and surface area of the MG160-ir elements with respect to neurons with a non-fragmented GA. We consider that these alterations of the GA could be a consequence of the postmortem delay time before fixation. In the case of CA1 hippocampal neurons, the percentage of neurons with an altered GA was almost 25%—markedly higher than in the neocortical neurons. This observation could be related to the deeper position of CA1 compared to the more superficial location of the neocortex and the consequent longer period prior to fixation of the hippocampus by immersion or it may be that the hippocampal neurons are more sensitive to postmortem factors than the neocortex.
Our results also show that, in both temporal neocortex and hippocampus, the percentage of neurons with a fragmented GA was higher in brain samples from AD patients than in human control tissue. This is in general agreement with previous studies showing GA fragmentation in neurons from AD patients, which suggested an important role of GA fragmentation in the neuropathology and pathogenesis of AD (Baloyannis, 2014, Salehi et al., 1995, Stieber et al., 1996). GA fragmentation in our material was associated with important reductions in the volume and surface area of the MG160-expressing elements of the GA. Considering that (1) there was a significant difference in age between the control group and the AD patients used both in previous reports (Stieber et al., 1995) and in the present study (control, mean ± SD: 50.6 ± 9.8 years; AD, mean ± SD 83.1 ± 4.6 years) and (2) a decrease in volume and surface area of MG160-ir GA elements was observed in aged mice in CA1 in the present work, we cannot rule out the possibility that the decreases observed in AD patients—especially those in the CA1 region—could be in part due to aging of AD patients and not a consequence of the disease itself. However, this is unlikely since fragmentation of the neuronal GA from AD patients was also reported in a study using groups of control subjects and AD patients with no differences in age (Salehi et al., 1995). Postmortem time prior to fixation also affect the morphological features of the GA (present results). However, in the present study, there were no differences in this parameter between the control and AD cases and they is therefore not considered to be a factor contributing to the observed differences in GA volume and surface area.
4.1. GA fragmentation and immunostaining for AT8
The present work also showed that GA fragmentation and significant reductions in the volume and surface area of the MG160-expressing elements of the GA were more pronounced in neurons with a progressive accumulation of phospho-tau (that is, pretangle and tangle states, as revealed by AT8 antibodies that detect phosphorylations in Ser202/Thr205) than in AT8-negative neurons. This observation supports the idea that the aggregation of hyperphosphorylated tau may exert a specific deleterious effect on the morphological features of the GA. This is in partial disagreement with pioneering studies in AD patients reporting that the alterations (including fragmentation and decreases in size) of the GA of hippocampal neurons are unrelated to the content of intracellular NFT in the CA1 hippocampal field (Salehi et al., 1995) and preferentially associated with neurons devoid of NTF in the subiculum and entorhinal cortex (Stieber et al., 1996). Methodological differences might contribute to the discrepancies between the present work and these two studies as they were carried out in paraffin-embedded brain tissue blocks from AD patients with heterogeneous postmortem periods and fixed in formaldehyde for over 1 month. Furthermore, the size of the GA was estimated in 2D in 5-6 μm-thick sections, and therefore the cell body of the pyramidal cells examined was likely incomplete as the diameter of the soma of the majority of pyramidal cells is larger than 5 μm, whereas we made full 3D reconstructions using confocal microscopy. In addition, the identification of the GA with conventional light microscopy relied on its immunocytochemical staining using light opaque chromogens as well as the subsequent (Salehi et al., 1995) or simultaneous (Stieber et al., 1996) staining of intracellular NFTs using, respectively, the silver Bodian technique or histochemical methods with a different colored chromogen. The fixation procedures used in these studies might have affected the preservation of the GA and the sectioning and staining methods used may have interfered with the measurement of the size of the GA or the assessment of the level of tau hyperphosphorylation.
