Abstract
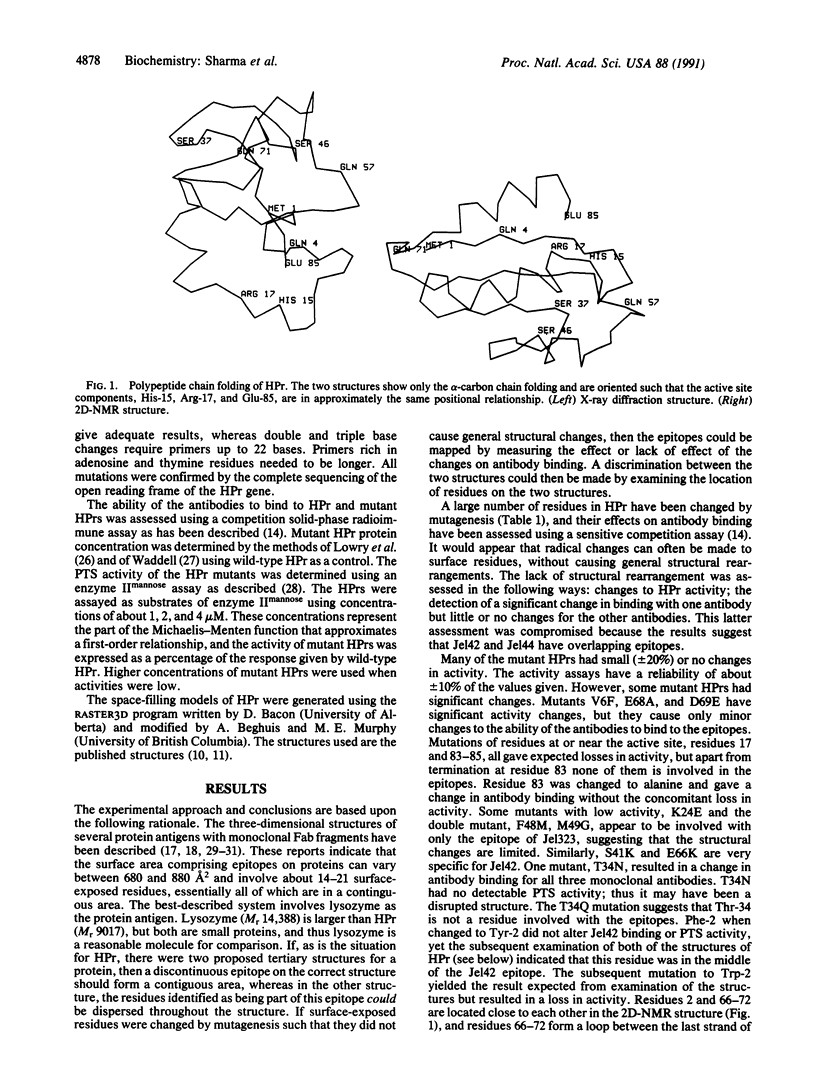
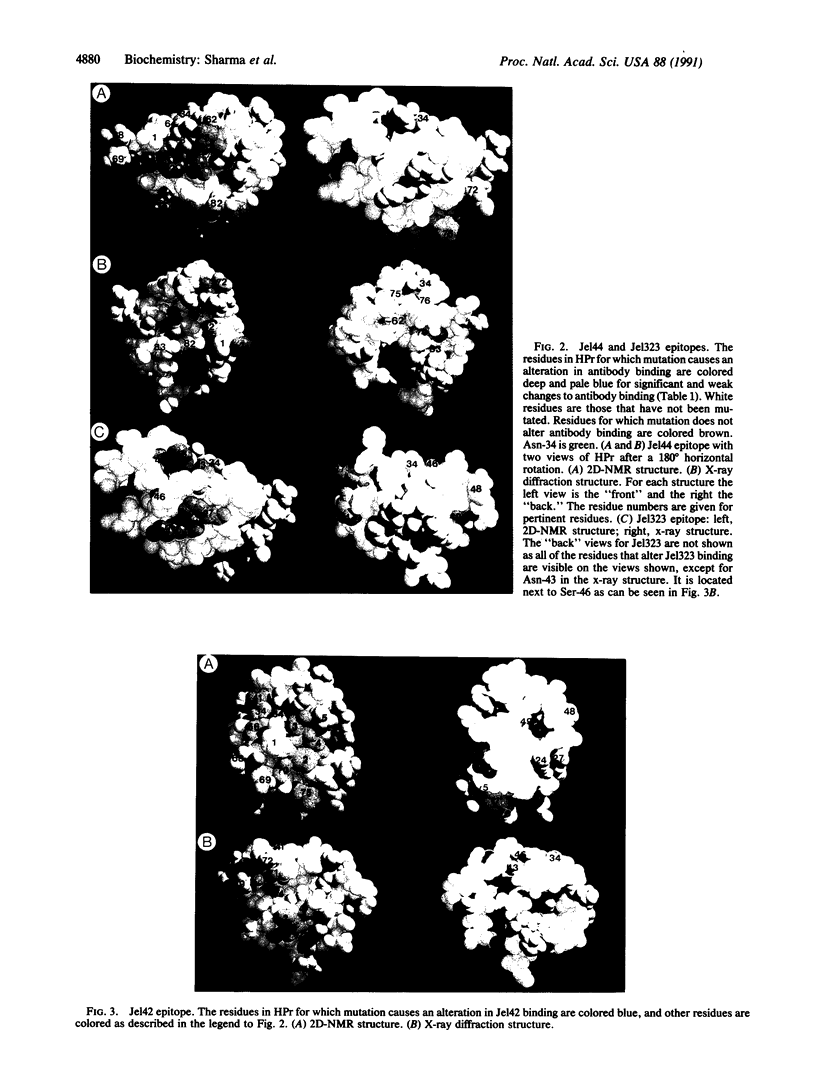
Thirty-four of the 85 residues of the histidine-containing protein HPr of the Escherichia coli phosphoenolpyruvate:sugar phosphotransferase system have been changed by site-directed mutagenesis. Many of the mutations have wild-type activity suggesting an unaltered tertiary structure but have altered binding to three monoclonal antibodies: Jel42, Jel44, and Jel323. This altered binding defines the residues that are involved in the epitopes of HPr. At present, two different three-dimensional structures have been determined for HPr, one from two-dimensional nuclear magnetic resonance spectra and the other from x-ray diffraction of HPr crystals. The epitope mapping for Jel42 does not distinguish between the tertiary structures. However, only the HPr structure derived from two-dimensional nuclear magnetic resonance spectra is consistent with a contiguous surface binding site that can be defined as the epitope for Jel44. Thus the x-ray structure may represent a partially unfolded HPr.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science. 1986 Aug 15;233(4765):747–753. doi: 10.1126/science.2426778. [DOI] [PubMed] [Google Scholar]
- Anderson B., Weigel N., Kundig W., Roseman S. Sugar transport. 3. Purification and properties of a phosphocarrier protein (HPr) of the phosphoenolpyruvate-dependent phosphotransferase system of Escherichia coli. J Biol Chem. 1971 Nov 25;246(22):7023–7033. [PubMed] [Google Scholar]
- Colman P. M., Laver W. G., Varghese J. N., Baker A. T., Tulloch P. A., Air G. M., Webster R. G. Three-dimensional structure of a complex of antibody with influenza virus neuraminidase. 1987 Mar 26-Apr 1Nature. 326(6111):358–363. doi: 10.1038/326358a0. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Padlan E. A., Sheriff S. Antibody-antigen complexes. Annu Rev Biochem. 1990;59:439–473. doi: 10.1146/annurev.bi.59.070190.002255. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Sheriff S., Padlan E. A. Antibody-antigen complexes. J Biol Chem. 1988 Aug 5;263(22):10541–10544. [PubMed] [Google Scholar]
