Pancreatic cancer affects approximately 6000 people in the United Kingdom every year and is one of the 10 most common causes of cancer death in the western hemisphere.1,2 There is a slight male preponderance in the incidence of the disease, with peak incidence in the seventh and eighth decades (box 1).1 The vast majority of patients with pancreatic cancer die within one year of diagnosis,w1 and the overall five year survival rate ranges from 0.4% to 4%, the lowest for any cancer.2 Currently, surgical resection offers the best chance of cure; however, more than 80% of patients present with advanced and unresectable disease,3 which accounts for the low rates of resection and survival. The key to increased resection rates lies with early diagnosis. In this article we describe the clinical features of pancreatic cancer and the various modalities used to diagnose this debilitating disease.
Sources and selection criteria
We did an internet based search of the Medline and Science Citation Index databases by using the keywords “pancreatic cancer,” “diagnosis,” and “pancreatic imaging.” We included reviews and evidence based studies in major journals from surgery, gastroenterology, and radiology published from January 1999 to June 2004, as well as key early papers.
Symptoms and signs
Box 2 summarises the signs and symptoms of pancreatic cancer. The early symptoms of pancreatic cancer are usually non-specific and are often ignored by the patient and doctor. These include epigastric bloating, flatulence, general malaise, diarrhoea, vomiting, and constipation. As the disease progresses, patients present with painless jaundice and weight loss. The prevalence of symptoms varies with the site and extent of the tumour. The presence of jaundice with a tumour in the body or tail of the pancreas is invariably associated with late presentation as well as inoperability due to hepatic or hilar nodal metastases.
Abdominal pain is the most frequently encountered late symptom (80% of patients) and is primarily caused by invasion of the coeliac and superior mesenteric plexuses.4 w2 It is usually epigastric in location and diffuse in nature in the early stages, becoming more localised later. Radiation of the pain to the back indicating retroperitoneal invasion of the splanchnic nerve plexus by the tumour occurs in a quarter of patients.4
Summary points
Pancreatic cancer is diagnosed in approximately 6000 people in the United Kingdom every year
Patients commonly present with painless jaundice and weight loss
Serological markers such as carbohydrate antigen 19-9 are not useful for screening but may be used in patients who have symptoms as well as for assessing prognosis
Computed tomography is the current modality of choice for diagnosis and staging of pancreatic carcinoma
Endoscopic retrograde cholangiopancreatography is an important investigation in patients with obstructive jaundice to obtain cytology or biopsy specimens and insert biliary stents to relieve biliary obstruction
Computed tomography or endoscopic ultrasound guided fine needle aspiration or trucut biopsy is used for cytological or histological diagnosis
Endoscopic ultrasonography is the most sensitive technique for detection of cancer, but its sensitivity in detecting early lesions has not yet been evaluated
The weight loss associated with pancreatic cancer is probably multifactorial. An increase in resting energy expenditure and serum concentrations of proinflammatory cytokines such as tumour necrosis factor α and interleukin 6 and a decrease in food intake, as well as a component of fat malabsorption, contribute to weight loss. The onset of diabetes mellitus due to impaired function of β islet cells may also herald pancreatic cancer and can be observed in up to 40% of patients.4 However, many studies seem to show that diabetes is not a risk factor for pancreatic cancer but one of the sequelae of the disease.5 Diabetes may be more common in patients with resectable tumours than in those with unresectable or advanced disease,4 so early recognition is important in detecting potentially resectable tumours. Patients may also present with acute cholecystitis, acute pancreatitis, upper gastrointestinal haemorrhage, neuropsychiatric disturbances, polyarthritis, painful skin nodules, pyrexia of unknown origin, steatorrhoea, or migratory thrombophlebitis (Trousseau's sign).w2
Box 1: Epidemiology of pancreatic cancer
Incidence: 10 in 100 000 population
Median age at diagnosis: 69 years
Male to female ratio: 1.2-1.5 to 1
Overall one year survival: 12%
Overall five year survival: 0.4-4%
Stage at presentation
Stage I: 20%
Stage II: 40%
Stage III-IV: 40%
Important physical signs include the presence of an upper abdominal mass, icterus and scratch marks, hepatomegaly, palpable gallbladder (Courvoisier's sign), supraclavicular lymphadenopathy (rare) (Virchow's node, Troisier's sign), splenomegaly (due to portal or splenic vein compression, thrombosis, or diffuse liver metastases), periumbilical nodules (Sister Mary Joseph's node), ascites or peripheral oedema (including obstruction of the inferior vena cava), and thrombophlebitis. Unfortunately, the presence of these physical signs usually indicates advanced and unresectable disease. Other conditions that may mimic pancreatic cancer include chronic pancreatitis and choledocholithiasis.