In the present study, the 3D segmentation of the GA elements was based on the observed threshold selection of fluorescent labeling. The threshold selection was adjusted slightly for each case, although the quality of immunostaining was similar in all cases. In addition, the threshold that was set for each confocal stack of images equally affected the AT8 − and AT8 + neurons contained in each microscopic field. Since labeling intensities influence the perceived size, the present methodology might induce some bias in the measurement of absolute values of GA surface area and volume. However, the main observation of the present study—showing a progressive reduction of the GA size associated with the increase in the accumulation of phospho-tau (from pretangle to neurofibrillary tangle-bearing neurons)—was consistently observed in all cases (see Fig. 5).
This suggests that the presence of phospho-tau aggregates might exert a progressive damage on the GA of human neurons. This is supported by the fact that GA alterations in AD patients were more pronounced in pyramidal neurons from CA1 than from the temporal neocortex (present work) and according to Braak and Braak studies (Braak and Braak, 1995) during the stereotypical spatiotemporal progression of AD, NFTs appear at earlier stages and progresses earlier in CA1 than in neocortex. In addition, the specific structural alterations in the GA observed in AT8 + NFT-bearing neocortical and hippocampal neurons from the brain of a non-demented patient who had not been diagnosed with AD and in the absence of β-amyloid plaques (present work) also support the idea that the presence of NFT is by itself enough to induce alterations on the structure of the GA. Interestingly, the relationship between tau hyperphosphorylation and GA alterations is in agreement with different lines of evidence and has also been established in previous studies using animal models of AD (Liazoghli et al., 2005). For example, the transitory GA fragmentation that mitotic dividing cells undergo is concomitant to an increase in tau phosphorylation, as revealed by antibodies that also recognize neurofibrillary tangles in AD such as AT8 (Delobel et al., 2002, Illenberger et al., 1998, Pope et al., 1994, Preuss et al., 1995, Preuss and Mandelkow, 1998, Vincent et al., 1996). In addition, the reversible alterations in volume and surface area of MG160-ir elements of the GA during the torpor phase of hibernation has also been related to the phospho-tau content, revealed with AT8 antibodies (Anton-Fernandez et al., 2015).
4.2. Temporal relationship between GA fragmentation and tau hyperphosphorylation
Regarding the temporal relationship between GA fragmentation and tau hyperphosphorylation, it is known that, among other factors, the integrity of the microtubule cytoskeleton determines the position and morphological properties of the GA (Burkhardt, 1998, Cole et al., 1996). It was suggested that tau binds Golgi membranes and microtubules (Farah et al., 2006). Therefore, morphological features of the GA might be potentially altered by the microtubule alterations induced by tau hyperphosphorylation. It has been proposed that in AD, tau pathology is an upstream event that precedes GA fragmentation since tau overexpression led to the fragmentation of the neuronal GA (Liazoghli et al., 2005, Lin et al., 2003). Nevertheless, tau hyperphosphorylation has been reported in HEK293/tau cells—after treatments with the microtubule-depolymerizing agent, nocodazole or the Golgi-disturbing agent, brefeldin A—which would indicate that fragmentation of GA is an upstream event required to trigger tau hyperphosphorylation (Jiang et al., 2014, Sutterlin et al., 2002).
Therefore, further studies should be carried out to unravel the time-relation of tau-phosphorylation and GA alterations, due to its crucial relevance for understanding pathology progression in AD.
4.3. Alterations in the GA AT8 − neurons
The present results have revealed alterations in the GA of AT8 − neurons. This is in line with the reported GA fragmentation in neurons without NFT, identified using silver staining techniques (Baloyannis, 2014, Salehi et al., 1995) or immunostaining for bFGF (Stieber et al., 1996). It is possible that some AT8 − neurons with a fragmented or altered GA might have tau that is hyperphosphorylated at residues other than Ser202/Thr205 (which are recognized by AT8 antibodies). Whether or not the degree of accumulation of phospho-tau that is hyperphosphorylated at different sites is correlated with the extent of GA alterations should be explored in further studies using PHF1 or AT180 antibodies which recognize tau that is hyperphosphorylated at other sites (Goedert et al., 1994).
