Abstract
The invasion of red blood cells (RBCs) by Plasmodium falciparum is dependent on multiple molecular interactions between erythrocyte receptors and parasite ligands. Invasion studies using culture-adapted parasite strains have indicated significant receptor heterogeneity. It is not known whether this heterogeneity reflects the parasite invasion arsenal in the field. We have studied the invasion phenotypes of 14 distinct field isolates from the Legal Amazon areas of Brazil by using erythrocyte invasion assays to investigate invasion into normal, enzyme-treated, and clinical-mutant RBCs. Analysis of these isolates revealed four distinct invasion profiles. Using En(a−) cells to get an unequivocal estimate of the use of glycophorin A (GPA) as a receptor, we found that the 175-kDa erythrocyte-binding antigen (EBA-175)/GPA pathway was used by a minority of the parasite isolates studied. Although polymorphism of region II domains at specific amino acid positions in both EBA-140 and EBA-181 was found in these field isolates, this did not correlate with invasion profiles and thus receptor selectivity. These studies have further confirmed the existence of a significant diversity of invasion pathways in nature and suggest that additional parasite ligands will have to be targeted to devise global vaccines that will work in the field.
The human red blood cell (RBC) serves as the host vehicle for the malaria parasite Plasmodium falciparum for the entire erythrocytic phase of the parasite's life cycle. Invasion of erythrocytes by malaria parasites is a multistep process involving several specific interactions between receptors on the RBCs and parasite ligands. As invasion is the central point in the erythrocytic life cycle of the malaria parasite, the presence of multiple invasion pathways is believed to be a survival strategy of the malaria parasite. In P. falciparum, five major invasion pathways have been identified. Of these, only two have been well characterized, one involving glycophorin A (GPA) and the 175-kDa erythrocyte-binding antigen (EBA-175) (5, 24) and the second utilizing glycophorin C (GPC) and a 140-kDa paralogue of EBA-175, EBA-140 (also called PfEBP-2 and baebl) (13, 14, 15, 19, 27). At least five additional receptors on the erythrocyte surface, including glycophorin B (GPB) and four as yet unidentified receptors, X, Y, E, and Z, have been postulated to play a role in GPA-independent pathways of invasion, termed alternative pathways (7, 8). Receptor X is neuraminidase resistant but trypsin sensitive (11), Y and E are trypsin resistant and neuraminidase and chymotrypsin sensitive (8, 22), and receptor Z is resistant to neuraminidase and trypsin but sensitive to chymotrypsin (9). In addition to EBA-175 and EBA-140, three other merozoite ligands, EBA-181 (jesebl), PfNBP1, and PfNBP2b, have been characterized (9, 10, 22); however, the identities of their RBC receptors remain unknown. Thus, although parasite invasion has attracted considerable study, the molecules and the basic mechanisms responsible for the GPA-independent alternative pathways remain elusive.
Laboratory-maintained malaria strains can be divided into those that preferentially use the sialic acid-dependent pathway of invasion (Dd2, FCR3, and Camp) and those that also use sialic acid-independent invasion pathways (Dd2-NM, HB3, 7G8, and 3D7) (6, 18). As invasion studies have focused mainly on pathways used by laboratory-maintained strains of P. falciparum, not much is known about the repertoire of invasion pathways used by parasites in the field. A study of field isolates in India has shown that 12 of 15 fresh isolates used the alternative invasion pathways and only three isolates were dependent on sialic acid and GPA for invasion (20). This finding has challenged the focus on EBA-175 as the sole vaccine candidate that could induce inhibition of RBC invasion. However, a study of invasion phenotypes in The Gambia revealed a high dependence on sialic acid and trypsin-sensitive proteins on the RBC surface and thus, the authors concluded, a high utilization of the GPA pathway (8). These contrasting results, from two distinct regions of endemicity, highlighted the need for additional studies of invasion pathways used by field isolates in other regions where malaria is endemic.
In this study, we sought to validate the importance of the different invasion pathways (usage of specific RBC receptor and parasite ligand combinations) in field isolates from Brazil. We have chosen to study this area as 7G8, which was the first parasite line shown to utilize the alternative pathway receptor(s) X, was isolated in Brazil (11). Moreover, in order to reach an unequivocal conclusion on the use of GPA as a parasite receptor in the field, rates of invasion of mutant cells lacking GPA by field isolates that were sensitive to both neuraminidase and trypsin were assessed for the first time. Recently, receptor selectivity in laboratory strains of the parasite has also been linked to polymorphism in defined amino acid positions in the binding domains of the two parasite ligands EBA-140 and EBA-181 (16, 17). We documented some of the same polymorphism in the Brazilian field isolates but found no correlation with the invasion phenotypes of the corresponding parasites.