Haematological and biochemical investigations
The laboratory findings in patients with pancreatic cancer are usually non-specific. Anaemia and hypoalbuminaemia may reflect the chronic nature of the neoplastic process and its nutritional sequelae. Patients presenting with obstructive jaundice show increases in serum bilirubin, alkaline phosphatase, and γ glutamyl transferase. Disproportionate increase of the transaminases may be associated with extensive liver metastases. Prolongation of prothrombin time results from exclusion of bile from the gastrointestinal tract leading to malabsorption of fat soluble vitamin K and decreased hepatic production of vitamin K dependent clotting factors. Glucose tolerance is also impaired in many patients; published evidence points to as many as 70% of patients with diagnosed pancreatic cancer having frank diabetes or impaired glucose tolerance.w3
Box 2: Signs and symptoms of pancreatic cancer
Symptoms
Jaundice
Weight loss
Nausea
Vomiting
Abdominal pain (late)
Anorexia
Diarrhoea
Signs
Jaundice
Palpable liver
Palpable gall bladder (Courvoisier's sign)
Abdominal tenderness
Ascites
Thrombophlebitis
Serological markers
Serological markers for pancreatic cancer may be classified as serum enzymes, tumour related antigens, ectopically produced regulatory peptides, and hormones. Tumour related antigens offer the best possibility of serodiagnosis of pancreatic malignancy, but as yet they are neither tumour specific nor pancreatic cancer specific and are only reliably positive in advanced or disseminated disease.6 Carbohydrate antigen 19-9 is the most commonly used marker. The efficacy of carbohydrate antigen 19-9 in comparison with other tumour markers (carcinoembryonic antigen, carbohydrate antigen 50, cellular adhesion molecule 17.1) in detecting pancreatic carcinoma has been widely studied; most studies have concluded that carbohydrate antigen 19-9, with its combination of high sensitivity (around 80%) and high specificity (60-70%), is the best tumour marker currently available for the detection of pancreatic carcinoma.6,7 It is normally found in medium or large duct cells and on the luminal surface of cancer cells. Increased concentrations of the marker are commonly found in patients with pancreatic cancers measuring ≥ 3 cm,w4 which limits its value in detecting potentially resectable tumours. The marker may also be increased in other malignancies of the gut involving the stomach, colon, or biliary tree, as well as in benign conditions such as pancreatitis, hepatitis, and cirrhosis.8 Nevertheless, carbohydrate antigen 19-9 may have a role in estimating the prognosis and response to treatment of patients having pancreatic resection and chemotherapy.6
Studies have also compared the efficacy of tumour markers with other investigations in detecting pancreatic cancers. Although tumour markers could be as sensitive as imaging, the presence of jaundice in many patients significantly lowered specificity.w5 w6 Novel markers such as “a disintegrin and metalloprotease” (ADAM9)w7 and liver-intestine cadherinw8 are being actively investigated with a view to a role in diagnosis. Despite the expense, radiological investigation will remain the diagnostic modality of choice for the near future owing to the poor specificity of tumour markers in a clinical context.
