Abstract
Background
Spitz nevi, atypical Spitz tumors and spitzoid melanomas (“spitzoid lesions”) represent controversial and poorly understood cutaneous melanocytic lesions that are difficult to diagnose histologically. It is unknown how these terms are used by pathologists.
Methods
We describe use of Spitz-related terminology using data from the Melanoma Pathology (M-Path) study database comprising pathologists’ interpretations of biopsy slides, a nation-wide study evaluating practicing U.S. pathologists’ (N=187) diagnoses of melanocytic lesions (8,976 independent diagnostic assessments on 240 total test cases, with one slide per case).
Results
Most pathologists (90%) used the Spitz-related terminology. However, significant variation exists in which specific lesions were diagnosed as spitzoid and in the corresponding treatment recommendations. Recommendations ranged from ‘no further treatment’ to ‘wide excision of 10 mm or greater’ with no category capturing more than 50% of responses. For spitzoid melanoma diagnoses, 90% of pathologists recommended excision with ≥10 mm margin. Pathologists report less confidence in diagnosing these lesions compared with other melanocytic proliferations and are more likely to request second opinions and additional clinical information (all p<0.05).
Conclusions
Spitzoid lesions are often not classified in any standardized way, evoke uncertainty in diagnosis by pathologists, and elicit variability in treatment recommendations.
Keywords: Spitz, terminology, dermatopathology, Melanocytic nevus, melanoma
INTRODUCTION
Spitzoid melanocytic neoplasms are a controversial and poorly understood category of melanocytic lesions which, despite controversy, are considered distinctive by some authorities.(1) They occur commonly, but not exclusively, in young individuals. (1) These tumors are distinguished from other types of melanocytic lesions not only by clinical and pathological features but also increasingly by an associated “landscape” of genomic structural alterations.(2-5) The controversy stems from their frequent confusion with melanoma and an inability of pathologists to predict their biological behavior. Importantly, these lesions account for the majority of so-called “melanomas” seen in children and adolescents.
Credit is given to Sophie Spitz for describing in some detail this class of melanocytic lesions, which she termed “juvenile melanoma.” She suggested that these tumors are associated with reduced lethality compared with melanomas of the conventional adult type. (6) Over the subsequent decades, a general consensus emerged, but not without controversy, that spitzoid neoplasms appear to represent a histopathological and biological continuum of lesions, ranging from benign to malignant. Utilizing a reductionist approach, many have advocated for a binary classification as simply “Spitz nevus” or “malignant melanoma,” leaving no room for an intermediate group of lesions that blatantly defy categorization. (7) In an effort to accommodate this group of intermediate lesions, the term “atypical Spitz tumor” was introduced by Reed et al. to describe tumors with histological features at variance with the conventional Spitz nevus yet insufficient for malignancy. (8) Over the past two to three decades, a more realistic approach has been embraced by dermatopathologists under the rubric of three-tiered scheme: 1) Spitz nevus, 2) atypical Spitz tumor, and 3) spitzoid melanoma, with the caveat of inherent uncertainty about the biological nature of many lesions so classified. (9-13)
As experts in the field have difficulty achieving consensus in diagnosis and establishing the prognosis for many spitzoid lesions, ongoing discordance and controversy exists with respect to terminology for these lesions. (12, 13) In order to gain insight into this challenging clinical problem, we describe here the utilization of Spitz-related nomenclatures amongst practicing U.S. pathologists who interpret melanocytic lesions in their own clinical practice. We report from the Melanoma Pathology (M-Path) study database how frequently pathologists employ these diagnostic terms when interpreting a test set of cases. We also describe their perceptions about the degree of atypia in these lesions and their suggestions concerning management.
MATERIALS AND METHODS
Development of Standard Test Sets
Melanocytic skin lesions biopsied from patients 20 years of age or older between January 1, 2010 and December 31, 2011 were obtained from a pathology practice in Washington State (Dermatopathology Northwest). Cases were selected utilizing the dermatopathology practice's internal computer database to identify a range of different types of skin lesions, including benign, atypia, nevus, melanoma in situ, and invasive melanoma. Shave, punch, and excisional biopsies were included, while consultative cases and re-excisions were excluded.
Eligible skin lesions (n=27,481) were divided into five diagnostic categories that mapped to corresponding clinical management suggestions using the Melanocytic Pathology Assessment Tool & Hierarchy for Diagnosis (MPATH-Dx) (14), based on the original pathologist's interpretation: 1) nevus /mild atypia (no further treatment required); 2) moderate atypia (narrow but complete re-excision); 3) severe atypia/melanoma in situ (repeat excision with at least 5 mm margins); 4 & 5) T1a melanoma (wide excision) and T1b melanoma (wide excision with additional treatment required). Age categories (20-49; 50-64; ≥65) were stratified to ensure cases were representative according to age.