MATERIALS AND METHODS
P. falciparum field isolates.
P. falciparum field isolates were collected from malaria patients in Peixoto de Azevedo, a municipality of Mato Grosso state located in the southern part of the Amazon region in Brazil. The individuals were usually migrant workers without documented ethnicity who had come to the local health service center, run by the Ministry of Health, and were found to be P. falciparum positive and P. vivax negative. Blood from consenting infected patients was cryopreserved in liquid nitrogen. Parasites were thawed and cultured for approximately five cycles before use in invasion assays. Parasite cultures were maintained in A+ RBCs at a hematocrit of 5% in RPMI 1640 supplemented with 10% human serum (A+) as previously described (28).
Enzymatic treatment of erythrocytes.
A+ erythocytes were washed three times in RPMI 1640 before treatment with neuraminidase, trypsin, or α-chymotrypsin (13). Briefly, 0.1 ml of packed RBCs was treated with either 1 ml of 19.4-mg/ml trypsin treated with TPCK ([N-tosyl-l-phenylalanine chloromethyl ketone]; Sigma), 1 mg of α-chymotrypsin (Sigma)/ml, or 0.1 ml of 0.1-IU/ml neuraminidase (Sigma) for 30 min at 37°C. The RBCs were washed extensively in phosphate-buffered saline and RPMI 1640 before use in invasion assays. Efficacy of each enzyme treatment was assessed in the Laboratory of Immunohematology, New York Blood Center, New York, by assaying for loss of RBC agglutinability by using a lectin and a panel of monoclonal antibodies against antigenic determinants on different blood group proteins that are sensitive to the enzymatic treatments. For assessment of the efficacy of neuraminidase treatment, lectin from the peanut, Arachis hypogaea, was used to detect the T antigen, exposed after sialic acid residues had been cleaved by neuraminidase treatment of the RBCs. For assessment of the efficacy of trypsin and chymotrypsin treatment, anti-M and anti-S antibodies were used. The M antigen is present on GPA and trypsin cleaves GPA at residues 31 and 39, leading to loss of the M antigen. Similarly, the S antigen is present on GPB and GPB is cleaved by α-chymotrypsin at residue 34, leading to loss of the S antigen.
In vitro RBC invasion assay.
P. falciparum trophozoites were obtained after two rounds of sorbitol treatment (12) followed by another 14 h of culturing and purification on Percoll gradient. To each well of a 96-well flat-bottom plate, 20 μl of test RBCs of a defined enzymatic phenotype (6 × 106 cells/μl) was added. Infected RBCs (parasitemia, >95%) were added to each well to yield a parasitemia of ∼0.2% in a total volume of 300 μl. Samples were analyzed at different time points (every 48 h for 7 days) for parasitemia and stage of development from Giemsa-stained smears. A minimum of 1,000 cells were counted from each well. In each experiment, invasion assays were done in triplicate. RBCs from rhesus monkeys, which are resistant to P. falciparum invasion, were used as a negative control. Invasion efficiencies were determined by subtracting parasitemia in rhesus cells on day 7 from test parasitemia on day 7 in three independent experiments, taking care to ensure that all parasites were still in the log phase of growth. Parasites were grouped into invasion profiles based on their sensitivities to a panel of enzymes. If day-7 parasitemia in enzyme-treated RBCs was less than 50% of the day-7 parasitemia in wild-type RBCs, the parasite isolate was considered to be sensitive to that enzyme treatment.
Similarly, invasion of En(a−) cells and age- and storage-matched control normal RBCs was assessed in three independent experiments. Parasitemia was initiated at 0.2%, and final parasitemia was recorded after 48 h.
Genotyping of field isolates and analysis of polymorphism in binding regions of EBA-135 and EBA-181.
Field isolates were assayed for the presence of single versus multiple clones by PCR amplification of gene fragments encoding msp-1 block 2 and msp-2 block 3. Oligonucleotide primers based on conserved sequences flanking these polymorphic regions were used and have been described previously (25, 26). Isolates with single bands for each PCR were typed as single clones.
Genomic DNA was used as a template for amplification of 1.8-kb fragments corresponding to the region II domains of EBA-140 and EBA-181 with primers designed from the reference sequences (GenBank accession numbers AF332918 and AF461096). The forward and reverse primers for EBA-140 were 5′-TATCGTTTTTTTATGAGCAT-3′ and 5′-GTCAGAATAGGTACAATATT-3′. The forward and reverse primers for EBA-181 were 5′-TACATAGATATCCAGTTAGT-3′ and 5′-TCACAGAATTGTGATTTACA-3′. The elongase amplification system (Invitrogen) was used to ensure high fidelity of replication. Purified PCR products were sequenced by cycle sequencing in both directions. All visibly ambiguous positions were resequenced from new PCR products to confirm their accuracy.
Nucleotide sequence accession numbers.
Gene sequences reported for field isolates have been submitted to GenBank under accession numbers AY652780 through AY652807.
RESULTS
Enzyme treatment of RBCs.