Imaging studies
Considerable improvements in non-invasive cross sectional radiological imaging in the past decade have greatly enhanced the ability to diagnose pancreatic cancer and plan appropriate treatment for patients. Accurate radiological staging of the disease allows for appropriate clinical decision making and ensures that surgery is limited to those patients who will benefit. “Diagnostic” laparotomy is now rarely undertaken, except as the final arbiter in cases of equivocal resectability.
Ultrasonography
Transabdominal ultrasonography is usually the initial screening investigation in patients presenting with jaundice. It can provide information, non-invasively, about the size, site, and characteristics of the primary tumour, the diameters of the biliary and pancreatic ducts, and the site of the obstruction. The presence or absence of lymph nodes or hepatic metastatic disease and the proximity of tumour to major vessels can also be determined.9 w5 Ultrasonography may even be as sensitive and specific as computed tomography in identifying hepatic metastases. The use of Doppler ultrasonography gives a reasonably reliable measure of vascular patency and can improve accuracy in measuring vascular invasion.9 The technique, however, is not without its limitations. Accurately assessing the pancreas, the size of the mass, and the extent of spread can often be difficult. The frequency of identification of the cancer by ultrasonography varies from 57% to 81%.10 w5 Ultrasonography is operator dependent and may be inaccurate more than one third of the time as a result of factors such as large body habitus, presence of ascites, or overlying bowel gas (in as many as 20% of patients10).
More recently, the use of echo enhanced power Doppler sonography (power Doppler sonography after injection of a contrast agent) has increased the sensitivity (to 87%) and specificity (to 94%) of this diagnostic modality.11 Coded phase inversion harmonic ultrasonography is another new technique that enables real time visualisation of slow flow in minute vessels in tumours with the use of a sonographic contrast agent and has been shown to have a sensitivity of 95% in detecting pancreatic tumours < 2 cm in diameter.12 These techniques are not freely available at present but may have an important role in aiding the diagnosis of pancreatic cancer in the future.
Computed tomography
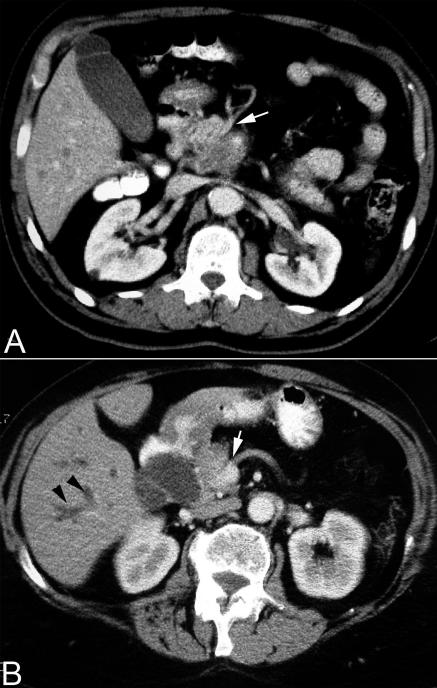
Although transabdominal ultrasonography is often the first imaging modality used, the current method of choice for diagnosis and staging of pancreatic cancer is thin section (3-5 mm cuts), contrast enhanced, dual phase multidetector computed tomography (fig 1).8,13,14 Computed tomography provides better tumour definition than does ultrasonography, although small hepatic or periportal metastases may still be missed.
Fig 1.
Contrast enhanced abdominal computed tomography scan showing (A) a large mass in the head of pancreas with encasement of the superior mesenteric artery (white arrow) and (B) dilated intrahepatic ducts (black arrowheads) and encasement of the superior mesenteric vein (white arrow)
Current computed tomography criteria for unresectability include the presence of metastatic disease (for example, liver, peritoneum), invasion of adjacent organs such as the stomach or colon, and encasement or occlusion of the peripancreatic vasculature. Encasement of the portal vein alone may not indicate inoperability as it may be resected with the tumour and then reconstructed. With these criteria, computed tomography has been shown to be almost 100% accurate in predicting unresectable disease.w9 However, the positive predictive value of the test is low, and approximately 25-50% of patients predicted to have resectable disease on computed tomography turn out to have unresectable lesions at laparotomy.11 The identification by preoperative imaging of patients who would not benefit from surgical exploration remains a challenge. The most common causes of unresectability are small peritoneal or liver tumour metastases (< 1 cm) and vascular involvement by the tumour.9 The advent of the multidetector dual phase computed tomography, along with three dimensional image reconstruction, has helped in improving the preoperative determination of surgical resectability, particularly in relation to vascular invasion.