- Delbaere L. T., Bruse L. M., Waygood E. B. Preliminary x-ray data for the HPr protein of the phosphoenolpyruvate: sugar phosphotransferase system of Escherichia coli. J Mol Biol. 1982 May 5;157(1):161–162. doi: 10.1016/0022-2836(82)90519-8. [DOI] [PubMed] [Google Scholar]
- Delbaere L. T., Vandonselaar M., Quail J. W., Waygood E. B., Lee J. S. Crystallization of the complex of a monoclonal Fab fragment with the histidine-containing protein of the phosphoenolpyruvate: sugar phosphotransferase system of Escherichia coli. J Biol Chem. 1989 Nov 5;264(31):18645–18646. [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevit R. E., Drobny G. P., Waygood E. B. Two-dimensional 1H NMR studies of histidine-containing protein from Escherichia coli. 1. Sequential resonance assignments. Biochemistry. 1986 Nov 18;25(23):7760–7769. doi: 10.1021/bi00371a071. [DOI] [PubMed] [Google Scholar]
- Klevit R. E., Waygood E. B. Two-dimensional 1H NMR studies of histidine-containing protein from Escherichia coli. 3. Secondary and tertiary structure as determined by NMR. Biochemistry. 1986 Nov 18;25(23):7774–7781. doi: 10.1021/bi00371a073. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee L. G., Britton P., Parra F., Boronat A., Kornberg H. Expression of the ptsH+ gene of Escherichia coli cloned on plasmid pBR322. A convenient means for obtaining the histidine-containing carrier protein HPr. FEBS Lett. 1982 Nov 29;149(2):288–292. doi: 10.1016/0014-5793(82)81119-8. [DOI] [PubMed] [Google Scholar]
- Lévy S., Zeng G. Q., Danchin A. Cyclic AMP synthesis in Escherichia coli strains bearing known deletions in the pts phosphotransferase operon. Gene. 1990 Jan 31;86(1):27–33. doi: 10.1016/0378-1119(90)90110-d. [DOI] [PubMed] [Google Scholar]
- Meadow N. D., Fox D. K., Roseman S. The bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu Rev Biochem. 1990;59:497–542. doi: 10.1146/annurev.bi.59.070190.002433. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad L., Vandonselaar M., Lee J. S., Delbaere L. T. Structure determination of a monoclonal Fab fragment specific for histidine-containing protein of the phosphoenolpyruvate: sugar phosphotransferase system of Escherichia coli. J Biol Chem. 1988 Feb 15;263(5):2571–2574. [PubMed] [Google Scholar]