To assay for efficacy of enzyme treatment, agglutination assays were done on the treated cells, and results of these assays are presented in Table 1. It is clear that the three enzymes worked efficiently since treatment with trypsin, which cleaves GPA (23), resulted in the loss of the M antigen and thus loss of agglutination by the anti-M antibodies. However, GPB, which bears the S antigen, is not affected by trypsin and thus agglutination using the anti-S antibody occurred as in untreated RBCs. Similarly, treatment of the RBCs with α-chymotrypsin resulted in the loss of the S antigen present on GPB (23) and thus loss of agglutination using anti-S antibodies. GPA is not cleaved by α-chymotrypsin and hence agglutination by anti-M antibody was normal as in untreated cells. Neuraminidase treatment results in the loss of sialic acid residues, which exposes the T epitope on the RBC. The Arachis hypogaea lectin binds to the T epitope and causes agglutination of the RBCs (23).
TABLE 1.
Agglutination scores for RBCs after various enzyme treatments
| Treatment enzyme | Scorea determined using:
|
||
|---|---|---|---|
| Arachis hypogaea lectin | Anti-M | Anti-S | |
| Neuraminidase | +++ | ND | ND |
| Trypsin | − | − | +++ |
| Chymotrypsin | − | +++ | − |
| None | − | +++ | +++ |
Agglutination was scored on a scale of − to +++, where − is no agglutination and +++ is maximum agglutination. ND, not determined.
Invasion profiles of P. falciparum field isolates.
Heterogeneity among P. falciparum lines can be assayed by demonstration of invasion by and growth of the parasite in enzyme-treated and mutant RBCs (21). Thus, we first typed the 14 Brazilian field isolates from Mato Grosso for invasion and growth in normal cells and cells treated with neuraminidase, trypsin, and α-chymotrypsin, which cleave different parts of various erythrocytic surface proteins. Invasion profiles for these isolates are shown in Table 2. Representative laboratory strains Dd2, 7G8, and 3D7 that exhibit differential invasion profiles that have been well characterized (3, 4, 6, 20) were also included in this study to facilitate analysis of the field isolates. Four invasion phenotypes were found in these isolates. Invasion by seven isolates was inhibited by treatment of RBCs with both neuraminidase and trypsin (type 1, designated NsTsCr [neuraminidase sensitive, trypsin sensitive, and α-chymotrypsin resistant]) (Table 2), similar to invasion by the 3D7 laboratory isolate, indicating a dependence of these isolates on the presence of both sialic acid and trypsin-sensitive proteins on the RBC surface for invasion. Five isolates did not invade neuraminidase-treated cells, just as Dd2 does not (type 2, designated NsTrCr [neuraminidase sensitive, trypsin resistant, and α-chymotrypsin resistant]) (Table 2). These isolates seemed to utilize only sialic acid moieties for invasion. In only one isolate was the ability to invade RBCs predominately sensitive to trypsin treatment of the RBCs (type 3, designated NrTsCr [neuraminidase resistant, trypsin sensitive, and α-chymotrypsin resistant]), a profile characteristic of the Brazilian 7G8 lab isolate (Table 2). Another isolate did not grow in RBCs treated with any of the three enzymes (type 4, designated NsTsCs [neuraminidase sensitive, trypsin sensitive, and α-chymotrypsin sensitive]) (Table 2), and thus this was the only isolate that we found to be sensitive to α-chymotrypsin treatment.
TABLE 2.
Invasion of enzyme-treated RBCs by P. falciparum isolates
| Invasion profile type | Isolate | Rate of invasion (%)a of RBCs treated with:
|
||
|---|---|---|---|---|
| Neuraminidase | Trypsin | Chymotrypsin | ||
| 1, NsTsCr | ||||
| 3D7 | 42 ± 9.5 | 34 ± 33 | 74 ± 26 | |
| JSL | 49 ± 18 | 49 ± 22 | 103 ± 20 | |
| ALR | 12 ± 0 | 5 ± 0 | 61 ± 0 | |
| PSS1 | 25 ± 0 | 41 ± 8 | 76 ± 36 | |
| BHZ | 43 ± 12 | 24 ± 3 | 100 ± 36 | |
| 04Q | 30 ± 23 | 38 ± 2 | 81 ± 6 | |
| FFS | 35 ± 2 | 37 ± 2.4 | 121 ± 36 | |
| GVM | 37.5 ± 12.5 | 42 ± 4.5 | 134 ± 18 | |
| 2, NsTrCr | ||||
| Dd2 | 8.6 ± 2.8 | 70 ± 4 | 56 ± 4 | |
| GOS | 3.6 ± 2.8 | 100 ± 30 | 69 ± 10 | |
| LANA | 0 ± 0 | 50 ± 3.5 | 125 ± 17 | |
| NS | 1 ± 1 | 125 ± 27 | 102 ± 16 | |
| IPN | 10.4 ± 3.9 | 63 ± 15 | 78 ± 16 | |
| 35Q | 25 ± 0 | 150 ± 50 | 165 ± 35 | |
| 3, NrTsCr | ||||
| 7G8 | 65 ± 8 | 9 ± 4.6 | 52 ± 11 | |
| 21Q | 77.5 ± 2.5 | 45 ± 5 | 98 ± 17 | |
| 4, NsTsCs | ||||
| RPN | 36 ± 11 | 21 ± 6.5 | 27 ± 1.5 | |
Invasion rates are expressed as percentages of invasion of test RBCs relative to invasion of normal RBCs. Data are means±standard deviations. Each experiment was repeated at least three times.