However, other factors such as the presence of β-amyloid plaques could be responsible for the GA fragmentation reported here in pyramidal neurons that were not immunoreactive for AT8. Indeed, trafficking and maturation of APP, its processing enzymes and Aβ production have been shown to be dependent on the integrity of the GA (Burgos et al., 2010, Choy et al., 2012, Greenfield et al., 1999, Huse et al., 2002, Joshi et al., 2015, Joshi and Wang, 2015). In addition, in the APPswe/PS1ΔE9 animal model of AD, it has been reported that the accumulation Aβ peptide in neurons might induce GA fragmentation, thereby accelerating the traffic of APP and the production of Aβ (Joshi et al., 2015, Joshi and Wang, 2015).
It has also been reported that approximately 50% of AD patients may also develop TDP-43 pathology in certain brain regions (see Wilson et al., 2011). This pathology has been found to correlate with GA fragmentation in spinal cord neurons in patients with Amyotrophic lateral sclerosis (Fujita and Okamoto, 2005). Therefore, although TDP-43 pathology might potentially contribute to GA alterations in neurons from AD patients, this was not the case in the four out of seven patients used in this study for which information regarding TDP43 inclusions was available.
Regardless of additional possible factors that might induce GA alterations, the present study revealed that, in neocortical and hippocampal neurons from AD patients, the changes in the microtubule network related to tau hyperphosphorylation was associated with the fragmentation and reduction in volume and surface area of the GA.
Since the level of cell activity has been directly related to the size of the GA (Lucassen et al., 1993, Salehi et al., 1995), the alterations of the GA observed in the present study would likely result in a deregulation of neuronal calcium levels (Li et al., 2013) and alterations in protein synthesis, modification, and sorting (Salehi et al., 1995, Wang et al., 2008, Xiang et al., 2013). This suggests that the defects in the GA are likely to contribute to the neurotoxicity associated with AD.
Acknowledgements
The authors declare no conflict of interest. This work was supported by grants from the following entities: SAF 2015-66603-P from the Ministerio de Economía y Competitividad; Centro de Investigación en Red sobre Enfermedades Neurodegenerativas (CIBERNED, CB06/05/0066, Spain); and a grant from the Alzheimer's Association (ZEN-15-321663).
This project received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 720270 (Human Brain Project).
References
- Anton-Fernandez A. Changes in the Golgi apparatus of neocortical and hippocampal neurons in the hibernating hamster. Front. Neuroanat. 2015;9:157. doi: 10.3389/fnana.2015.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloyannis S.J. Golgi apparatus and protein trafficking in Alzheimer's disease. J. Alzheimers Dis. 2014;42(Suppl. 3):S153–S162. doi: 10.3233/JAD-132660. [DOI] [PubMed] [Google Scholar]
- Blazquez-Llorca L. Pericellular innervation of neurons expressing abnormally hyperphosphorylated tau in the hippocampal formation of Alzheimer's disease patients. Front. Neuroanat. 2010;4:20. doi: 10.3389/fnana.2010.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez-Llorca L. Abnormal tau phosphorylation in the thorny excrescences of CA3 hippocampal neurons in patients with Alzheimer's disease. J. Alzheimers Dis. 2011;26:683–698. doi: 10.3233/JAD-2011-110659. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol. Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. (discussion 278-84) [DOI] [PubMed] [Google Scholar]