A study set of 240 melanocytic cases was developed as previously described using a modified Delphi approach. (14) For this test set development, each patient case had three slides containing one histologic section. A panel of three experienced pathologists (DEE, RLB, MWP) individually reviewed slides for each patient case, followed by a consensus meeting where the panel divided the patient cases into five categories according to the MPATH-Dx mapping tool. (14, 15) Agreement was obtained in every case although in some cases the extent of agreement was limited to a descriptive category such as “Melanocytic tumor of uncertain malignant potential” (MELTUMP).
All procedures were HIPAA compliant and approval was obtained from the Institutional Review Boards of the University of Washington, Fred Hutchinson Cancer Research Center, Oregon Health Sciences University, Rhode Island Hospital, and Dartmouth College. Informed consent was obtained from participating pathologists.
Pathologists Recruitment and Interpretation
Pathologists in 10 states (CA, CT, HI, IA, KY, LA, NJ, NM, UT, and WA) were invited to participate in the study. Eligible pathologists were those who had completed their pathology training, interpreted melanocytic skin biopsies within the previous year, and expected to continue interpreting melanocytic skin lesions for the next two years. Each pathologist was sent one test set of 48 glass slides, with slides to be reviewed independently and in a random order. Pathologists directly selected their interpretations for each case and suggested treatment recommendations through the online MPATH-Dx diagnosis form. The diagnoses and treatments considered were selected from pull-down menus of terms that were designed to be as inclusive as possible. The only clinical information provided was the patient's age, patient's gender, biopsy type (shave, punch, or excision), and biopsy site.
Statistical Analysis
This study describes the M-Path cases where the pathologists rendered diagnoses that included at least one of the following six terms: Spitz nevus, pigmented spindle cell nevus, atypical/dysplastic Spitz lesion/tumor, atypical pigmented spindle cell lesion/tumor, Spitz-like invasive melanoma, and pigmented spindle cell-like invasive melanoma. For analysis, we grouped the first two diagnoses as “Spitz nevus,” the middle two as “atypical Spitz tumor,” and the last two as “spitzoid melanoma” (Table 1). Descriptive statistics computed frequencies and percentages of the use of the terms across treatment recommendations.
Table 1.
Terminologies used for spitzoid lesions.
| Spitzoid melanocytic proliferation |
| Spitz nevus (MPATH Dx Category 2) |
| - Spitz nevus conventional, (junctional, compound, or intradermal) |
| - Pigmented spindle cell nevus, (junctional, compound) |
| Atypical Spitz tumor (MPATH Dx Category 3) |
| - Atypical/dysplastic Spitz lesion, (junctional, compound, or dermal) |
| - Atypical pigmented spindle cell lesions, (junctional, compound) |
| Spitzoid melanoma (MPATH Dx Category 4/5) |
| - Spitz-like invasive melanoma, (a melanoma that resembles a Spitz nevus/tumor) |
| - Pigmented spindle cell-like invasive melanoma (a melanoma resembling pigmented spindle cell nevus or plexiform spindle cell nevus) |
To determine if the use of these terms differed compared to other terms extant in melanoma pathology, we compared the three grouped categories of spitzoid lesions with other types of melanocytic proliferations sharing similar MPATH-Dx assignments.(16) Accordingly, we compared the Spitz nevus lesions to other MPATH-Dx category 2 lesions, atypical Spitz tumors to other category 3 cases, and spitzoid melanomas to other MPATH-Dx category 4 or 5 lesions. Each of these comparisons was assessed across variables reflecting physician perception of case difficulty. Perceptions were assessed by asking the pathologist if he/she would request a second opinion for the case, would request additional clinical information, would order special stains, considered the diagnosis to be borderline between neighboring diagnostic categories, felt the case was challenging, and had confidence in his/her diagnosis. The Cochran-Mantel-Haenszel (CMH) test stratified across pathologists to account for the multiple readings was used to determine significant differences in the use of terms. All analyses and statistics were produced using SAS software, version 9.4, Cary, NC, USA.
RESULTS
Of 301 eligible responding pathologists, 207 consented and 187 proceeded to complete the study interpretations. The average age of pathologists was 51 (range: 33-79) with 114 (61%) pathologists male and 73 (39%) female. The pathologists provided a total of 8,976 individual interpretations with 542 (6%) primary diagnoses within the spectrum of spitzoid melanocytic proliferations defined above. At least one test case was interpreted as a spitzoid lesion per 48-case test set by the majority of pathologists (168/187; 90%) (Appendix 1). Among the pathologists who used nomenclatures under the category of spitzoid lesions, 151/168 (90%) used the terms 1 to 5 times; 14 pathologists (8%) used the terms 6-8 times; and only 3 (2%) used the terms 9 or 10 times when interpreting their 48 test cases.