Magnetic resonance imaging
Magnetic resonance imaging has recently been increasingly used in the evaluation of pancreatic tumours, although with the introduction of multidetector computed tomography the value of magnetic resonance imaging may be questionable.15 A study comparing magnetic resonance imaging and computed tomography in the imaging of pancreatic neoplasms concluded that magnetic resonance imaging offers no significant diagnostic advantage over computed tomography.16 However, the anatomy of the biliary tree and pancreatic duct are shown better with magnetic resonance cholangiopancreatography than with computed tomography. Magnetic resonance cholangiopancreatography has been shown to be as sensitive as endoscopic retrograde cholangiopancreatography in detecting pancreatic carcinomas.17 The resolution of magnetic resonance images of pancreatic pathology can be improved by using intravenous gadolinium enhancement. Pancreatic masses, biliary or pancreatic duct dilatation, and hepatic metastases can be shown in great detail.9,13 Additionally, contrast enhanced magnetic resonance angiography or venography can show vascular involvement with the tumour and obviate the need for conventional angiography.
Endoscopic retrograde cholangiopancreatography and percutaneous transhepatic cholangiography
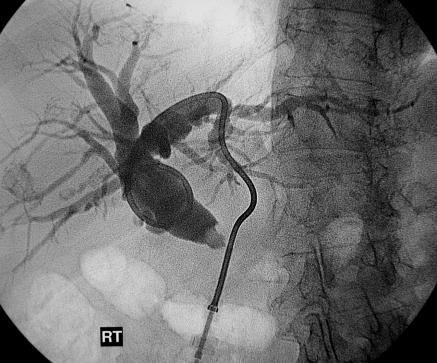
Before the widespread availability of endoscopic retrograde cholangiopancreatography, percutaneous transhepatic cholangiography was often used to delineate the biliary anatomy preoperatively (fig 2). Endoscopic retrograde cholangiopancreatography has largely replaced percutaneous transhepatic cholangiography as it has several major advantages. Preoperative duct delineation is usually necessary to confirm the exact site of obstruction, exclude concurrent pathology, and exclude obstruction at multiple levels.w10
Fig 2.
Percutaneous transhepatic cholangiogram showing a catheter in a dilated common bile duct with an abrupt, irregular stricture at the lower end, indicative of a pancreatic cancer
Endoscopic retrograde cholangiopancreatography, when used appropriately, can provide a definitive diagnosis. This is important, as only a third of tumours less than 2 cm and half of tumours less than 3 cm will be detected by conventional computed tomography.18 The advantages of endoscopic retrograde cholangiopancreatography over percutaneous transhepatic cholangiography are that it avoids liver puncture with the accompanying risk of bile leakage and haemorrhage and allows exclusion of other gastroduodenal disease, diagnosis of periampullary tumours, and imaging of the pancreatic duct. Brushing and biopsy specimens can also obtained for cytological and histological examination.
Both endoscopic retrograde cholangiopancreatography and percutaneous transhepatic cholangiography allow the insertion of biliary stents, and when access is difficult a combined approach may be necessary. However, preoperative biliary stenting has been embroiled in controversy. Sewnath et al suggested that it did not offer any benefit and should not be routinely carried out.19 Stenting provides ideal palliation for patients with jaundice who have unresectable or metastatic disease or are not fit for surgical resection. Expandable metal stents offer excellent palliation.w10 On the basis of current evidence, endoscopic retrograde cholangiopancreatography or percutaneous transhepatic cholangiography with stenting should not be used routinely in patients with resectable tumours as it may increase the rate of septic postoperative complications.19 Pragmatically, stenting may be necessary if surgery is not anticipated for several weeks or if the serum bilirubin concentration is rising rapidly.
