Abstract
Mitochondrial bioenergetics, mitochondrial reactive oxygen species (ROS) and cellular levels of nucleotides have been hypothesized as early indicators of Alzheimer’s disease (AD). Utilizing relative decline of cognitive ability as a predictor of AD risk, we evaluated the correlation between change of cognitive ability and mitochondrial bioenergetics, ROS and cellular levels of deoxyribonucleotides. Change of cognitive abilities, scored at ages of approximately 20 and 57 was determined for a cohort of 1985 male participants. Mitochondrial bioenergetics, mitochondrial ROS and whole-cell levels of deoxyribonucleotide triphosphates were measured in peripheral blood mononuclear cells (PBMCs) from a total of 103 selected participants displaying the most pronounced relative cognitive decline and relative cognitive improvement. We show that relative cognitive decline is associated with higher PBMC content of deoxythymidine-triphosphate (dTTP) (20%), but not mitochondrial bioenergetics parameters measured in this study or mitochondrial ROS. Levels of dTTP in PBMCs are indicators of relative cognitive change suggesting a role of deoxyribonucleotides in the etiology of AD.
Keywords: Alzheimer’s disease, Cognitive decline, Mitochondrial bioenergetics, Mitochondrial ROS, Deoxyribonucleotide levels
1. Introduction
Dementia is a condition involving serious loss of global cognitive function. It is frequent in the elderly, affecting 6–7% of the Western population aged 65 and older (Lobo et al., 2000). The major cause of dementia is Alzheimer’s disease (AD).
Mitochondrial dysfunction has been reported in brain tissue from patients suffering from AD (reviewed in (Swerdlow et al., 2014)), and we and others have demonstrated impaired mitochondrial bioenergetics in lymphocytes and platelets in patients diagnosed with AD (Leuner et al., 2012) (Maynard et al., in press). Furthermore, we have demonstrated increased levels of deoxythymidine triphosphate (dTTP) (indicative) and deoxyadenosine triphosphate (dATP) (significant) but not deoxycytidine triphosphate (dCTP) or deoxyguanosine triphosphate (dGTP) in lymphocytes of age matched AD patients (Maynard et al., in press). Both system-wide impairment of mitochondrial function and alterations of cellular nucleotide levels have been proposed to manifest early in the etiology of AD (Coskun et al., 2010; Pesini et al., 2014; Swerdlow et al., 2014), suggesting that these factors could be indicative for increased risk of AD.
Mild cognitive impairment (MCI) is a transitional state between the cognitive changes of normal aging and very early dementia and is becoming increasingly recognized as a risk factor for AD (Grundman et al., 2004). In this study, we designate relative cognitive decline as statistically extreme decline in cognitive function between young adulthood and middle age and we define the term as characteristic of unhealthy brain aging, and as an early indicator of increased risk of dementia and AD. In peripheral blood mononuclear cells (PBMCs), we measured the mitochondrial basal respiration, ATP turnover, and reserve respiratory capacity as well as levels of mitochondrial reactive oxygen species (ROS), but found no significant correlation to relative cognitive decline. Interestingly, we show a significant increase in whole-cell dTTP levels in PBMCs in individuals displaying relative cognitive decline when compared to individuals displaying relative cognitive improvement.
2. Materials and methods
2.1. Cohort selection and vitality score evaluation
Individuals for the present study were selected among 1985 members of the Metropolit Cohort (Osler et al., 2012) who participated in the Copenhagen Aging and Midlife Biobank (CAMB) 2009–10 follow-up (Avlund et al., 2014). Boerge Prien’s Proeve (BPP) was administered at the conscript examination at around age 20 and a short version of the Intelligenz-Struktur-Test (I-S-T 2000R) was administered as part of the CAMB follow-up. Based on the results of the 1985 CAMB participants, a linear regression with BPP scores as predictor and IST scores as outcome was conducted. Standardized residuals from this regression were used to select, 56 men with the largest negative residuals and 47 men with the largest positive residuals were selected for inclusion. In this paper, these groups are referred to as individuals displaying relative cognitive decline and improvement respectively. None have been diagnosed with MCI, dementia or AD. The CAMB data collection was approved by the Ethical Review Committee of the Capital Region of Copenhagen (No: H-A-2008-126). This study was conducted according to the ethical principles of the Helsinki II declaration and approved by the Danish Data Protection Agency (No: 2008-41-2938-41-2001-41-2377; 2008-331-0172; 2008-41-2377).
