Abstract
Objective To estimate the protection against death provided by vaccination against influenza.
Design Prospective cohort follow up supplemented by weekly national counts of influenza confirmed in the community.
Setting Primary care.
Participants 24 535 patients aged over 75 years from 73 general practices in Great Britain.
Main outcome measure Death.
Results In unvaccinated members of the cohort daily all cause mortality was strongly associated with an index of influenza circulating in the population (mortality ratio 1.16, 95% confidence interval 1.04 to 1.29 at 90th centile of circulating influenza). The association was strongest for respiratory deaths but was also present for cardiovascular deaths. In contrast, in vaccinated people mortality from any cause was not associated with circulating influenza. The difference in patterns between vaccinated and unvaccinated people could not easily be due to chance (P = 0.02, all causes).
Conclusions This study, using a novel and robust approach to control for confounding, provides robust evidence of a protective effect on mortality of vaccination against influenza.
Introduction
A randomised trial showed the effectiveness of vaccination against laboratory confirmed clinical influenza to be 58%,1 but mortality is too rare an end point for reduction in mortality to be clearly established. Observational studies, mostly on people aged 65 and over, have estimated effects on mortality but are subject to confounding.2-7 Confounding “by indication,” whereby sicker people may be selected for vaccination, biases estimates of effectiveness downwards.6-9 However, people vaccinated may be healthier than those not vaccinated, potentially biasing estimates upwards.8,9 Problems also exist in identifying deaths due to influenza. Deaths certified with influenza as underlying cause are known to be a small fraction of deaths due to influenza,10 leading researchers to prefer all deaths during influenza epidemics and due to respiratory or cardiovascular disease as an outcome measure. However, these deaths include many not caused by influenza, and this lack of specificity reduces estimates of the clinical effectiveness of vaccination, particularly as cold related deaths and other non-influenzal infections such as respiratory syncytial virus often occur in temperate climates at the same time of the year as influenza.11
We sought to overcome these problems by measuring vaccine effectiveness as the extent to which increases in mortality during periods of high circulating influenza are diminished in vaccinated people, rather than by direct comparison of mortality in vaccinated and unvaccinated people.
Methods
We included all 24 535 people invited to participate in a randomised trial of targeted screening versus universal screening of patients aged over 75 years from 73 general practices in Great Britain.12 (We did not use the remaining 33 of the 106 practices in the trial because data on dates of vaccination against influenza were unavailable for any of the study period—January 1996 to August 2000.) As a weekly index of influenza circulating in the population we used the number of clinical specimens reported to the UK Public Health Laboratory Service in which influenza A virus was found, according to date of provision of sample. These counts are known to underestimate very substantially the actual numbers of people infected by influenza, but under-ascertainment was likely to be fairly constant over the study period. Thus the specimen count series reasonably accurately reflects the week to week variation in cases of influenza.13 During this period influenza A H3N2 was the predominant virus subtype in circulation. We linked daily regional meteorological and pollution data to the cohort data to allow control for their potential confounding effect on mortality from influenza. Here we report on deaths (ascertained by the Office for National Statistics) to the end of 2000.
We calculated numbers of deaths and cohort members at risk for each day of the study (January 1996-August 2000), subdividing the cohort according to vaccination status. Because we sought to compare (within the vaccinated and unvaccinated groups) mortality in periods of high circulating influenza with that in periods of low circulating influenza, precision of the risk estimates depended in part on duration of the baseline period of low circulating influenza. We therefore did not restrict analysis to the conventional influenza season, but included the period January-August. We excluded the period September-December, because nearly all (99%) vaccinations took place in this period. Dividing this period individually for each participant according to exact date of vaccination would have been complex and may have introduced errors. We thus reassigned vaccination status to participants each 1 January, according to whether they had been vaccinated during the previous four months.
Details of the statistical methods are given on bmj.com and summarised here. We used Poisson regression to obtain estimates, separately for vaccinated and unvaccinated people, of association of mortality with circulating influenza adjusted for confounding by seasonal factors and weather, broadly following methods developed for daily time series studies of air pollution and mortality.14 We chose this approach to analysis to avoid reliance on an assumption that vaccinated people had similar mortality to unvaccinated people outside of influenza periods. The approach thus did not require adjustment for factors that might affect this “baseline” mortality. From this model we estimated the fraction of mortality attributable to influenza when circulating influenza was at its 90th centile.15 We estimated vaccine effectiveness, the proportion of deaths attributable to influenza that were apparently prevented by the vaccine, from these fractions.