GPA and GPC are both sensitive to neuraminidase and trypsin and resistant to chymotrypsin treatment (23). The seven parasite isolates (type 1) (Table 2) whose growth in cells treated with neuraminidase and trypsin was inhibited may potentially be using either glycophorins or another as yet unidentified receptor with a similar enzymatic profile. To conclusively show whether this profile represents a dependence on or independence from GPA, we tested the invasion and growth of all parasites belonging to type 1 (Table 2) in clinical-mutant En(a−) cells which lack GPA. Means of results of these assays from three experiments are shown in Table 3. Interestingly, only two of the seven isolates, FFS and ALR, could not invade and grow in the En(a−) cells, and this finding challenges the notion that the GPA/EBA-175 pathway of invasion is the one of choice for most isolates. We could not test the use of GPC as a receptor for these isolates as Leach cells, which lack GPC, are not available.
TABLE 3.
Invasion of normal cells (WT), cells lacking GPA [En(a−)], and wild-type cells age- and storage-matched to En(a−) cells (FrC) by P. falciparum field isolates
| Isolate | % Parasitemia ± SD in:
|
||
|---|---|---|---|
| WT | FrC | En(a−) | |
| 3D7 | 2.3 ± 0.30a | 1.5 ± 0.05 | 0.8 ± 0 |
| ALR | 1.4 ± 0.10 | 1.2 ± 0.08 | 0.05 ± 0.01b |
| JSL | 1.8 ± 0 | 1.6 ± 0.16 | 0.9 ± 0.06 |
| GVM | 1.4 ± 0.04 | 1.0 ± 0.20 | 0.9 ± 0.12 |
| FFS | 0.8 ± 0.10 | 0.8 ± 0.10 | 0.15 ± 0.04b |
| 04Q | 1.3 ± 0.35 | 1.5 ± 0.23 | 0.9 ± 0.26 |
| BHZ | 1 ± 0.25 | 0.9 ± 0.06 | 0.7 ± 0.13 |
| PSS1 | 1.3 ± 0.16 | 1.2 ± 0.05 | 1.2 ± 0.03 |
Percent parasitemia (percentage of infected cells) was calculated using the average number from triplicate wells, of infected erythrocytes.
Results for the two strains that are postulated to use GPA as the primary receptor are given in bold.
Differentiation between single and multiple clone infection.
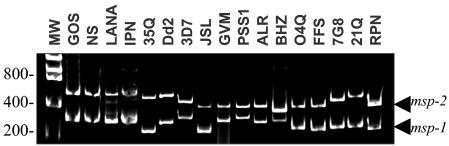
It was important to determine whether the field isolates in this study were clonal or a composite of multiple parasite lines. Each blood sample was subjected to PCR amplification of two different polymorphic loci: msp-1 (block 2) and msp-2 (block 3). As these loci are highly variable (25, 26), PCR amplification should have demonstrated the presence of multiple genotypes within each isolate sample. As can be seen in Fig. 1, each field isolate yielded a single PCR product with both msp-1 and msp-2 primers, thus suggesting that each isolate was clonal. As four of the isolates showed similarly sized products for both msp-1 and msp-2, a microsatellite analysis of 15 different loci was done for the four samples, and this analysis showed differences in many of the alleles (data not shown), confirming that these isolates were also unique.
FIG. 1.
PCR typing of P. falciparum field isolates based on msp-1 and msp-2. PCR products were electrophoretically separated on 2% agarose gels and visualized by staining with ethidium bromide. MW, standards corresponding to molecular weight (sizes are shown in base pairs). The top bands correspond to the msp-2 product, and the lower bands correspond to the msp-1 product.
Association between sequence polymorphism in the binding domains of EBA-140 and EBA-181 and invasion phenotype.
Region II is a 600-amino acid, cysteine-rich region in the N-terminal portion of the erythrocyte-binding ligand (EBL) protein family and is implicated in erythrocyte binding (12). Other investigators have looked unsuccessfully for an association between sequence polymorphism in EBA-175 and invasion phenotypes in field isolates (4). Recent studies of laboratory isolates of P. falciparum have identified a set of nonsynonymous changes in region II domains of both EBA-140 and EBA-181, and these changes were shown to have a functional significance as they changed the specificity of the erythrocyte receptor to which the mutated recombinant proteins bound (15, 16). Analyses of variants among field isolates were not done. Since EBA-175 did not seem to be the primary ligand used by the Brazilian field isolates (Tables 2 and Table 3) and its variants were shown not to be linked to different invasion phenotypes, we focused on the analysis of polymorphism of the other two ligands, EBA-140 and EBA-181. The entire region II domains of both EBA-140 and EBA-181 were sequenced in all 14 field isolates and in the laboratory isolates 7G8, Dd2, and 3D7. 4 shows the results of the analyses of differences among the isolates. Although we confirmed the presence of the previously defined polymorphisms for both ligands, we found only two of five EBA-140 variants and four of eight EBA-181 variants in the Brazilian isolates, and most importantly, no association between the presence of a given variant and a dominant invasion phenotype was found.