2.2. PBMC isolation and levels of mitochondrial bioenergetics and ROS
PBMC were isolated from blood and mitochondrial bioenergetics and levels of mitochondrial ROS were determined as earlier described (Maynard et al., 2013).
2.3. Determination of whole cell levels of dTTP, dATP, dCTP and dGTP
The whole-cell levels of dTTP, dATP, dCTP and dGTP were extracted from 2 × 106 PBMCs and determined using the DNA polymerase assay previously described (Desler et al., 2007).
2.4. Determination of relative mtDNA levels and sequence variants
Genomic DNA was extracted from frozen PBMC pellets from respectively 9 and 8 individuals with lower and higher than average measured dTTP levels. DNA was extracted using the NucleoSpin Tissue kit (Macherey-Nagel). Relative mtDNA levels were determined from the 17 samples by real-time qPCR using the StepOnePlus real-time PCR system (Applied Biosystems) and primers for the mitochondrial gene encoding tRNA-Leucine 1 and the nuclear gene encoding beta-2 microglobulin. mtDNA from 16 samples was sequenced at Genotypic Technology, Bangalore, India, using the Ion Torrent and the Mitopanel developed by the company.
2.5. Statistics
Statistical comparisons of mitochondrial bioenergetics, production of mitochondrial ROS as well as whole-cell levels of dTTP, dATP, dCTP and dGTP between individuals displaying relative cognitive decline and relative cognitive improvement, as well as comparison of relative mtDNA levels and sequence variants were performed using Student’s t-test for independent samples. P < 0.05 was considered significant.
3. Results
3.1. Mitochondrial bioenergetics, ROS production and whole-cell levels of dTTP, dATP, dCTP and dGTP
We measured mitochondrial basal respiration (Fig. 1a), ATP turnover (Fig. 1b), reserve respiratory capacity (Fig. 1c) and levels of mitochondrial ROS (Fig. 1d) and whole cell levels of dTTP, dATP, dCTP and dGTP (Fig. 2a), and found no significant difference between individuals displaying relative cognitive decline (n = 35) and relative cognitive improvement (n = 39) for the three mitochondrial bioenergetics parameters: basal rate (P = 0.52), ATP turnover (P = 0.97), and reserve respiratory capacity (P = 0.39). Similarly, we found no significant difference between individuals displaying relative cognitive decline (n = 20) and relative cognitive improvement (n = 29) for mitochondrial ROS (P = 0.28). Interestingly, the levels of dTTP in PBMCs of individuals displaying relative cognitive decline (n = 56) were 20% higher (P = 0.02) than in PBMCs of individuals displaying relative cognitive improvement (n = 47).
Fig. 1.
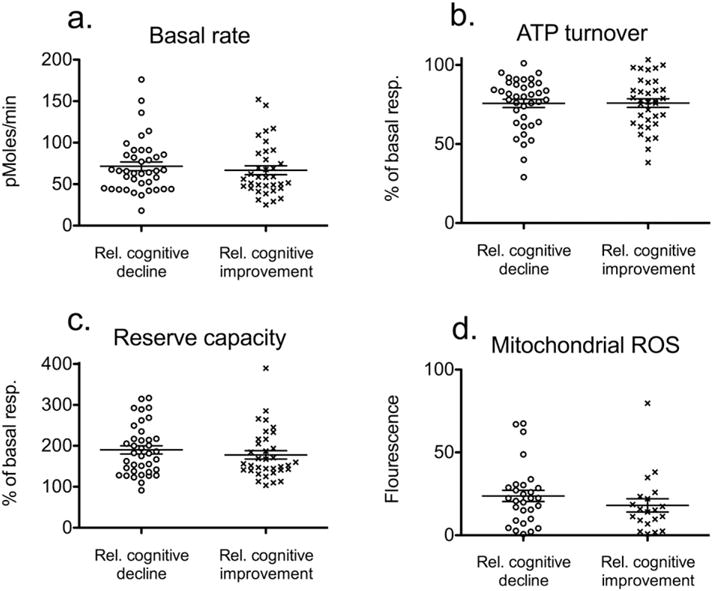
Mitochondrial bioenergetics and mitochondrial reactive oxygen species (ROS) of peripheral blood mononuclear cells (PBMC) from individuals displaying relative cognitive decline and improvement. (a) Basal rate of PBMCs measured in pMoles consumed oxygen per minute (b) The difference in rate of oxygen consumption before and after treatment of PBMCs with the ATP synthase inhibitor, oligomycin equals the ATP turnover. Rates are expressed as a percentage of the basal rate (c) Reserve respiratory capacity is calculated as the difference between rate of oxygen consumption before and after treatment of PBMCs with the uncoupling agent carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP). Rates are expressed as a percentage of the basal rate (d) Mitochondrial ROS in peripheral blood mononuclear cells (PBMC) from individuals displaying relative cognitive decline and improvement.(error-bars indicate SEM).