To avoid loss of information due to an arbitrary “flu period” dichotomy and to control for confounding, we regressed daily mortality rates on influenza counts in the previous week, using a Poisson model with separate regression lines in vaccinated and unvaccinated participants. We adjusted for dependence of mortality on year, season (month), and low temperature by including terms for these effects in the model. We also adjusted for a slight tendency for mortality in vaccinated people to converge with that in unvaccinated people over time by including, for the vaccinated group, an additional linear term in days from 1 January. Adjustment for conventional confounders such as age at entry and sex was unnecessary because we drew information only from comparisons between periods of high and low circulating influenza, over which these variables do not change appreciably.
Results
Mortality (second and third columns of table) was lower in vaccinated people than in unvaccinated people even outside influenza periods, suggesting that vaccinated people were healthier than unvaccinated people. However, the difference was greater during influenza periods, suggesting an effect of vaccination independent of the selection of healthier people for vaccination.
Table 1.
Association of mortality with influenza circulating in population: vaccinated and unvaccinated people
|
No (rate*) of deaths
|
|||||
|---|---|---|---|---|---|
| Cause of death | In high influenza period | Outside high influenza period | Association of mortality (95% CI) with influenza index† | Modification of “influenza” effect by vaccination‡ | Deaths (%) attributable to influenza§ |
| All causes | |||||
| Unvaccinated | 564 (13.9) | 2305 (9.4) | 1.16 (1.04 to 1.29) | 1 | 13.4 |
| Vaccinated | 346 (9.7) | 1630 (7.7) | 1.02 (0.90 to 1.16) | 0.89 (0.80 to 0.98) | 2.2 |
| Cardiovascular | |||||
| Unvaccinated | 226 (5.6) | 1050 (4.3) | 1.19 (1.05 to 1.36) | 1 | 16.3 |
| Vaccinated | 145 (4.1) | 772 (3.7) | 1.03 (0.85 to 1.26) | 0.87 (0.73 to 1.02) | 3.2 |
| Respiratory | |||||
| Unvaccinated | 156 (3.9) | 380 (1.6) | 1.31 (0.92 to 1.87) | 1 | 23.9 |
| Vaccinated | 97 (2.7) | 293 (1.4) | 1.05 (0.75 to 1.47) | 0.80 (0.69 to 0.93) | 5.1 |
| Other | |||||
| Unvaccinated | 182 (4.5) | 875 (3.6) | 1.00 (0.86 to 1.16) | 1 | 0 |
| Vaccinated | 104 (2.9) | 565 (2.7) | 0.98 (0.80 to 1.21) | 0.98 (0.82 to 1.17) | 0 |
Crude annual mortality (%).
Exponentiated regression coefficient scaled to represent mortality at 90th centile relative to zero influenza period. Adjusted for month (indicators for 40 strata), temperature (linear term for two week mean of degrees below 20), and number of days since 31 December separately in vaccinated and unvaccinated people.
Ratio (95% confidence interval) of exponentiated coefficients in previous column, estimating impact of vaccination on tendency for mortality to increase during periods of high counts of circulating influenza.
Fraction of deaths attributable to influenza at 90th centile of circulating influenza.
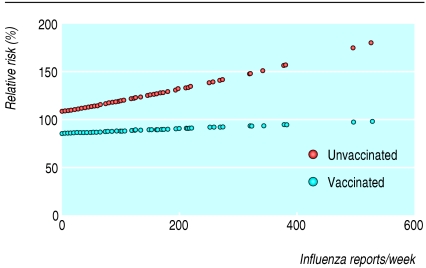
The fitted regression lines (fig) confirm the lower mortality in vaccinated people, even when no influenza was present in the population. They also show that as population influenza counts increased, mortality in vaccinated people remained stable, whereas mortality in unvaccinated people increased. The fourth column of the table shows the slopes of the graphs in the figure scaled to represent the mortality at the 90th centile of weekly population influenza counts relative to that when there was no circulating influenza. (The choice of the 90th centile is arbitrary but common practice; it does not affect whether confidence intervals include the null ratio of one.) The fifth column of the table shows the ratio of the two scaled slopes, providing a robust estimate of the modification by vaccination of the association of mortality with influenza. The low ratio for all cause mortality (0.89), indicating a protective effect of vaccination, could not easily be explained by chance (P = 0.02). A similar pattern, but less precisely estimated, occurred for deaths due to cardiovascular disease (P = 0.09), with a more pronounced effect for deaths due to respiratory disease (P = 0.002). We found no association of deaths from other causes with population rates of influenza in either vaccinated or unvaccinated people.
Figure 1.