As depicted in Table 2, recommendations for Spitz nevus and atypical Spitz tumors ranged from ‘no further treatment’ to ‘wide excision of 10 mm or greater’ with no recommendation category capturing more than 50% of responses. The most frequent recommendation was “excision <5 mm margin” for Spitz nevi (48%) and atypical Spitz tumors (44%). If a diagnosis of atypical Spitz tumor was made, 5% of pathologists recommended no further treatment, 44% recommended excision with < 5mm margin, 37% recommended excision with 5-9 mm margins, and 15% recommended wide excision with ≥ 10mm. There was high consensus in treating spitzoid melanoma, with 90% of pathologists recommending excision with ≥ 10 mm margin, 9% recommended excision with 5-9 mm margin, and 1% recommended excision with <5 mm margin (Table 2).
Table 2.
Participants' diagnoses and recommendation
| Recommendation | |||||
|---|---|---|---|---|---|
| Diagnosis | No further treatment | Excision <5mm margin | Excision 5-9mm margin | Wide excision 10mm or greater and/or other | Number of assessments |
| Spitz nevus - Spitz nevus conventional - Pigmented spindle cell nevus |
48 (44.9%) | 51 (47.7%) | 6 (5.6%) | 2 (1.9%) | 107 (100.0%) |
| Atypical Spitz tumor - Atypical/dysplastic Spitz lesions - Atypical pigmented spindle cell lesion |
12 (4.7%) | 110 (43.5%) | 94 (37.2%) | 37 (14.6%) | 253 (100.0%) |