- Reizer J., Saier M. H., Jr, Deutscher J., Grenier F., Thompson J., Hengstenberg W. The phosphoenolpyruvate:sugar phosphotransferase system in gram-positive bacteria: properties, mechanism, and regulation. Crit Rev Microbiol. 1988;15(4):297–338. doi: 10.3109/10408418809104461. [DOI] [PubMed] [Google Scholar]
- Roseman S., Meadow N. D. Signal transduction by the bacterial phosphotransferase system. Diauxie and the crr gene (J. Monod revisited). J Biol Chem. 1990 Feb 25;265(6):2993–2996. [PubMed] [Google Scholar]
- Sheriff S., Silverton E. W., Padlan E. A., Cohen G. H., Smith-Gill S. J., Finzel B. C., Davies D. R. Three-dimensional structure of an antibody-antigen complex. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8075–8079. doi: 10.1073/pnas.84.22.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- Waygood E. B., Erickson E., el Kabbani O. A., Delbaere L. T. Characterization of phosphorylated histidine-containing protein (HPr) of the bacterial phosphoenolpyruvate:sugar phosphotransferase system. Biochemistry. 1985 Nov 19;24(24):6938–6945. doi: 10.1021/bi00345a028. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Meadow N. D., Roseman S. Modified assay procedures for the phosphotransferase system in enteric bacteria. Anal Biochem. 1979 May;95(1):293–304. doi: 10.1016/0003-2697(79)90219-7. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Reiche B., Hengstenberg W., Lee J. S. Characterization of mutant histidine-containing proteins of the phosphoenolpyruvate:sugar phosphotransferase system of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1987 Jun;169(6):2810–2818. doi: 10.1128/jb.169.6.2810-2818.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waygood E. B., Steeves T. Enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system of Escherichia coli. Purification to homogeneity and some properties. Can J Biochem. 1980 Jan;58(1):40–48. doi: 10.1139/o80-006. [DOI] [PubMed] [Google Scholar]
- Wright P. E. What can two-dimensional NMR tell us about proteins? Trends Biochem Sci. 1989 Jul;14(7):255–260. doi: 10.1016/0968-0004(89)90058-3. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]
- el-Kabbani O. A., Waygood E. B., Delbaere L. T. Tertiary structure of histidine-containing protein of the phosphoenolpyruvate:sugar phosphotransferase system of Escherichia coli. J Biol Chem. 1987 Sep 25;262(27):12926–12929. [PubMed] [Google Scholar]