Mortality versus population influenza rate by vaccination status. Fitted regression lines for daily mortality by number of specimens with influenza A reported in the United Kingdom during the previous week, using Poisson model controlling for month, temperature, and number of days since 31 December. Mortality is scaled to be relative to the total cohort average
The proportion of deaths attributable to influenza during periods of influenza (final column of table) for all cause mortality was 13.4% in unvaccinated people (1 - RR-1 = 1 - 1.16-1) and 2.2% in vaccinated people (1 - 1.02-1). The derived estimate of vaccine effectiveness against deaths associated with influenza, given by a comparison of these as described above and in the detailed methods on bmj.com, was (13.4 - 2.2)/13.4 = 83% (95% confidence interval 9% to 100%). The derived estimate of vaccine effectiveness against death from cardiovascular disease was 80%, and that for respiratory deaths was 79%, but these were very imprecisely estimated, with 95% confidence intervals spanning the entire meaningful range (0 to 100%).
Discussion
In the vaccinated cohort compared with the unvaccinated cohort, the tendency of mortality to rise in periods of high influenza infection rates was clearly reduced. This is not easily explicable by chance or confounding. We have substantially reduced vulnerability to confounding by avoiding direct comparison of mortality in vaccinated and unvaccinated groups in favour of comparing vulnerability within each group to increasing mortality associated with high circulation of influenza. It is hard to envisage confounding that would cause spurious patterns of sharply reduced mortality in vaccinated people specifically during the high influenza periods.
This approach also improves specificity of outcome. By estimating deaths attributable to influenza statistically, we avoided a choice between reliance on information from death certificates or settling for a non-specific outcome. A somewhat similar idea has been used in the study of malaria.16 The robustness and improved outcome specificity of our indirect approach is at the cost of low precision. Only for all cause mortality and respiratory mortality was the protection by vaccination statistically significant at conventional benchmarks. Estimation of vaccine effectiveness, still more demanding of information under this approach, was even less precise. Furthermore, the approach is not immune to information bias. Errors in the index of circulating influenza would have reduced associations of circulating influenza with mortality in both vaccinated and unvaccinated people. Misclassification of vaccination status would have blurred differences between the groups.17
Several recent conventional observational studies have reported vaccine effectiveness against all cause mortality in the influenza season. A UK study of people aged 55 and over in 1989-90 reported a vaccine effectiveness of 75% (95% confidence interval 21% to 92%).3 Among studies of vaccine effectiveness against all cause mortality in people aged 65 and over, vaccine effectiveness was 57% (55% to 60%) in a Swedish cohort study in 1998-2000,8 24% (3% to 40%) in 1996-7 in the Netherlands,7 and 50% in the United States in 1998-2000.6 A meta-analysis of 20 earlier observational studies found mean vaccine effectiveness for all cause mortality of 68% (56% to 76%).4
These estimates were all lower than our estimate of 83%. However, these fractions were of all deaths in the influenza season, rather than of the excess associated with high influenza periods measured by the more specific estimate of vaccine effectiveness we used in our study. Furthermore, because no comparison was made with a non-influenza season, estimates were more vulnerable to confounding and may have been overestimated owing to vaccine recipients being healthier than non-recipients. Control for confounding is possible in conventional studies, but only for the limited variables measured. One study noted a 12% (8% to 16%) protective effect against acute respiratory mortality averaged over a several influenza seasons (1989-90 to 1998-9). Mortality outside the influenza season, during which vaccination had no effect on respiratory mortality, was also investigated. An apparent protective effect against all cause mortality was seen.9 The authors concluded that the estimate of vaccine effectiveness against all cause mortality during influenza seasons was probably upwardly biased due to the “healthy vaccinee” effect. Our study also shows the presence of the healthy vaccinee effect (second column of table), but we controlled for this by comparing effects of influenza rather than mortality in vaccinated and unvaccinated people, as described earlier.
What is already known on this topic
Randomised trials have shown effectiveness of vaccination against influenza, but mortality is too rare an end point for a reduction to be clearly established
Observational studies have estimated effects on mortality but are subject to confounding and to problems in identifying deaths due to influenza
What this study adds
Mortality in periods of high circulating influenza was clearly increased in unvaccinated people but not in vaccinated people, strongly suggesting a protective effect of vaccination
By avoiding direct comparisons of mortality in vaccinated and unvaccinated people in favour of comparisons of responses to circulating influenza, we avoided most confounding
The difference in the point estimates of vaccine effectiveness between the results in the literature and our estimate could thus be due to differences in methods as discussed above or to chance, given our wide confidence intervals. In particular, the greater specificity of outcome from the examination of mortality during periods of high influenza activity and adjustment for cold weather may account for the higher effectiveness seen here. In conclusion, this research adds to evidence that influenza vaccination protects against mortality from influenza, although estimates of vaccine effectiveness are imprecise. The novel method we adopted offers improved control of confounding at the cost of some precision and is applicable to most studies of effects of episodic infections on mortality.