- Burgos P.V. Sorting of the Alzheimer's disease amyloid precursor protein mediated by the AP-4 complex. Dev. Cell. 2010;18:425–436. doi: 10.1016/j.devcel.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt J.K. The role of microtubule-based motor proteins in maintaining the structure and function of the Golgi complex. Biochim. Biophys. Acta. 1998;1404:113–126. doi: 10.1016/s0167-4889(98)00052-4. [DOI] [PubMed] [Google Scholar]
- Burrus L.W. Identification of a cysteine-rich receptor for fibroblast growth factors. Mol. Cell. Biol. 1992;12:5600–5609. doi: 10.1128/mcb.12.12.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy R.W. Amyloid precursor protein (APP) traffics from the cell surface via endosomes for amyloid beta (Abeta) production in the trans-Golgi network. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E2077–E2082. doi: 10.1073/pnas.1208635109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole N.B. Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol. Biol. Cell. 1996;7:631–650. doi: 10.1091/mbc.7.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M.C. The Golgi apparatus and the pathogenesis of Alzheimer's disease. Am. J. Pathol. 1996;148:355–360. [PMC free article] [PubMed] [Google Scholar]
- Delobel P. Abnormal Tau phosphorylation of the Alzheimer-type also occurs during mitosis. J. Neurochem. 2002;83:412–420. doi: 10.1046/j.1471-4159.2002.01143.x. [DOI] [PubMed] [Google Scholar]
- Egea G. Actin dynamics at the Golgi complex in mammalian cells. Curr. Opin. Cell Biol. 2006;18:168–178. doi: 10.1016/j.ceb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Ellisman M.H. Diagnostic levels of ultrasound may disrupt myelination. Exp. Neurol. 1987;98:78–92. doi: 10.1016/0014-4886(87)90073-2. [DOI] [PubMed] [Google Scholar]
- Fan J. Golgi apparatus and neurodegenerative diseases. Int. J. Dev. Neurosci. 2008;26:523–534. doi: 10.1016/j.ijdevneu.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Farah C.A. Tau interacts with Golgi membranes and mediates their association with microtubules. Cell Motil. Cytoskeleton. 2006;63:710–724. doi: 10.1002/cm.20157. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Okamoto K. Golgi apparatus of the motor neurons in patients with amyotrophic lateral sclerosis and in mice models of amyotrophic lateral sclerosis. Neuropathology. 2005;25:388–394. doi: 10.1111/j.1440-1789.2005.00616.x. [DOI] [PubMed] [Google Scholar]
- Fujita Y. The Golgi apparatus is fragmented in spinal cord motor neurons of amyotrophic lateral sclerosis with basophilic inclusions. Acta Neuropathol. 2002;103:243–247. doi: 10.1007/s004010100461. [DOI] [PubMed] [Google Scholar]
- Fujita Y. Fragmentation of Golgi apparatus of nigral neurons with alpha-synuclein-positive inclusions in patients with Parkinson's disease. Acta Neuropathol. 2006;112:261–265. doi: 10.1007/s00401-006-0114-4. [DOI] [PubMed] [Google Scholar]
- Glick B.S. Can the Golgi form de novo? Nat. Rev. Mol. Cell Biol. 2002;3:615–619. doi: 10.1038/nrm877. [DOI] [PubMed] [Google Scholar]
- Goedert M. Epitope mapping of monoclonal antibodies to the paired helical filaments of Alzheimer's disease: identification of phosphorylation sites in tau protein. Biochem. J. 1994;301(Pt 3):871–877. doi: 10.1042/bj3010871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas N.K. Fragmentation of the Golgi apparatus of motor neurons in amyotrophic lateral sclerosis. Am. J. Pathol. 1992;140:731–737. [PMC free article] [PubMed] [Google Scholar]