Endoscopic ultrasonography
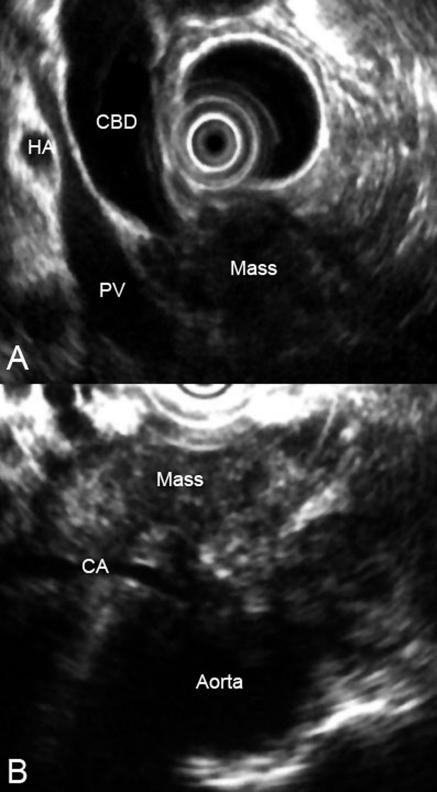
Endoscopic ultrasonography (fig 3) is a relatively new technique that is used to produce high resolution images of the pancreas by allowing the placement of a high frequency ultrasound probe in the stomach and duodenum in close proximity to the pancreas. Endoscopic ultrasonography may be the most accurate test for the diagnosis of pancreatic cancer. Studies comparing it with computed tomography have shown that endoscopic ultrasonography has a higher sensitivity and specificity for this diagnosis, particularly in evaluating tumours < 3 cm in diameter.20 In addition, endoscopic ultrasonography is highly accurate for detecting local invasion and nodal metastases from pancreatic cancer, although results are similar when compared with dual phase multislice multidetector computed tomography. Computed tomography does provide additional information about hepatic metastases.21 The side viewing duodenoscope that delivers the ultrasound probe also permits the detection of ampullary and duodenal carcinomas. However, the shortage of equipment, finances (an estimated £4.2 ($7.5; €6.2) million is needed to provide this facility nationwide22), and adequately trained endoscopists means that most patients in the United Kingdom do not have endoscopic ultrasonography.
Fig 3.
Endoscopic ultrasound image showing mass in the head of pancreas in relation to adjacent structures (CBD=common bile duct; HA=hepatic artery; PV=portal vein; CA=coeliac axis)
Angiography
In the past, preoperative angiography was commonly used to predict resectability and to give information on vascular anatomy. Some studies have shown that angiography adds to the reliability of computed tomography, whereas other studies have shown that multidetector computed tomography can predict unresectability better than angiography alone.14 Conventional angiography is not currently part of the diagnostic protocol in most centres.
Positron emission tomography
Positron emission tomography is a non-invasive imaging tool that provides metabolic rather than morphological information on tumours. This diagnostic method is based on greater use of glucose by tumour cells than by normal pancreatic parenchyma.w11 A radioactive glucose analogue termed fluorodeoxyglucose is administered intravenously, followed by detection by the scanner of uptake of fluorodeoxyglucose by the tumour. Malignant tissues will show a higher uptake of fluorodeoxyglucose than normal surrounding tissues. Positron emission tomography is useful in diagnosing small tumours (< 2 cm) and detecting extrapancreatic disease such as peritoneal or omental metastases.9 At present it is not routinely used in the diagnosis of pancreatic cancer because of the lack of anatomical detail. With the advent of combined positron emission tomography and computed tomography scanning, both anatomical and functional imaging can be obtained simultaneously.23 w12
Additional educational resources
Further reading
Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet 2004;363: 1049-57
Yeo TP, Hruban RH, Leach SD, Wilentz RE, Sohn TA, Kern SE, et al. Pancreatic cancer. Curr Probl Cancer 2002;26: 176-275
DiMagno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. Gastroenterology 1999;117: 1464-84
O'Reilly E, Kelvin JF, Harty JR, McCully J. 100 questions and answers about pancreatic cancer. Subbury, MA: Jones and Bartlett, 2003
Useful websites
Cancer Research UK (www.cancerhelp.org.uk/trials/trials/default.asp)—Provides information on current clinical trials in cancer treatment, including those involving pancreatic cancer
Doctor's Guide (free registration required) (www.docguide.com)—American website for clinicians.