Supplementary Material
 Details of statistical methods are on bmj.com
Details of statistical methods are on bmj.com
We thank the respiratory division of CDSC, Public Health Laboratory Service (since April 2003 the Health Protection Agency) for access to laboratory reports of influenza. AMcM now works at the National Centre for Epidemiology and Population Health, Australian National University, Canberra.
Contributors: BGA was involved in study design, statistical analysis, and preparation of the manuscript. PM was involved in collection of data on vaccination and influenza and preparation of the manuscript. AF was involved in study design and was principal investigator for this study and the parent study. SK was involved in collection of data on vaccination, influenza, and weather. AMcM and PW were involved in study design. SP was involved in statistical analysis. All contributors commented on manuscript drafts and participated in study progress meetings. BGA is the guarantor of the paper. He accepts full responsibility for the conduct of the study, had access to the data and controlled the decision to publish in consultation with the other authors.
Funding: This study was supported by the UK Medical Research Council. PM was funded by the Wellcome Foundation (grant number 051637) during this work. PW is supported by a public health career scientist award (NHS Executive, CCB/BS/PHCS031).
Competing interests: None declared.
Ethical approval: The study was approved by the relevant local research ethics committees.
References
- 1.Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals: a randomized double-blind placebo-controlled trial. JAMA 1994;272: 1661-5. [PubMed] [Google Scholar]
- 2.Christenson B, Lundbergh P, Hedlund J, Ortqvist A. Effects of a large-scale intervention with influenza and 23-valent pneumococcal vaccines in adults aged 65 years or older: a prospective study. Lancet 2001;357: 1008-11. [DOI] [PubMed] [Google Scholar]
- 3.Fleming DM, Watson JM, Nicholas S, Smith GE, Swan AV. Study of the effectiveness of influenza vaccination in the elderly in the epidemic of 1989-90 using a general practice database. Epidemiol Infect 1995;115: 581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons: a meta-analysis and review of the literature. Ann Intern Med 1995;123: 518-27. [DOI] [PubMed] [Google Scholar]
- 5.Hedlund J, Christenson B, Lundbergh P, Ortqvist A. Effects of a large-scale intervention with influenza and 23-valent pneumococcal vaccines in elderly people: a 1-year follow-up. Vaccine 2003;21: 3906-11. [DOI] [PubMed] [Google Scholar]
- 6.Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med 2003;348: 1322-32. [DOI] [PubMed] [Google Scholar]
- 7.Voordouw BC, van der Linden PD, Simonian S, van der Lei J, Sturkenboom MC, Stricker BH. Influenza vaccination in community-dwelling elderly: impact on mortality and influenza-associated morbidity. Arch Intern Med 2003;163: 1089-94. [DOI] [PubMed] [Google Scholar]
- 8.Christenson B, Lundbergh P. Comparison between cohorts vaccinated and unvaccinated against influenza and pneumococcal infection. Epidemiol Infect 2002;129: 515-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangtani P, Cumberland P, Hodgson C, Roberts J, Cutts F, Hall AJ. A cohort study of the effectiveness of influenza vaccine in older people, performed using the United Kingdom general practice research database. J Infect Dis 2004;190: 1-10. [DOI] [PubMed] [Google Scholar]
- 10.Ashley J, Smith T, Dunnell K. Deaths in Great Britain associated with the influenza epidemic of 1989/90. Population Trends 1991;65: 16-20. [Google Scholar]
- 11.Zambon MC, Stockton JD, Clewley JP, Fleming DM. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet 2001;358: 1410-6. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher AE, Jones DA, Bulpitt CJ, Tulloch AJ. The MRC trial of assessment and management of older people in the community: objectives, design and interventions [ISRCTN23494848]. BMC Health Serv Res 2002;2: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goddard NL, Kyncl J, Watson JM. Appropriateness of thresholds currently used to describe influenza activity in England. Commun Dis Public Health 2003;6: 238-45. [PubMed] [Google Scholar]
- 14.Schwartz J, Spix C, Touloumi G, Bacharova L, Barumamdzadeh T, le Tertre A, et al. Methodological issues in studies of air pollution and daily counts of deaths or hospital admissions. J Epidemiol Community Health 1996;50(suppl 1): S3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C. Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol 1985;122: 904-14. [DOI] [PubMed] [Google Scholar]
- 16.Smith T, Schellenberg JA, Hayes R. Attributable fraction estimates and case definitions for malaria in endemic areas. Stat Med 1994;13: 2345-58. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong BG. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup Environ Med 1998;55: 651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pattenden S, Nikiforov B, Armstrong BG. Mortality and temperature in Sofia and London. J Epidemiol Community Health 2003;57: 628-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong BG. Fixed factors that modify the effects of time-varying factors: applying the case-only approach. Epidemiology 2003;14: 467-72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.