TABLE 4.
Lack of association between sequence polymorphism in EBA-140 and EBA-181 and invasion phenotypes
| Invasion profile | Isolate | Amino acid (codon) at EBA-140 region IIa position:
|
Amino acid (codon) at EBA-181 region IIb position:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 185 | 239 | 261 | 285 | 359 | 363 | 414 | 443 | 637 | ||
| NsTsCr | 3D7 | I (ATT) | N (AAT) | K (AAG) | K (AAA) | R (AGG) | V (GTT) | N (AAT) | K (AAA) | N (AAT) |
| ALR | V (GTT) | S (AGT) | T (ACG) | K (AAA) | R (AGG) | V (GTT) | N (AAT) | K (AAA) | N (AAT) | |
| JSL | V (GTT) | S (AGT) | T (ACG) | K (AAA) | R (AGG) | V (GTT) | N (AAT) | K (AAA) | K (AAA) | |
| GVM | V (GTT) | S (AGT) | T (ACG) | K (AAA) | K (AAG) | V (GTT) | I (ATT) | Q (CAA) | N (AAT) | |
| PSS1 | I (ATT) | N (AAT) | K (AAG) | K (AAA) | K (AAG) | V (GTT) | I (ATT) | Q (CAA) | N (AAT) | |
| BHZ | V (GTT) | S (AGT) | T (ACG) | K (AAA) | R (AGG) | V (GTT) | N (AAT) | Q (CAA) | N (AAT) | |
| O4Q | V (GTT) | S (AGT) | T (ACG) | K (AAA) | R (AGG) | V (GTT) | N (AAT) | Q (CAA) | N (AAT) | |
| FFS | V (GTT) | S (AGT) | T (ACG) | K (AAA) | R (AGG) | V (GTT) | N (AAT) | Q (CAA) | N (AAT) | |
| NsTrCr | Dd2 | V (GTT) | S (AGT) | T (ACG) | K (AAA) | R (AGG) | V (GTT) | I (ATT) | Q (CAA) | N (AAT) |
| GOS | V (GTT) | S (AGT) | T (ACG) | K (AAA) | R (AGG) | V (GTT) | I (ATT) | Q (CAA) | N (AAT) | |
| NS | V (GTT) | S (AGT) | T (ACG) | K (AAA) | R (AGG) | V (GTT) | I (ATT) | Q (CAA) | N (AAT) | |
| LANA | V (GTT) | S (AGT) | T (ACG) | K (AAA) | R (AGG) | V (GTT) | I (ATT) | Q (CAA) | N (AAT) | |
| IPN | V (GTT) | S (AGT) | T (ACG) | K (AAA) | R (AGG) | V (GTT) | I (ATT) | Q (CAA) | N (AAT) | |
| 35Q | I (ATT) | N (AAT) | K (AAG) | K (AAA) | R (AGG) | V (GTT) | N (AAT) | K (AAA) | N (AAT) | |
| NrTsCr | 7G8 | V (GTT) | S (AGT) | T (ACG) | K (AAA) | R (AGG) | V (GTT) | N (AAT) | Q (CAA) | N (AAT) |
| 21Q | V (GTT) | S (AGT) | T (ACG) | K (AAA) | R (AGG) | V (GTT) | N (AAT) | K (AAA) | N (AAT) | |
| NsTsCs | RPN | I (ATT) | N (AAT) | K (AAG) | K (AAA) | R (AGG) | V (GTT) | N (AAT) | K (AAA) | N (AAT) |
The(INKK(variant(is(bolded(to(distinguish(it(from(the(VSTK(variant.
The(RVIQN(variant(is(italicized,(the(RVNKN(variant(is(bolded,(the(RVNKK(variant(is(underlined,(and(the(KVIQN(variant(is(bolded(and(italicized(to(distinguish them(from(the(RVNQN(variant.