Can be set up to provide the latest news, clinical trial information, and news on advances in basic sciences for a variety of medical and surgical conditions
The Pancreatic Duct (www.acor.org/pancreas/)—An interesting website set up by a patient with pancreatic cancer for people diagnosed as having the disease
Other tests
Percutaneous and endoscopic ultrasound guided aspiration cytology
Fine needle aspiration cytology of the pancreas has been one of the major advances in the management of patients with pancreatic tumours. When imaging findings are of an unresectable tumour or metastatic disease, fine needle aspiration guided by computed tomography or endoscopic ultrasonography is indicated for histological confirmation, unless a palliative surgical procedure is being contemplated.9 Computed tomography guided biopsy has been used for more than 20 years and has proved to be a safe and reliable procedure with good sensitivity. It is, however, associated with the risk of peritoneal and cutaneous seeding of the cancer, a complication avoided by endoscopic ultrasound guided biopsy. With greater availability, an attempt should be made to obtain preoperative endoscopic ultrasound guided fine needle aspirates or trucut biopsies in all patients with pancreatic cancer.
Laparoscopy or laparotomy
If the findings of imaging are inconclusive, a staging laparoscopy or laparotomy may be done before definitive surgery. Laparoscopy and laparotomy can be combined with intraoperative ultrasonography to define pancreatic lesions and to exclude subtle liver metastases that may have been missed by other imaging modalities.24 However, routine laparoscopy is not currently recommended as it influences the management in less than 14% of patients with pancreatic cancer.10
Staging
Pancreatic cancers are staged using the TNM (tumour, node, metastasis) classification. Accurate staging has a vital role in the management of pancreatic tumours now that non-operative palliative options are available. Computed tomography is widely used in the preoperative staging of pancreatic neoplasms. With recent advances in magnetic resonance imaging and endoscopic ultrasonography, the accuracy of preoperative staging has improved, especially with respect to local invasion and regional node involvement.
Conclusions
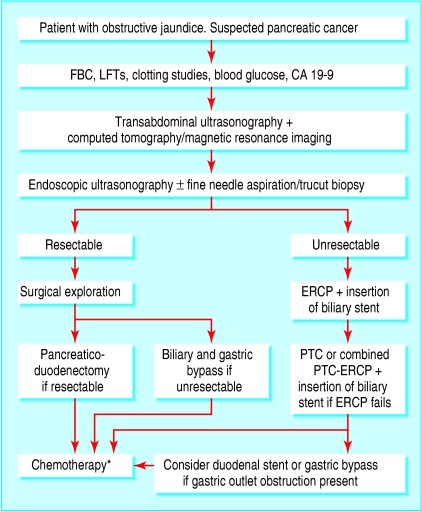
Several imaging modalities are available to the clinician to diagnose and stage pancreatic cancer, and figure 4 suggests a treatment algorithm. When diagnostic techniques are combined appropriately, the rate of unnecessary surgical explorations can be reduced. Studies comparing the various imaging modalities used in the diagnosis of pancreatic cancer have shown that it is difficult to diagnose on the basis of computed tomography alone. Further examinations or biopsy are often needed to confirm the diagnosis. Although computed tomography is the imaging modality of choice at present, endoscopic ultrasonography and positron emission tomography are likely to become vital in the detection of small tumours. In general, endoscopic retrograde cholangiopancreatography should be reserved for treatment in patients who are found to have unresectable or metastatic disease. Despite the rapid advances in imaging techniques, the overall impact of these modalities on the survival of patients with pancreatic cancer is debatable as most patients still present with locally advanced disease. A need thus exists for the development of biomarkers and techniques for the early diagnosis of the disease in symptomatic patients as well as for use in screening tests in symptom-free people at risk of developing pancreatic carcinoma. The table summarises the recent and ongoing clinical trials in pancreatic cancer.