Fig. 2.
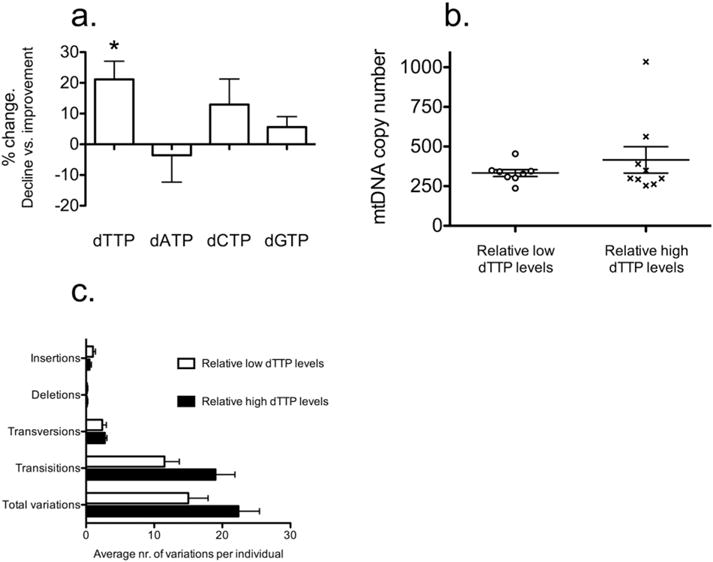
(a) Whole-cell level of dTTP in PBMC is associated with relative changes of cognitive ability. Percentage-wise change of dTTP, dATP, dCTP and dGTP levels in PBMC from individuals displaying relative cognitive decline and improvement (n = 56 and n = 47 respectively; error-bars indicate SEM). (b) Relative levels of mitochondrial DNA in peripheral blood mononuclear cells from a subgroup of 9 individuals with dTTP levels higher than average as well as 8 individuals with dTTP levels lower than average. Error-bars represent SEM. (c) Variants in mtDNA of 8 individuals with lower than average measured dTTP levels and 8 individuals with higher than average measured dTTP levels. Error-bars represent SEM.
3.2. mtDNA levels and mtDNA sequence variants
Since pyrimidine levels have been demonstrated to be regulated by mitochondrial functions (Desler et al., 2007), we determined the mtDNA level relative to nuclear DNA (nDNA) level by real-time qPCR in eight individuals with PBMC levels of dTTP higher than average and nine individuals with dTTP level lower than average. We found no significant differences in mtDNA/nDNA levels between the two groups (Fig. 2b). We quantified the mtDNA mutation load by determining the mtDNA sequence variants by deep sequencing for eight individuals with lower and higher than average measured PBMC level of dTTP, respectively. There was no significant difference in the occurrence or type of variants in these individuals (Fig. 2c).
4. Discussion
In this study, we have correlated change of cognitive ability of individuals from young adulthood to late midlife with mitochondrial bioenergetics, mitochondrial ROS, and whole-cell levels of dTTP, dATP, dCTP and dGTP in PBMCs. None of the participating individuals had been diagnosed with MCI, but the mean residual of individuals displaying relative cognitive decline corresponded to the 7th percentile of the distribution of residuals for the 1985 members of the CAMB cohort, while the mean residual of the individuals displaying relative cognitive improvement corresponded to the 91th percentile. Therefore, the relative cognitive change in the two groups is substantial and unusual and we define extreme relative cognitive decline as a characteristic of unhealthy brain aging, and an early indicator of conditions such as dementia and AD.
4.1. Mitochondrial bioenergetics and ROS are not early indicators of relative cognitive decline
Several studies suggest an early role of mitochondrial dysfunction in the etiology of AD (Coskun et al., 2010; Hirai et al., 2001; Lin et al., 2002; Swerdlow et al., 2014). Studies of mitochondrial function of peripheral tissue suggest mitochondrial alterations as early indicator of AD. However, these studies have been performed on patients already clinically diagnosed with AD or probable AD (Leuner et al., 2012; Podlesniy et al., 2013). When comparing basal mitochondrial respiration, ATP turnover, and respiratory capacity as well as mitochondrial ROS between individuals displaying relative cognitive decline and improvement, we could not demonstrate any significant differences. Our results, therefore, suggest that mitochondrial bioenergetics and mitochondrial ROS production in PBMCs are not clinically relevant indicators of relative cognitive decline.