Mayer et al. (16) reported the following variations: VSTK, VSKK, ISKK, INRE, and INKK for EBA-140 (baebl) at amino acid positions 185, 239, 261, and 285. We found only a subset of polymorphisms in our field population, those corresponding to amino acids VSTK and INKK at these positions. Mayer et al. (16) also found that depending on the polymorphism present, the binding specificity of recombinant region II for RBCs with enzymatically defined receptors changes such that VSTK confers ligand-receptor binding that is sensitive to trypsin and neuraminidase and dependent on the presence of intact GPC on the RBCs. Using the invasion assay as a marker for receptor selectivity, we have found that parasites that exhibited VSTK or INKK belonged to more than one invasion group and that each group demonstrated different invasion profiles (types 1 to 3) (Table 2), thus establishing that there is no association between these EBA-140 region II variants and the ability of the parasite to invade defined erythrocytic phenotypes. For example, VSTK was the polymorphism found in ALR and FFS, which depended on GPA (a neuraminidase- and trypsin-sensitive receptor) for invasion (Table 4); however, 21Q, a parasite isolate that invaded via a receptor that is sensitive only to trypsin (potentially receptor X), also had a similar EBA-140 variant. INKK was found in PSS1 and 3D7, which were sensitive to treatment of RBCs with neuraminidase and trypsin, but also in 35Q, which was sensitive only to neuraminidase, as is Dd2, which is characterized by the VSTK variant, and in RPN, which was sensitive to all three enzymes.
In EBA-181, amino acid substitutions at positions 359, 363, 414, 443, and 637 have been reported (17), leading to eight different sets of point mutations: KDIQK, KDIQN, KVIQN, RVIQN, RVNQN, RVNKN, RVNKK, and KHIQN. These variants conferred different profiles of binding of the corresponding recombinant region II of the ligand to enzymatically treated RBCs. For instance, recombinant-protein region II of RVIQN parasites could bind to trypsin-treated RBCs but not to other treated RBCs, binding of region II of RVNKN parasites to RBCs was not inhibited by any treatment of the RBCs, and binding of recombinant-protein region II of RVNKK parasites was sensitive to all three treatments. In our analysis, we found only five combinations of amino acid substitutions in the fresh isolates: RVIQN, RVNQN, RVNKN, KVIQN, and RVNKK. Once again, these were distributed among parasites that exhibited different invasion profiles (Table 4), thus showing no association between EBA-181 polymorphisms and defined invasion phenotypes. For example, RVNKN was associated with binding of EBA-181 to an RBC receptor that is resistant to treatment with all three enzymes, but the Brazilian isolate ALR that was dependent on GPA, which is sensitive to neuraminidase and trypsin, exhibited the genotype corresponding to RVNKN, and the other GPA-dependent isolate, FFS, exhibited the genotype corresponding to RVNQN. Moreover, the ability of RPN to invade RBCs was sensitive to all three enzyme treatments, but binding to RBCs of recombinant ligand bearing the genotype corresponding to RVNKN did not depend on any protein moieties that are sensitive to the three enzymes.
DISCUSSION
Results from this study on invasion profiles of Brazilian field isolates lend support to previous reports of diversity in the utilization of receptors on the erythrocyte surface by the malaria parasite (11, 21). We have found that the Brazilian field isolates could be classified into four major groups of parasites, those whose ability to invade was sensitive to neuraminidase alone (NsTrCr), those whose ability to invade was sensitive to trypsin alone (NrTsCr), those whose profile was a combination of the first two (NsTsCr), and one whose ability to invade was sensitive to all three enzymes used, neuraminidase, trypsin, and α-chymotrypsin (NsTsCs). Unfortunately, previous studies examining the invasion profiles of fresh isolates from India (20) and The Gambia (3) have not exploited the use of α-chymotrypsin, which is the only enzyme that can cleave GPB (23) and thus shed light on the relative use of GPB as a receptor by the other field isolates. In this study, we found the invasion ability of only one parasite line, RPN, to be sensitive to α-chymotrypsin treatment of the RBCs. However, this parasite was sensitive to trypsin and neuraminidase too, and this finding suggests that the parasite is capable of using a variety of receptors for invasion.
The field isolates that were used in this study were all typed as single clones based on two polymorphic markers, msp-1 (block 2) and msp-2 (block 3). One would have expected to see multiple genotypes from a single isolate. We speculate that our results are because all lines were grown for five to six cycles before use in invasion and typing assays and this process may have eliminated slower-growing parasites, leaving the dominant parasite as the residual genotype in the parasite line. An additional explanation may be that Mato Grosso is an area where malaria transmission is hypoendemic and thus we may be seeing the results of low numbers of circulating parasites in mosquitoes and consequently in infected people, with the isolated parasites being the predominant ones sampled. PCR typing of the parasites and sequencing of the region II domains of EBA-140 and EBA-181 clearly showed that the field isolates are unique and independent. For the four isolates that had similar PCR profiles and region II sequences, microsatellite analysis at 10 different loci confirmed their uniqueness.