- Gonatas J.O. MG-160, a membrane sialoglycoprotein of the medial cisternae of the rat Golgi apparatus, binds basic fibroblast growth factor and exhibits a high level of sequence identity to a chicken fibroblast growth factor receptor. J. Cell Sci. 1995;108(Pt 2):457–467. doi: 10.1242/jcs.108.2.457. [DOI] [PubMed] [Google Scholar]
- Gonatas J.O. Truncations of the C-terminal cytoplasmic domain of MG160, a medial Golgi sialoglycoprotein, result in its partial transport to the plasma membrane and filopodia. J. Cell Sci. 1998;111(Pt 2):249–260. doi: 10.1242/jcs.111.2.249. [DOI] [PubMed] [Google Scholar]
- Gonatas N.K. The involvement of the Golgi apparatus in the pathogenesis of amyotrophic lateral sclerosis, Alzheimer's disease, and ricin intoxication. Histochem. Cell Biol. 1998;109:591–600. doi: 10.1007/s004180050257. [DOI] [PubMed] [Google Scholar]
- Gonatas N.K. Fragmentation of the Golgi apparatus in neurodegenerative diseases and cell death. J. Neurol. Sci. 2006;246:21–30. doi: 10.1016/j.jns.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Greenfield J.P. Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer beta-amyloid peptides. Proc. Natl. Acad. Sci. U. S. A. 1999;96:742–747. doi: 10.1073/pnas.96.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z. The study of Golgi apparatus in Alzheimer's disease. Neurochem. Res. 2007;32:1265–1277. doi: 10.1007/s11064-007-9302-4. [DOI] [PubMed] [Google Scholar]
- Huse J.T. Beta-secretase processing in the trans-Golgi network preferentially generates truncated amyloid species that accumulate in Alzheimer's disease brain. J. Biol. Chem. 2002;277:16278–16284. doi: 10.1074/jbc.M111141200. [DOI] [PubMed] [Google Scholar]
- Illenberger S. The endogenous and cell cycle-dependent phosphorylation of tau protein in living cells: implications for Alzheimer's disease. Mol. Biol. Cell. 1998;9:1495–1512. doi: 10.1091/mbc.9.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q. Golgin-84-associated Golgi fragmentation triggers tau hyperphosphorylation by activation of cyclin-dependent kinase-5 and extracellular signal-regulated kinase. Neurobiol. Aging. 2014;35:1352–1363. doi: 10.1016/j.neurobiolaging.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Joshi G., Wang Y. Golgi defects enhance APP amyloidogenic processing in Alzheimer's disease. BioEssays. 2015;37:240–247. doi: 10.1002/bies.201400116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi G. Golgi fragmentation in Alzheimer's disease. Front. Neurosci. 2015;9:340. doi: 10.3389/fnins.2015.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine T.P. Mitotic disassembly and reassembly of the Golgi apparatus. Cold Spring Harb. Symp. Quant. Biol. 1995;60:549–557. doi: 10.1101/sqb.1995.060.01.058. [DOI] [PubMed] [Google Scholar]
- Li L.H. The Golgi apparatus: panel point of cytosolic Ca2 + regulation. Neurosignals. 2013;2013:272–284. doi: 10.1159/000350471. [DOI] [PubMed] [Google Scholar]
- Liazoghli D. Fragmentation of the Golgi apparatus induced by the overexpression of wild-type and mutant human tau forms in neurons. Am. J. Pathol. 2005;166:1499–1514. doi: 10.1016/S0002-9440(10)62366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.L. Ultrastructural neuronal pathology in transgenic mice expressing mutant (P301L) human tau. J. Neurocytol. 2003;32:1091–1105. doi: 10.1023/B:NEUR.0000021904.61387.95. [DOI] [PubMed] [Google Scholar]
- Lucassen P.J. Activation of the human supraoptic and paraventricular nucleus neurons with aging and in Alzheimer's disease as judged from increasing size of the Golgi apparatus. Brain Res. 1993;632:105–113. doi: 10.1016/0006-8993(93)91144-h. [DOI] [PubMed] [Google Scholar]