| Spitzoid melanoma - Spitz-like invasive melanoma - Pigmented spindle cell-like invasive melanoma |
0 (0.0%) | 2 (1.1%) | 17 (9.3%) | 163 (89.6%) | 182 (100.0%) |
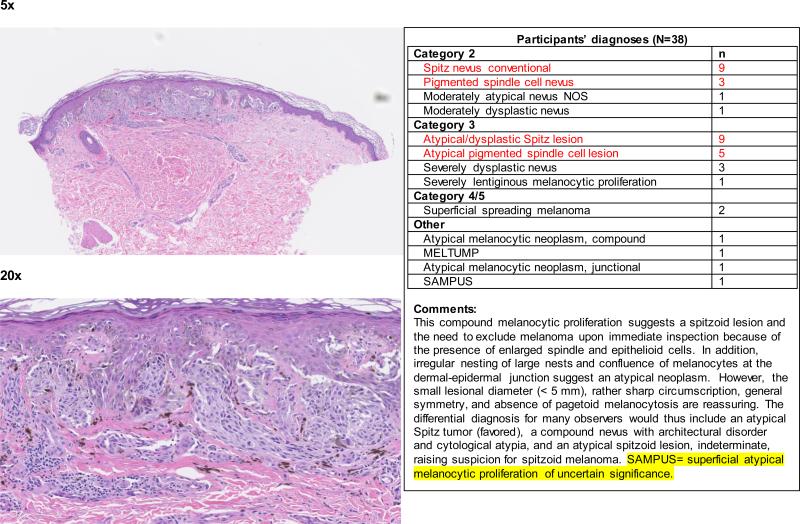
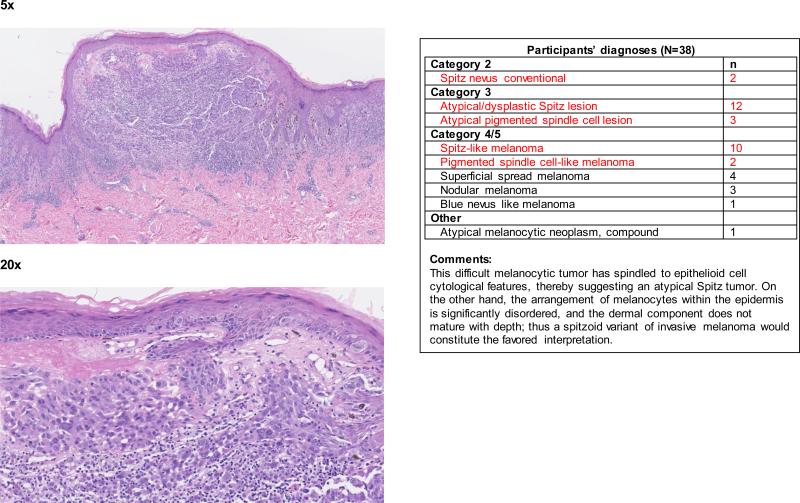
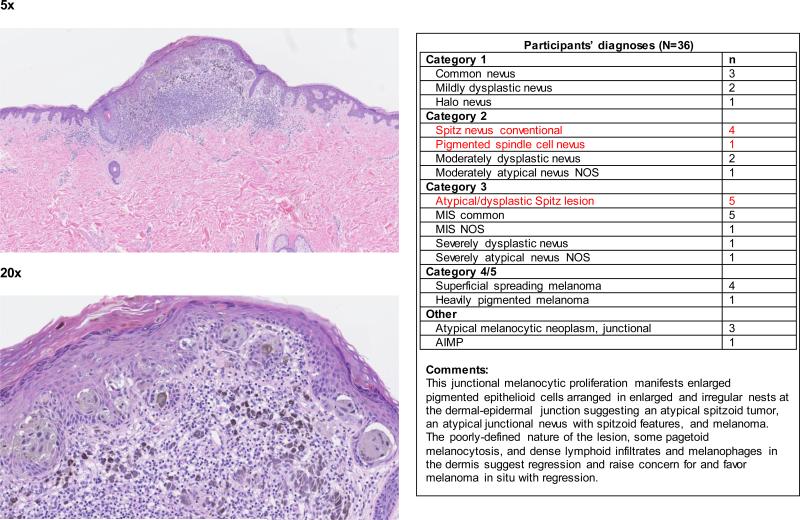
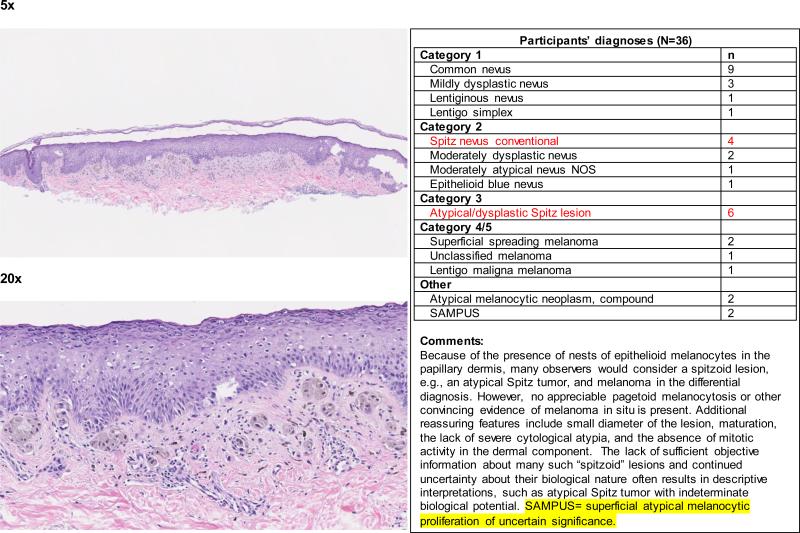
When the data were considered at the level of the individual 240 cases, the term spitzoid melanocytic proliferation was applied by at least one pathologist to half of the test cases (119 of 240 cases; 50%). Each 48-case test set was evaluated by between 36 and 39 independent pathologists, depending on the random allocation process. Forty-two (18%) cases were diagnosed as a type of spitzoid lesion by at least 4 pathologists (Figure 1 and Appendix 1). In 14/42 (33%) cases, the interpretations crossed the entire spectrum from Spitz nevus to spitzoid melanoma. We illustrate four cases demonstrating an atypical Spitz tumor (Figure 2a), a spitzoid melanoma (Figure 2b), and cases with significantly less interobserver agreement (Figure 2c and 2d).
Figure 1.
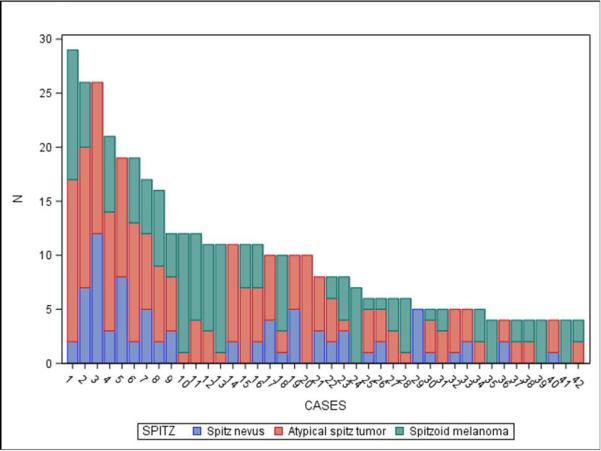
Interpretations of Spitz nevus, atypical Spitz tumor, and spitzoid melanoma in each case with a least 4 Spitz-type assessments
Figure 2a.
Teaching case 1. 5x (top panel) and 20x (bottom panel)
Figure 2b.
Teaching case 2. 5x (top panel) and 20x (bottom panel)
Figure 2c.
Teaching case 3. 5x (top panel) and 20x (bottom panel)
Figure 2d.