The studies using field isolates in India and in The Gambia have provided opposing conclusions on the importance of the GPA/EBA-175 invasion pathway in the field. We based our field study in the Brazilian Amazon as it is characterized by a hypoendemic pattern of infection, due mainly to its low demographic index, and thus provides an excellent field to test hypotheses on the importance of various invasion pathways in clonal fresh isolates. Additionally, 7G8, the first parasite shown to utilize alternative pathways of invasion, originates from Brazil. Using En(a−) cells in our invasion assays enabled us to unambiguously estimate the importance of the classical GPA-dependent pathway in Brazilian isolates whose ability to invade was sensitive to trypsin and neuraminidase, paralleling the sensitivity of the GPA receptor to these enzymes. Our findings that only two of the seven parasites within this group could not invade GPA-deficient cells contrast with the finding that in The Gambia the GPA-dependent pathway is the predominant pathway used and thus have important implications for a vaccine that targets only EBA-175, the parasite ligand that binds to GPA.
This study was also an excellent opportunity to confirm for the first time the presence of previously documented polymorphisms in two known EBL family members, EBA-140 and EBA-181 (16, 17), in field isolates and to investigate whether there is an association between the use of a specific invasion pathway, which would be characterized by a specific profile of invasion of enzyme-treated RBCs, and the presence of a variant region II in the corresponding parasites. Previous data on the EBA-140 and EBA-181 variants came from laboratory isolates of P. falciparum. Typing of the 14 Brazilian isolates revealed the presence of polymorphisms in both EBA-140 and EBA-181, though only two of the five EBA-140 variants and five of eight EBA-181 variants were found. Notably, we found no association between the sequence polymorphisms and the ability of the parasite to invade specific enzyme-treated erythrocytes. As invasion is a complex event that may involve many receptor-ligand interactions (1, 2), it appears that polymorphisms in a single ligand may not be sufficient for determining the final result and the selection of a particular invasion pathway by a parasite isolate. The assay used to study receptor selectivity in previous studies (16, 17) was based on an in vitro assay in which the rosetting of wild-type or treated RBCs is measured using transfected cells expressing recombinant region II containing the different polymorphisms. Thus, the polymorphisms in EBA-140 and EBA-181 do not seem to be functionally important as interpreted by receptor selectivity in our invasion assay.
In summary, field isolates from Mato Grosso, Brazil, were found to use a variety of erythrocyte receptors, with only two isolates dependent on GPA. Thus, studies of protective immunity should also focus on parasite ligands other than EBA-175 to devise a vaccine that can be used in areas around the world where malaria is endemic.
Acknowledgments
This work was supported by grant number P50 HL 54459 from the National Institutes of Health.
We thank Phyllis Walker from the Blood Centers of the Pacific, San Francisco, Calif., for providing the En(a−) cells, Jill Storry for running the enzyme efficacy assays, and Susan Fetics of the Nucleic Acid Analysis Laboratory for her work in DNA sequencing.
Editor: W. A. Petri, Jr.
Footnotes
This paper is dedicated to the memory of Karla de Frazao.
REFERENCES
- 1.Adams, J. H., P. L. Blair, O. Kaneko, and D. S. Peterson. 2001. An expanding ebl family of Plasmodium falciparum. Trends Parasitol. 17:297-299. [DOI] [PubMed] [Google Scholar]
- 2.Adams, J. H., B. K. Sim, S. A. Dolan, X. Fang, D. C. Kaslow, and L. H. Miller. 1992. A family of erythrocyte binding proteins of malaria parasites. Proc. Natl. Acad. Sci. USA 89:7085-7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baum, J., M. Pinder, and D. J. Conway. 2003. Erythrocyte invasion phenotypes of Plasmodium falciparum in the Gambia. Infect. Immun. 71:1856-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binks, R. H., and D. J. Conway. 1999. The major allelic dimorphisms in four Plasmodium falciparum merozoite proteins are not associated with alternative pathways of erythrocyte invasion. Mol. Biochem. Parasitol. 103:123-127. [DOI] [PubMed] [Google Scholar]
- 5.Camus, D., and T. J. Hadley. 1985. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 230:553-556. [DOI] [PubMed] [Google Scholar]
- 6.Dolan, S. A., L. H. Miller, and T. E. Wellems. 1990. Evidence for a switching mechanism in the invasion of erythrocytes by Plasmodium falciparum. J. Clin. Investig. 86:618-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolan, S. A., J. L. Proctor, D. W. Alling, Y. Okubo, T. E. Wellems, and L. H. Miller. 1994. Glycophorin B as an EBA-175 independent Plasmodium falciparum receptor of human erythrocytes. Mol. Biochem. Parasitol. 64:55-63. [DOI] [PubMed] [Google Scholar]