- Mirra S.S. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mizuno Y. Familial Parkinson's disease. Alpha-synuclein and parkin. Adv. Neurol. 2001;86:13–21. [PubMed] [Google Scholar]
- Pope W.B. Microtubule-associated protein tau is hyperphosphorylated during mitosis in the human neuroblastoma cell line SH-SY5Y. Exp. Neurol. 1994;126:185–194. doi: 10.1006/exnr.1994.1057. [DOI] [PubMed] [Google Scholar]
- Preuss U., Mandelkow E.M. Mitotic phosphorylation of tau protein in neuronal cell lines resembles phosphorylation in Alzheimer's disease. Eur. J. Cell Biol. 1998;76:176–184. doi: 10.1016/S0171-9335(98)80032-0. [DOI] [PubMed] [Google Scholar]
- Preuss U. Cell cycle-dependent phosphorylation and microtubule binding of tau protein stably transfected into Chinese hamster ovary cells. Mol. Biol. Cell. 1995;6:1397–1410. doi: 10.1091/mbc.6.10.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C., Haase G. Editorial: Golgi pathology in neurodegenerative diseases. Front. Neurosci. 2015;9:489. doi: 10.3389/fnins.2015.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A. Fragmentation of the Golgi apparatus of the ballooned neurons in patients with corticobasal degeneration and Creutzfeldt-Jakob disease. Acta Neuropathol. 2000;100:270–274. doi: 10.1007/s004010000182. [DOI] [PubMed] [Google Scholar]
- Salehi A. Decreased activity of hippocampal neurons in Alzheimer's disease is not related to the presence of neurofibrillary tangles. J. Neuropathol. Exp. Neurol. 1995;54:704–709. doi: 10.1097/00005072-199509000-00013. [DOI] [PubMed] [Google Scholar]
- Stieber A. MG160, a membrane protein of the Golgi apparatus which is homologous to a fibroblast growth factor receptor and to a ligand for E-selectin, is found only in the Golgi apparatus and appears early in chicken embryo development. Exp. Cell Res. 1995;219:562–570. doi: 10.1006/excr.1995.1265. [DOI] [PubMed] [Google Scholar]
- Stieber A. In Alzheimer's disease the Golgi apparatus of a population of neurons without neurofibrillary tangles is fragmented and atrophic. Am. J. Pathol. 1996;148:415–426. [PMC free article] [PubMed] [Google Scholar]
- Sutterlin C. Fragmentation and dispersal of the pericentriolar Golgi complex is required for entry into mitosis in mammalian cells. Cell. 2002;109:359–369. doi: 10.1016/s0092-8674(02)00720-1. [DOI] [PubMed] [Google Scholar]
- Vincent I. Mitotic mechanisms in Alzheimer's disease? J. Cell Biol. 1996;132:413–425. doi: 10.1083/jcb.132.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Golgi cisternal unstacking stimulates COPI vesicle budding and protein transport. PLoS One. 2008;3:e1647. doi: 10.1371/journal.pone.0001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A.C. TDP-43 in aging and Alzheimer's disease - a review. Int. J. Clin. Exp. Pathol. 2011;4:147–155. [PMC free article] [PubMed] [Google Scholar]
- Xiang Y. Regulation of protein glycosylation and sorting by the Golgi matrix proteins GRASP55/65. Nat. Commun. 2013;4:1659. doi: 10.1038/ncomms2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi F. Identification of MG-160, a FGF binding medial Golgi sialoglycoprotein, in brain tumors: an index of malignancy in astrocytomas. Int. J. Oncol. 2003;22:1045–1049. [PubMed] [Google Scholar]
- Zhou Z. Identification and characterization of a fibroblast growth factor (FGF) binding domain in the cysteine-rich FGF receptor. J. Biol. Chem. 1997;272:5167–5174. doi: 10.1074/jbc.272.8.5167. [DOI] [PubMed] [Google Scholar]
- Zuber M.E. Cysteine-rich FGF receptor regulates intracellular FGF-1 and FGF-2 levels. J. Cell. Physiol. 1997;170:217–227. doi: 10.1002/(SICI)1097-4652(199703)170:3<217::AID-JCP1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]