Teaching case 4. 5x (top panel) and 20x (bottom panel)
Pathologists’ approaches to and perceptions of diagnosing spitzoid lesions are summarized in Table 3. Since melanocytic lesions with different malignant potentials often require different diagnostic evaluations, we compared the three groupings of spitzoid lesions (Table 1) with lesions having a similar MPATH-Dx assignment: Spitz nevus vs. MPATH-Dx category 2 lesions, atypical Spitz tumor vs. MPATH-Dx category 3 lesions, and spitzoid melanoma vs. MPATH-Dx category 4 or 5 lesions. The majority of pathologists (71-92%) indicated that they would request a second opinion for lesions they classified into one of the three spitzoid groupings in a real-life situation. When evaluating atypical Spitz tumors or spitzoid melanomas, pathologists are more likely to request additional clinical information (36% vs 23% for atypical Spitz tumor, 20% vs 15% for spitzoid melanoma), to diagnose them as borderline lesions (54% vs 43% for atypical Spitz tumor, 29% vs 13% for spitzoid melanoma), to consider the cases to be more challenging (79% vs 50% for atypical Spitz tumor, 50% vs 32% for spitzoid melanoma), and to feel less confident in assessed diagnosis (51% vs 27% for atypical Spitz tumor, 25% vs 17% for spitzoid melanoma), compared to assessing other melanocytic lesions within the same MPATH-Dx mapping category. When ordering special stains, Ki-67 was more likely indicated for Spitz nevus, atypical Spitz tumor, and spitzoid melanomas (9-22%) compared to assessing other melanocytic lesions within the same MPATH-Dx mapping category.
Table 3.
Pathologist perceptions and approaches to diagnosing spitzoid lesions compared to other melanocytic proliferations
| Diagnostic category | ||||||||
|---|---|---|---|---|---|---|---|---|
| MPATH-Dx Category I | Spitz Nevus | Other MPATH-Dx Category II | Atypical Spitz | Other MPATH-Dx Category III | Spitzoid melanoma | Other MPATH-Dx Category IV/V | Total | |
| Total Assessments | 2618 | 107 | 1057 | 253 | 1683 | 182 | 3076 | 8976 |
| Request 2nd opinion: | ||||||||
| For personal reasons | 649 (25%) | 67 (63%)* | 588 (56%) | 207 (82%)* | 1040 (62%) | 106 (58%)* | 1242 (40%) | 3899 |
| Practice policy | 273 (10%) | 20 (19%) | 191 (18%) | 64 (25%)* | 558 (33%) | 79 (43%)* | 1435 (47%) | 2620 |
| Any reason | 831 (32%) | 76 (71%)* | 687 (65%) | 234 (92%)* | 1361 (81%) | 152 (84%)* | 2256 (73%) | 5597 |
| Request additional information: | ||||||||
| Additional clinical information | 373 (14%) | 27 (25%) | 262 (25%) | 91 (36%)* | 391 (23%) | 36 (20%)* | 461 (15%) | 1641 |
| Order special stains (any) | 184 (7%) | 18 (17%) | 229 (22%) | 79 (31%) | 473 (28%) | 42 (23%) | 601 (20%) | 1626 |
| Ki-67 | 35 (1%) | 10 (9%)* | 50 (5%) | 55 (22%)* | 129 (8%) | 26 (14%)* | 262 (9%) | 567 |
| Mart-1 | 119 (5%) | 8 (7%) | 150 (14%) | 44 (17%) | 339 (20%) | 26 (14%) | 387 (13%) | 1073 |
| Perception: | ||||||||
| Case was challenging | 443 (17%) | 50 (47%) | 483 (46%) | 198 (79%)* | 835 (50%) | 91 (50%)* | 973 (32%) | 3073 |
| Low confidence in assessment | 278 (11%) | 26 (24%) | 275 (26%) | 130 (51%)* | 450 (27%) | 46 (25%)* | 530 (17%) | 1735 |
| Diagnosis is borderline | 317 (12%) | 30 (28%) | 349 (33%) | 136 (54%)* | 730 (43%) | 52 (29%)* | 393 (13%) | 2007 |
| Borderline diagnosis is in lower category than primary diagnosis | 6 (20%) | 59 (17%) | 28 (21%)* | 55 (8%) | 48 (92%)* | 256 (65%) | 452 | |
| Borderline diagnsosis is in same category as primary diagnosis | 189 (60%) | 5 (17%) | 36 (10%) | 36 (26%)* | 417 (57%) | 4 (8%)* | 137 (35%) | 824 |
| Borderline diagnosis in in higher category than primary diagnosis | 127 (40%) | 19 (63%) | 252 (73%) | 72 (53%)* | 257 (35%) | 727 | ||
indicates CMH p-value <0.05
a.Difficulty scored on a scale of 1-6 with 1-3 being easy and 4-6 being very challenging
b.Confident scored on a scale of 1 to 6 with 1-3 being very confident and 4-6 being not at all confident
d.Note: date 14jun16 - sas release: 9.4 - h010_table3_June_2016.sas - sue peacock (206) 744-9912 peacocks@uw.edu GIM
DISCUSSION
We performed a nation-wide study to assess U.S. practicing pathologists’ diagnoses and perceptions of spitzoid lesions. We found that the majority of pathologists acknowledge the controversial nature of the subject when using terms such as “atypical Spitz tumor” or “spitzoid melanoma.” There was wide variation among pathologists’ diagnoses and proposed surgical excisional margins. Pathologists who feel relatively less certain about their diagnoses are more likely to request second opinions when they encounter an atypical Spitz tumor or spitzoid melanoma case.