- 8.Duraisingh, M. T., T. Triglia, S. A. Ralph, J. C. Rayner, J. W. Barnwell, G. I. McFadden, and A. F. Cowman. 2003. Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes. EMBO J. 22:1047-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duraisingh, M. T., A. G. Maier, T. Triglia, and A. F. Cowman. 2003. Erythrocyte-binding antigen 175 mediates invasion in Plasmodium falciparum utilizing sialic acid-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 100:4796-4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilberger, T. W., J. K. Thompson, T. Triglia, R. T. Good, M. T. Duraisingh, and A. F. Cowman. 2003. A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. J. Biol. Chem. 278:14480-14486. [DOI] [PubMed] [Google Scholar]
- 11.Hadley, T. J., F. W. Klotz, G. Pasvol, J. D. Haynes, M. H. McGiniss, Y. Okubo, and L. H. Miller. 1987. Falciparum malaria parasites invade erythrocytes that lack glycophorin A and B (MkMk). Strain differences indicate receptor heterogeneity and two pathways for invasion. J. Clin. Investig. 80:1190-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 13.Lobo, C. A., M. Rodriguez, M. Reid, and S. Lustigman. 2003. Glycophorin C is the receptor for the Plasmodium falciparum erythrocyte binding ligand PfEBP-2 (baebl). Blood 101:4628-4631. [DOI] [PubMed] [Google Scholar]
- 14.Maier, A. G., M. T. Duraisingh, J. C. Reeder, S. S. Patel, J. W. Kazura, P. A. Zimmerman, and A. F. Cowman. 2003. Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat. Med. 9:87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer, D. C., O. Kaneko, D. E. Hudson-Taylor, M. E. Reid, and L. H. Miller. 2001. Characterization of a Plasmodium falciparum erythrocyte binding orotein paralogous to EBA-175. Proc. Natl. Acad. Sci. USA 98:5222-5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer, D. C., J. B. Mu, X. Feng, X. Z. Su, and L. H. Miller. 2002. Polymorphism in a Plasmodium falciparum erythrocyte-binding ligand changes its receptor specificity. J. Exp. Med. 196:1523-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer, D. C., J. B. Mu, O. Kaneko, J. Duan, X. Z. Su, and L. H. Miller. 2004. Polymorphism in the Plasmodium falciparum erythrocyte-binding ligand JESEBL/EBA-181 alters its receptor specificity. Proc. Natl. Acad. Sci. USA 101:2518-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell, G. H., T. J. Hadley, M. H. McGiniss, F. W. Klotz, and L. H. Miller. 1986. Invasion of erythrocytes by Plasmodium falciparum malaria parasites. Evidence for receptor heterogeneity and two receptors. Blood 67:1519-1521. [PubMed] [Google Scholar]
- 19.Narum, D. L., S. R. Fuhrman, T. Luu, and B. K. Sim. 2002. A novel Plasmodium falciparum erythrocyte binding protein-2 (EBP-2/BAEBL) involved in erythrocyte receptor binding. Mol. Biochem. Parasitol. 119:159-168. [DOI] [PubMed] [Google Scholar]
- 20.Okoyeh, J. N., C. R. Pillai, and C. E. Chitnis. 1999. Plasmodium falciparum field isolates commonly use erythrocyte invasion pathways that are independent of sialic acid residues of glycophorin A. Infect. Immun. 67:5784-5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perkins, M. E., and E. H. Holt. 1988. Erythrocyte receptor recognition varies in Plasmodium falciparum isolates. Mol. Biochem. Parasitol. 27:23-34. [DOI] [PubMed] [Google Scholar]
- 22.Rayner, J. C., E. Vargas-Serrato, C. S. Huber, M. R. Galinski, and J. W. Barnwell. 2001. A Plasmodium falciparum homologue of Plasmodium vivax reticulocyte binding protein (PvRBP1_) defines a trypsin-resistant erythrocyte invasion pathway. J. Exp. Med. 194:1571-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reid, M. E., and C. Lomas-Francis. 1997. The blood group antigen facts book. Academic Press, New York, N.Y.
- 24.Sim, B. K. L., C. E. Chitnis, K. Wasniowska, T. J. Hadley, and L. H. Miller. 1994. Receptor and ligand domains for invasion of erythocytes by Plasmodium falciparum. Science 264:1941-1944. [DOI] [PubMed] [Google Scholar]
- 25.Smythe, J. A., R. L. Coppel, K. P. Day, R. K. Martin, A. M. Oduola, D. J. Kemp, and R. F. Anders. 1991. Structural diversity in the Plasmodium falciparum merozoite surface antigen 2. Proc. Natl. Acad. Sci. USA 88:1751-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snewin, V. A., M. Herrera, G. Sanchez, A. Scherf, and G. Langsley. 1991. Polymorphism of the alleles of merozoite surface antigens MSA-1 and MSA-2 in Plasmodium falciparum wild isolates from Colombia. Mol. Biochem. Parasitol. 49:265-275. [DOI] [PubMed] [Google Scholar]
- 27.Thompson, J. K., T. Triglia, M. B. Reed, and A. F. Cowman. 2001. A novel ligand from Plasmodium falciparum that binds to a sialic acid-containing receptor on the surface of human erythrocytes. Mol. Microbiol. 41:47-58. [DOI] [PubMed] [Google Scholar]
- 28.Trager, W., and J. B. Jensen. 1976. Humanmalaria parasites in continuous culture. Science 193:675. [DOI] [PubMed]