Fig 4.
Suggested algorithm for the diagnosis and treatment of pancreatic cancer. CA=carbohydrate antigen; ERCP=endoscopic retrograde cholangiopancreatography; FBC=full blood count; LFTs=liver function tests; PTC=percutaneous transhepatic cholangiography. *The recommendation for adjuvant chemotherapy for patients with pancreatic cancer is based on a recent multicentre trial report that showed a survival advantage conferred by chemotherapy in patients with resected pancreatic cancerw13
Table 1.
Recent and current clinical trials in pancreatic cancer (further information can be found at www.cancerhelp.org.uk/trials/trials/)
| Trial | Phase | Details | Recruitment dates or results |
|---|---|---|---|
| GEM-CAP |
III |
Single or combination therapy. Comparing gemcitabine alone with a combination of gemcitabine and capecitabine |
April 2002 to March 2005 |
| ESPAC 1 |
III |
Compared survival in tumour resected patients having chemoradiotherapy, a combination of chemoradiotherapy and chemotherapy, and no adjuvant treatment |
Recently concluded trial showed adjuvant chemotherapy had a significant beneficial effect on survival, whereas chemoradiotherapy had a deleterious effectw13 |
| ESPAC 3 v2 |
III |
Comparing gemcitabine with a combination of fluorouracil and folinic acid |
July 2000 to July 2005 |
| MetXia-OB83 and cyclophosphamide | I | Patients will be given a combination of MetXia-OB83 (gene therapy) and cyclophosphamide | March 2004 to October 2005 |
Supplementary Material
 Extra references are on bmj.com
Extra references are on bmj.com
We thank Guruprasad P Aithal, consultant hepatologist, University Hospital, Queen's Medical Centre, Nottingham, for providing the images for figure 3.
Contributors: AST and PP reviewed the literature and prepared the initial and final drafts of the manuscript. RD reviewed the imaging aspects of the paper and was involved in writing the final draft. DNL reviewed the manuscript and provided intellectual input and overall supervision, in addition to preparing the figures. AST and DNL are guarantors.
Funding: None.
Competing interests: None declared.
References
- 1.Quinn M, Babb P, Brock A, Kirby L, Jones J. Cancer trends in England and Wales 1950-1999. London: Office for National Statistics, 2001.
- 2.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin 2003;53: 5-26. [DOI] [PubMed] [Google Scholar]
- 3.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet 2004;363: 1049-57. [DOI] [PubMed] [Google Scholar]
- 4.DiMagno EP. Pancreatic cancer: clinical presentation, pitfalls and early clues. Ann Oncol 1999;10(suppl 4): 140-2. [PubMed] [Google Scholar]
- 5.Gullo L. Diabetes and the risk of pancreatic cancer. Ann Oncol 1999;10(suppl 4): 79-81. [PubMed] [Google Scholar]
- 6.Yeo TP, Hruban RH, Leach SD, Wilentz RE, Sohn TA, Kern SE, et al. Pancreatic cancer. Curr Probl Cancer 2002;26: 176-275. [DOI] [PubMed] [Google Scholar]
- 7.Ozkan H, Kaya M, Cengiz A. Comparison of tumor marker CA 242 with CA 19-9 and carcinoembryonic antigen (CEA) in pancreatic cancer. Hepatogastroenterology 2003;50: 1669-74. [PubMed] [Google Scholar]
- 8.Tamm EP, Silverman PM, Charnsangavej C, Evans DB. Diagnosis, staging, and surveillance of pancreatic cancer. AJR Am J Roentgenol 2003;180: 1311-23. [DOI] [PubMed] [Google Scholar]