As previously mentioned, some critics have argued against the concept of an intermediate category of spitzoid lesions (i.e., atypical Spitz tumors), and believe that there should be a clear-cut distinction made between benign and malignant tumors only. (7) For many colleagues, the introduction of the term atypical Spitz tumor has engendered considerable confusion as to what this term means and the biological nature these tumors versus that of conventional Spitz nevus and bonafide melanoma. This reaction is understandable, and, as already discussed, the biology of this neoplastic system remains the subject of basic research. The goal of the current study has been to document the useage of Spitz-related terminology among pathologists and not to endorse any preferred nomenclature. Our study found, however, that most pathologists accepted the concept of intermediate lesions (i.e., atypical Spitz tumor) and used this intermediate classification term at least once in this study, and some pathologists employed it up to 10 times in a test set of 48 cases.
Our team of dermatopathologists and investigators designed the MPATH-Dx form with the intention to include as many diagnostic terms as possible within a five-category schema. It must be emphasized that this diagnostic classification system is in a preliminary stage of development. Further research and more extensive discussions with the general dermatological and dermatopathological communities will be needed before this system can be introduced into general usage. Because some pathologists may favor morphologically descriptive terms such as “pigmented spindle cell nevus” over eponyms, we included a range of options in our form to capture the diverse spectrum of terminology used in current practice. The general category of spitzoid lesions includes, inter alia, Spitz nevus, pigmented spindle cell nevus, atypical Spitz tumor, atypical pigmented spindle cell nevus, and spitzoid melanoma. It is possible and perhaps likely that some pathologists may use other terms, such as spindle and epithelioid cell nevus/tumor, atypical spitzoid melanocytic neoplasm, or melanocytic tumor of uncertain malignant potential (MELTUMP), in diagnosing spitzoid lesions, but the latter terms were not included in our analysis.
The evaluation of spitzoid lesions is based on the utilization of a constellation of histologic features without any single feature permitting the conclusive establishment of a diagnosis. A number of reviews have summarized the difficulties in diagnosing these proliferations, concluding that the diagnosis is complex and subjective. The bellwether publications over the past half-century asserting diagnostic standards have emphasized different histological criteria, and in fact some have come to essentially opposing conclusions regarding the time-honored criteria.(1, 6-13, 17) Not surprisingly, histological criteria in some analyses proved inaccurate in retrospectively identifying those lesions with clinically untoward outcomes. (17) As a pragmatic approach to the histological ambiguities, the proposal has been made to identify a lesion as “spitzoid” based on the finding of a large spindled and/or epithelioid cell constituency having eosinophilic, “ground glass” cytoplasm in the context of particular architectural configurations; however it seems that most other histologic features attributed to these proliferations seem too variable between lesions to confer reasonable specificity. (1, 6-13, 17) Nevertheless, recent genomic studies demonstrating a high prevalence of fusion genes in contrast to the activated oncogenes that prevail in usual nevi and melanomas suggest that this is a relatively distinct category of melanocytic neoplasms. (2, 3, 5)
Because of diagnostic and philosophical ambiguities, experts have difficulty in achieving consensus on diagnosis and risk stratification of atypical spitzoid lesions. (12, 13) Variations in interpretation relate to the lack of basic biological information about this class of melanocytic lesions and hence the lack of standardized diagnostic criteria, variability in pathologists’ diagnostic thresholds, and diverse perspectives from unique training environments and experience. In our study, even when the pathologists agreed on a particular spitzoid diagnosis, they did not agree on whether the lesions were benign, malignant, or atypical/indeterminate in many cases. This uncertainty is reflected in the high percentage of second opinions requested when pathologists apply the diagnostic term of a spitzoid lesion, and the finding that for the majority of second opinions requested, the reason stated by the pathologists was based on personal preference rather than policy requirements at their laboratory practice. Most pathologists considered these cases to be challenging and they commonly expressed some lack of confidence in their diagnosis.
As already emphasized, the uncertainty about many spitzoid lesions is related to the lack of sufficient diagnostic criteria and robust ancillary techniques for their definitive assessment. However, new diagnostic tools are beginning to emerge. Immunohistochemistry currently offers no biomarkers that are unequivocally definitive for the diagnosis of spitzoid lesions and for their risk stratification. Increasingly, elevated nuclear labeling by Ki-67, a proliferative marker (18), is being used to help stratify perceived malignant risk. Molecular techniques such as the analysis of chromosomal copy number alterations on array CGH and FISH testing hold promise, but their true predictive value for clinical outcome in spitzoid tumors remain uncertain.(3, 5, 19, 20) On the other hand, the detection of mutations in the TERT promoter region appears to identify a clinically high-risk subset of atypical spitzoid tumors but will require validation in a larger cohort of patients.(3) In our study, the pathologists did not order significantly more ancillary tests, such as immunostains, when they were diagnosing spitzoid lesions compared to other lesions with comparable grades of atypia.