4.2. Whole cell level of dTTP is increased in individuals displaying relative cognitive decline
Postmitotic cells, like neurons, do not require deoxyribonucleotides to the same extent as mitotic cells, however, high levels of both activity and immunoactivity of the enzyme dihydroorotate dehydrogenase (DHODH) have been demonstrated in neocortex and hippocampus of rats (Schaefer et al., 2010). DHODH is an integral enzyme of the de novo synthesis of pyrimidines. It has been suggested that pyrimidines produced in these tissues of the brain are utilized as substrates for the synthesis of phospholipids, glycolipids, and glycoproteins of neuronal membranes and also as uridine nucleotides functioning as neuronal signaling molecules (Schaefer et al., 2010). Damage to the neocortex and hippocampus are hallmarks of AD and it has, therefore, been hypothesized that a lack of pyrimidine degradation products is involved in the debilitation of these tissues and, thereby, the etiology of AD (Pesini et al., 2014). In this study, we demonstrated a significant 20% increase of whole-cell dTTP levels in PBMCs of individuals displaying relative cognitive decline. This suggests an association between dTTP levels in PBMCs and relative cognitive ability of the individual.
4.3. Correlation between dTTP levels and mitochondria
We cannot exclude dysfunction of the mitochondria in the analyzed individuals. DHODH is localized in the mitochondrial membrane and is dependent on the mitochondrial electron transport chain. We have shown that an inhibition of DHODH will alter the whole-cell pyrimidine levels without affecting mitochondrial bioenergetics (Supplementary Fig. 1). This opens for the possibility that a mitochondrial dysfunction exist in individuals displaying relative cognitive decline, but this independent of mitochondrial bioenergetics and ROS.
An imbalance of the cytosolic level of dTTP has been demonstrated to affect the replication of mtDNA and result in mutations and depletion of mtDNA (López et al., 2009). Furthermore, we have shown that imbalanced dTTP pools cause nuclear genomic instability (Desler et al., 2007), which could lead to diseases such as cancer and neurodegeneration. However, no significant difference was found in relative mtDNA levels, occurrence, or nature of mtDNA variants between individuals with high and low dTTP levels.
We will continue to follow the participants of this study and will, in follow-up studies over the next 1–2 years, determine the correlation be-tween participants who displayed relative cognitive decline in this study, and future onset of dementia and AD. Furthermore, we will continue to monitor the dTTP levels and mitochondrial bioenergetics to elucidate the role of these factors in the etiology of dementia and AD.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.mito.2015.09.002.
Supplementary Material
Acknowledgments
We thank Mads Bak for assistance with interpreting sequencing data.
This work was supported by a grant from Nordea-fonden (LJR, VB, ELM, ML) and the The Danish Council for Independent Research-Natural Sciences (LJR, CD).
Abbreviations
- AD
Alzheimer’s disease
- BPP
Boerge Prien’s Proeve
- CAMB
Copenhagen Aging and Midlife Biobank
- DHODH
dihydroorotate dehydrogenase
- I-S-T
Intelligenz-Struktur-Test
- MCI
mild cognitive impairment
- mtDNA
mitochondrial DNA
- nDNA
nuclear DNA
- PBMC
Peripheral blood mononuclear cells
- ROS
reactive oxygen species
- dATP
deoxyadenosine triphosphate
- dCTP
deoxycytidine triphosphate
- dGTP
deoxyguanosine triphosphate
- dTTP
deoxythymidinetriphosphate
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- Avlund K, Osler M, Mortensen EL, Christensen U, Bruunsgaard H, Holm-Pedersen P, Fiehn NE, Hansen AM, Bachkati SH, Meincke RH, Jepsen E, Molbo D, Lund R. Copenhagen aging and midlife biobank (CAMB): an introduction. J Aging Health. 2014;26:5–20. doi: 10.1177/0898264313509277. http://dx.doi.org/10.1177/0898264313509277. [DOI] [PubMed] [Google Scholar]