- 9.Clarke DL, Thomson SR, Madiba TE, Sanyika C. Preoperative imaging of pancreatic cancer: a management-oriented approach. J Am Coll Surg 2003;196: 119-29. [DOI] [PubMed] [Google Scholar]
- 10.Potter MW, Shah SA, McEnaney P, Chari RS, Callery MP. A critical appraisal of laparoscopic staging in hepatobiliary and pancreatic malignancy. Surg Oncol 2000;9: 103-10. [DOI] [PubMed] [Google Scholar]
- 11.Rickes S, Unkrodt K, Neye H, Ocran KW, Wermke W. Differentiation of pancreatic tumours by conventional ultrasound, unenhanced and echo-enhanced power Doppler sonography. Scand J Gastroenterol 2002;37: 1313-20. [DOI] [PubMed] [Google Scholar]
- 12.Kitano M, Kudo M, Maekawa K, Suetomi Y, Sakamoto H, Fukuta N, et al. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut 2004;53: 854-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalra MK, Maher MM, Mueller PR, Saini S. State-of-the-art imaging of pancreatic neoplasms. Br J Radiol 2003;76: 857-65. [DOI] [PubMed] [Google Scholar]
- 14.Vargas R, Nino-Murcia M, Trueblood W, Jeffrey RB Jr. MDCT in pancreatic adenocarcinoma: prediction of vascular invasion and resectability using a multiphasic technique with curved planar reformations. AJR Am J Roentgenol 2004;182: 419-25. [DOI] [PubMed] [Google Scholar]
- 15.Hanbidge AE. Cancer of the pancreas: the best image for early detection—CT, MRI, PET or US? Can J Gastroenterol 2002;16: 101-5. [DOI] [PubMed] [Google Scholar]
- 16.Steiner E, Stark DD, Hahn PF, Saini S, Simeone JF, Mueller PR, et al. Imaging of pancreatic neoplasms: comparison of MR and CT. AJR Am J Roentgenol 1989;152: 487-91. [DOI] [PubMed] [Google Scholar]
- 17.Adamek HE, Albert J, Breer H, Weitz M, Schilling D, Riemann JF. Pancreatic cancer detection with magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography: a prospective controlled study. Lancet 2000;356: 190-3. [DOI] [PubMed] [Google Scholar]
- 18.Graham RA, Bankoff M, Hediger R, Shaker HZ, Reinhold RB. Fine-needle aspiration biopsy of pancreatic ductal adenocarcinoma: loss of diagnostic accuracy with small tumors. J Surg Oncol 1994;55: 92-4. [DOI] [PubMed] [Google Scholar]
- 19.Sewnath ME, Karsten TM, Prins MH, Rauws EJ, Obertop H, Gouma DJ. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg 2002;236: 17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mertz HR, Sechopoulos P, Delbeke D, Leach SD. EUS, PET, and CT scanning for evaluation of pancreatic adenocarcinoma. Gastrointest Endosc 2000;52: 367-71. [DOI] [PubMed] [Google Scholar]
- 21.Midwinter MJ, Beveridge CJ, Wilsdon JB, Bennett MK, Baudouin CJ, Charnley RM. Correlation between spiral computed tomography, endoscopic ultrasonography and findings at operation in pancreatic and ampullary tumours. Br J Surg 1999;86: 189-93. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Guidance Group. Guidance on commissioning cancer services: improving outcomes in upper gastro-intestinal cancers: the manual. London: NHS Executive, 2001.
- 23.Hosten N, Lemke AJ, Wiedenmann B, Bohmig M, Rosewicz S. Combined imaging techniques for pancreatic cancer. Lancet 2000;356: 909-10. [DOI] [PubMed] [Google Scholar]
- 24.Pisters PW, Lee JE, Vauthey JN, Charnsangavej C, Evans DB. Laparoscopy in the staging of pancreatic cancer. Br J Surg 2001;88: 325-37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.