As with diagnostic ambiguity associated with these proliferations, there is no consensus on appropriate management. Since most lesions are completely excised, there are very few studies evaluating clinical outcomes. In general, lesions from adult patients are treated more aggressively compared to pediatric patients. According to the MPATH-Dx mapping tool, Spitz nevi are classified into category 2 (excision with < 5 mm margin); atypical Spitz tumors are classified into category 3 (excision with 5-9 mm margin), and spitzoid melanomas are classified into category 4 or 5 (wide excision with ≥ 1 cm margin +/− ancillary treatments). Sentinel lymph node biopsy is controversial. Even though approximately 40% of sentinel lymph nodes are involved in atypical spitzoid lesions, further metastases and resultant mortality are exceedingly rare. (21) There is no evidence to conclusively support or refute sentinel lymph node biopsy as a treatment strategy, however having a positive sentinel lymph node “does not seem to predict a poorer outcome for patients with atypical Spitz tumors.” (22) Our study showed that pathologists agree on treatment of spitzoid melanoma but had different opinions concerning treatment of other spitzoid melanocytic lesions.
There are several limitations of this study. We did not specifically or intentionally include any Spitz nevi with the stereotypical features promulgated by authoritative references in the field. The sample size was limited to 240 total test cases of melanocytic lesions. Our study set excluded the pediatric population in which Spitz nevi are very commonly seen, and this may explain why there were no cases where the participating pathologists rendered an overall consensus diagnosis of Spitz nevus. The design of the study differs from clinical practice, as pathologists were provided with limited clinical information (only age, sex, and anatomic site), no clinical image or lesional descriptors, only one section per specimen, and no option to order ancillary tests or to request second opinions. These are all important contributors to the pathologist's diagnostic process.
Our study establishes that the majority of practicing U.S. pathologists in this sampling use the terms “atypical Spitz tumor” (including atypical pigmented spindle cell nevus) and “spitzoid melanoma.” The variability in treatment recommendations that these pathologists assigned for the cases may reflect the controversy and lack of universally accepted guidelines for the management of spitzoid lesions. This underscores the importance of a reporting system, such as the MPATH-Dx form, which may enable pathologists to report, in a more standardized fashion, their perceived level of risk for given melanocytic lesions and the associated suggestion for management, thereby mitigating potentially harmful misunderstandings between pathologists and clinicians. Although the use of the terminologies “atypical Spitz tumor” and “spitzoid melanoma” appears generally accepted among pathologists, fundamental ambiguities remain in the reliability of their usage in risk assessment and in the appropriate treatment. In conclusion, although much more research is needed, comprehensive genomic studies currently underway should facilitate the definitive characterization of spitzoid lesions at the molecular level. Correlation of this fundamental information with clinical outcome should lead to a more simplified and coherent nomenclature and approach to the clinical management of these difficult melanocytic lesions.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Cancer Institute and National Institutes of Health under award numbers R01 CA 151306, K05 CA 104699 and 5T32AR056969.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Barnhill RL, Busam KJ. The Spitz Nevus and Variants. In: Barnhill R, Piepkorn M, Busam KJ, editors. Pathology of Melanocytic Nevi and Melanoma. 3rd ed. Springer; New York: 2014. pp. 205–69. [Google Scholar]
- 2.Wiesner T, He J, Yelensky R, Esteve-Puig R, Botton T, Yeh I, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014:5. doi: 10.1038/ncomms4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S, Barnhill RL, Dummer R, Dalton J, Wu J, Pappo A, et al. TERT Promoter Mutations Are Predictive of Aggressive Clinical Behavior in Patients with Spitzoid Melanocytic Neoplasms. Sci Rep. 2015;5:11200. doi: 10.1038/srep11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh I, Botton T, Talevich E, Shain AH, Sparatta AJ, de La Fouchardiere A, et al. Activating MET kinase rearrangements in melanoma and Spitz tumours. Nat Commun. 2015:6. doi: 10.1038/ncomms8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu G, Barnhill RL, Lee S, Li Y, Shao Y, Easton J, et al. The landscape of fusion transcripts in spitzoid melanoma and biologically indeterminate spitzoid tumors by RNA sequencing. Mod Pathol. 2016 doi: 10.1038/modpathol.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spitz S. Melanoma of childhood. American Journal of Pathology. 1948;24(3):591–609. [PMC free article] [PubMed] [Google Scholar]