- Coskun PE, Wyrembak J, Derbereva O, Melkonian G, Doran E, Lott IT, Head E, Cotman CW, Wallace DC. Systemic mitochondrial dysfunction and the etiology of Alzheimer’s disease and down syndrome dementia. J Alzheimers Dis. 2010;20(Suppl. 2):S293–S310. doi: 10.3233/JAD-2010-100351. http://dx.doi.org/10.3233/JAD-2010-100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desler C, Munch-Petersen B, Stevnsner T, Matsui SI, Kulawiec M, Singh KK, Rasmussen LJ. Mitochondria as determinant of nucleotide pools and chromosomal stability. Mutat Res. 2007;625:112–124. doi: 10.1016/j.mrfmmm.2007.06.002. http://dx.doi.org/10.1016/j.mrfmmm.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR, Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R, van Dyck CH, Mintzer J, Zamrini EY, Cahn-Weiner D, Thal LJ, Alzheimer’s Disease Cooperative Study Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. http://dx.doi.org/10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner K, Schulz K, Schütt T, Pantel J, Prvulovic D, Rhein V, Savaskan E, Czech C, Eckert A, Müller WE. Peripheral mitochondrial dysfunction in Alzheimer’s disease: focus on lymphocytes. Mol Neurobiol. 2012;46:194–204. doi: 10.1007/s12035-012-8300-y. http://dx.doi.org/10.1007/s12035-012-8300-y. [DOI] [PubMed] [Google Scholar]
- Lin MT, Simon DK, Ahn CH, Kim LM, Beal MF. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Hum Mol Genet. 2002;11:133–145. doi: 10.1093/hmg/11.2.133. [DOI] [PubMed] [Google Scholar]
- Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM, Copeland JR, Dartigues JF, Jagger C, Martinez-Lage J, Soininen H, Hofman A. Prevalence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54:S4–S9. [PubMed] [Google Scholar]
- López LC, Akman HO, García-Cazorla A, Dorado B, Marti R, Nishino I, Tadesse S, Pizzorno G, Shungu D, Bonilla E, Tanji K, Hirano M. Unbalanced deoxynucleotide pools cause mitochondrial DNA instability in thymidine phosphorylase-deficient mice. Hum Mol Genet. 2009;18:714–722. doi: 10.1093/hmg/ddn401. http://dx.doi.org/10.1093/hmg/ddn401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard S, Keijzers G, Gram M, Desler C, Bendix L, Budtz-Jørgensen E, Molbo D, Croteau DL, Osler M, Stevnsner T, Rasmussen LJ, Dela F, Avlund K, Bohr VA. Relationships between human vitality and mitochondrial respiratory parameters, reactive oxygen species production and dNTP levels in peripheral blood mononuclear cells. Aging (Albany NY) 2013;5:850–864. doi: 10.18632/aging.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard S, Hejl AM, Dinh TST, Keijzers G, Hansen ÅM, Desler C, Moreno-Villanueva M, Bürkle A, Rasmussen LJ, Waldemar G, Bohr VA. Defective mitochondrial respiration, altered dNTP pools and reduced AP endonuclease 1 activity in peripheral blood mononuclear cells of Alzheimer’s disease patients. Aging. 2015 doi: 10.18632/aging.100810. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osler M, Avlund K, Mortensen EL. Socio-economic position early in life, cognitive development and cognitive change from young adulthood to middle age. Eur J Pub Health. 2012 doi: 10.1093/eurpub/cks140. http://dx.doi.org/10.1093/eurpub/cks140. [DOI] [PubMed]
- Pesini A, Iglesias E, Garrido N, Bayona-Bafaluy MP, Montoya J, Ruiz-Pesini E. OXPHOS, pyrimidine nucleotides, and Alzheimer’s disease: a pharmacogenomics approach. J Alzheimers Dis. 2014;42:87–96. doi: 10.3233/JAD-140384. http://dx.doi.org/10.3233/JAD-140384. [DOI] [PubMed] [Google Scholar]
- Podlesniy P, Figueiro-Silva J, Llado A, Antonell A, Sanchez-Valle R, Alcolea D, Lleo A, Molinuevo JL, Serra N, Trullas R. Low cerebrospinal fluid concentration of mitochondrial DNA in preclinical Alzheimer disease. Ann Neurol. 2013;74:655–668. doi: 10.1002/ana.23955. http://dx.doi.org/10.1002/ana.23955. [DOI] [PubMed] [Google Scholar]
- Schaefer CM, Schäfer MKH, Löffler M. Region-specific distribution of dihydroorotate dehydrogenase in the rat central nervous system points to pyrimidine de novo synthesis in neurons. Nucleosides Nucleotides Nucleic Acids. 2010;29:476–481. doi: 10.1080/15257771003730128. http://dx.doi.org/10.1080/15257771003730128. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta. 2014;1842:1219–1231. doi: 10.1016/j.bbadis.2013.09.010. http://dx.doi.org/10.1016/j.bbadis.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