- 7.Mones JM, Ackerman AB. “Atypical” blue nevus, “malignant” blue nevus, and “metastasizing” blue nevus: a critique in historical perspective of three concepts flawed fatally. Am J Dermatopathol. 2004;26(5):407–30. doi: 10.1097/00000372-200410000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Reed RJ, Ichinose H, Clark WH, Jr., Mihm MC., Jr. Common and uncommon melanocytic nevi and borderline melanomas. Semin Oncol. 1975;2(2):119–47. [PubMed] [Google Scholar]
- 9.Smith KJ, Barrett TL, Skelton HG, 3rd, Lupton GP, Graham JH. Spindle cell and epithelioid cell nevi with atypia and metastasis (malignant Spitz nevus). Am J Surg Pathol. 1989;13(11):931–9. doi: 10.1097/00000478-198911000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Spatz A, Calonje E, Handfield-Jones S, Barnhill RL. Spitz tumors in children: a grading system for risk stratification. Arch Dermatol. 1999;135(3):282–5. doi: 10.1001/archderm.135.3.282. [DOI] [PubMed] [Google Scholar]
- 11.Barnhill RL. The Spitzoid lesion: rethinking Spitz tumors, atypical variants, 'Spitzoid melanoma' and risk assessment. Mod Pathol. 2006;19(Suppl 2):S21–33. doi: 10.1038/modpathol.3800519. [DOI] [PubMed] [Google Scholar]
- 12.Barnhill RL, Argenyi ZB, From L, Glass LF, Maize JC, Mihm MC, Jr., et al. Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum Pathol. 1999;30(5):513–20. doi: 10.1016/s0046-8177(99)90193-4. [DOI] [PubMed] [Google Scholar]
- 13.Cerroni L, Barnhill R, Elder D, Gottlieb G, Heenan P, Kutzner H, et al. Melanocytic Tumors of Uncertain Malignant Potential Results of a Tutorial Held at the XXIX Symposium of the International Society of Dermatopathology in Graz, October 2008. Am J Surg Pathol. 2010;34(3):314–26. doi: 10.1097/PAS.0b013e3181cf7fa0. [DOI] [PubMed] [Google Scholar]
- 14.Carney PA, Reisch LM, Piepkorn MW, Barnhill RL, Elder DE, Knezevich SR, et al. Achieving Consensus for the Histological Diagnosis of Melanocytic Lesions: Use of the Modified Delphi Approach. J Cutan Pathol. 2016 doi: 10.1111/cup.12751. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piepkorn M, RL B, Elder D, Knezevich S, Carney P, Reisch LM, et al. The MPATH-Dx reporting schema for melanocytic proliferations and melanoma. J Am Acad Dermatol. 2014;70(1):131–41. doi: 10.1016/j.jaad.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo S, Sepehr A, Tsao H. Spitz nevi and other Spitzoid lesions Part I. Background and diagnoses. Journal of the American Academy of Dermatology. 2011;65(6):1073–84. doi: 10.1016/j.jaad.2011.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piepkorn M. On the nature of histologic observations: the case of the Spitz nevus. J Am Acad Dermatol. 1995;32(2 Pt 1):248–54. doi: 10.1016/0190-9622(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 18.Vollmer RT. Use of Bayes rule and MIB-1 proliferation index to discriminate Spitz nevus from malignant melanoma. Am J Clin Pathol. 2004;122(4):499–505. doi: 10.1309/MFFF-06D5-CYXR-2F8T. [DOI] [PubMed] [Google Scholar]
- 19.Yazdan P, Cooper C, Sholl LM, Busam K, Rademaker A, Weitner BB, et al. Comparative analysis of atypical Spitz tumors with heterozygous versus homozygous 9p21 deletions for clinical outcomes, histomorphology, BRAF mutation, and p16 expression. Am J Surg Pathol. 2014;38(5):638–45. doi: 10.1097/PAS.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 20.Gerami P, Scolyer RA, Xu XW, Elder DE, Abraham RM, Fullen D, et al. Risk Assessment for Atypical Spitzoid Melanocytic Neoplasms Using FISH to Identify Chromosomal Copy Number Aberrations. Am J Surg Pathol. 2013;37(5):676–84. doi: 10.1097/PAS.0b013e3182753de6. [DOI] [PubMed] [Google Scholar]
- 21.Luo S, Sepehr A, Tsao H. Spitz nevi and other Spitzoid lesions Part II. Natural history and management. Journal of the American Academy of Dermatology. 2011;65(6):1087–92. doi: 10.1016/j.jaad.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lallas A, Kyrgidis A, Ferrara G, Kittler H, Apalla Z, Castagnetti F, et al. Atypical Spitz tumours and sentinel lymph node biopsy: a systematic review. Lancet Oncol. 2014;15(4):e178–83. doi: 10.1016/S1470-2045(13)70608-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.