Abstract
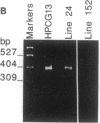
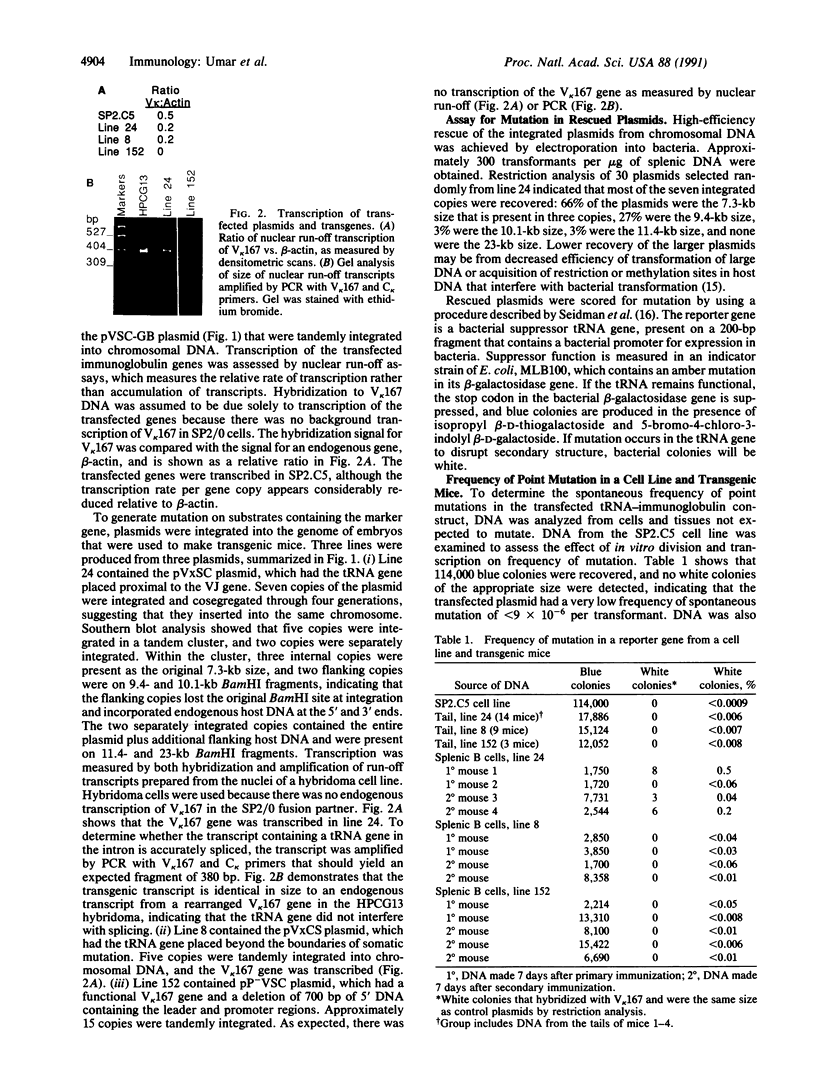
Somatic mutation in immunoglobulin genes is localized to a 2-kilobase region of DNA surrounding and including rearranged variable (V), diversity, and joining (J) gene segments encoding heavy and light chains. To examine the structural basis for targeted mutation, we developed an assay to score mutation on plasmid substrates by using a reporter gene: a bacterial gene encoding an amber-suppressor tRNA molecule was placed 3' of a rearranged kappa VJ gene within the boundaries of mutation. The reporter gene is exquisitely suited for mutational analysis because it is only 200 base pairs (bp), which should not greatly disrupt structure of the immunoglobulin locus, and gene function depends on secondary structure, which means mutation can be scored in many different nucleotide positions. The plasmid was used to make transgenic mice, which were then immunized. The shuttle vector was retrieved by plasmid rescue into an indicator strain of Escherichia coli that contained an amber mutation in its beta-galactosidase gene. Integrity of the tRNA molecule was monitored by colony color, which permitted many transformants to be screened visually. Mutations were not seen in DNA from a transfected B-cell line grown in vitro or in DNA from nonlymphoid tissue of transgenic mice, indicating that the reporter gene was stable during cell division and DNA manipulations. However, when the transgenic mice were immunized, DNA from splenic B cells contained point mutations in the reporter gene at a frequency of 10(-3) per transformant. Sequence analysis of 17 mutated transgenes revealed that the mutations were 1- and 2-bp deletions in the tRNA gene, and one plasmid had an additional 2-bp deletion in the V gene. In contrast, previous studies have shown that mutations in endogenous VJ genes are predominantly nucleotide substitutions and have only 6% deletions. Two other plasmid constructs were analyzed in transgenic lines: no mutations were found when the tRNA gene was placed distal to the VJ gene, and no mutations were seen when the immunoglobulin promoter was deleted. Although we lack direct evidence that the deletions in the tRNA gene are caused by the same mechanism that acts on VJ genes, we have shown that mutations in this assay occur in a manner consistent with immunoglobulin-specific mutation in that they are found in splenic B cells and not in tail tissue, depend on position next to the VJ gene, and require transcription of the VJ gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Both G. W., Taylor L., Pollard J. W., Steele E. J. Distribution of mutations around rearranged heavy-chain antibody variable-region genes. Mol Cell Biol. 1990 Oct;10(10):5187–5196. doi: 10.1128/mcb.10.10.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredberg A., Kraemer K. H., Seidman M. M. Restricted ultraviolet mutational spectrum in a shuttle vector propagated in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8273–8277. doi: 10.1073/pnas.83.21.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celano P., Baylin S. B., Casero R. A., Jr Polyamines differentially modulate the transcription of growth-associated genes in human colon carcinoma cells. J Biol Chem. 1989 May 25;264(15):8922–8927. [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durdik J., Gerstein R. M., Rath S., Robbins P. F., Nisonoff A., Selsing E. Isotype switching by a microinjected mu immunoglobulin heavy chain gene in transgenic mice. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2346–2350. doi: 10.1073/pnas.86.7.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French D. L., Laskov R., Scharff M. D. The role of somatic hypermutation in the generation of antibody diversity. Science. 1989 Jun 9;244(4909):1152–1157. doi: 10.1126/science.2658060. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Bogenhagen D. F. Clusters of point mutations are found exclusively around rearranged antibody variable genes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3439–3443. doi: 10.1073/pnas.80.11.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Rollini P., Mach B. Somatic mutations of immunoglobulin variable genes are restricted to the rearranged V gene. Science. 1983 Jun 10;220(4602):1179–1181. doi: 10.1126/science.6857243. [DOI] [PubMed] [Google Scholar]
- Grant S. G., Jessee J., Bloom F. R., Hanahan D. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4645–4649. doi: 10.1073/pnas.87.12.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett J., Jr, Rogerson B. J., O'Brien R. L., Storb U. Analysis of somatic mutations in kappa transgenes. J Exp Med. 1990 Jul 1;172(1):131–137. doi: 10.1084/jem.172.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Davis M., Sinn E., Patten P., Hood L. Antibody diversity: somatic hypermutation of rearranged VH genes. Cell. 1981 Dec;27(3 Pt 2):573–581. doi: 10.1016/0092-8674(81)90399-8. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Seidman M. M. Use of supF, an Escherichia coli tyrosine suppressor tRNA gene, as a mutagenic target in shuttle-vector plasmids. Mutat Res. 1989 Mar-May;220(2-3):61–72. doi: 10.1016/0165-1110(89)90011-0. [DOI] [PubMed] [Google Scholar]
- Lebecque S. G., Gearhart P. J. Boundaries of somatic mutation in rearranged immunoglobulin genes: 5' boundary is near the promoter, and 3' boundary is approximately 1 kb from V(D)J gene. J Exp Med. 1990 Dec 1;172(6):1717–1727. doi: 10.1084/jem.172.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Matthias P. D., Bernard H. U., Scott A., Brady G., Hashimoto-Gotoh T., Schütz G. A bovine papilloma virus vector with a dominant resistance marker replicates extrachromosomally in mouse and E. coli cells. EMBO J. 1983;2(9):1487–1492. doi: 10.1002/j.1460-2075.1983.tb01612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. B., Neuberger M. S. The immunoglobulin kappa locus contains a second, stronger B-cell-specific enhancer which is located downstream of the constant region. EMBO J. 1989 Jul;8(7):1959–1964. doi: 10.1002/j.1460-2075.1989.tb03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. L., Brinster R. L., Storb U. Somatic hypermutation of an immunoglobulin transgene in kappa transgenic mice. 1987 Mar 26-Apr 1Nature. 326(6111):405–409. doi: 10.1038/326405a0. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Morrison S. L., Herzenberg L. A., Berg P. Immunoglobulin gene expression in transformed lymphoid cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):825–829. doi: 10.1073/pnas.80.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roes J., Hüppi K., Rajewsky K., Sablitzky F. V gene rearrangement is required to fully activate the hypermutation mechanism in B cells. J Immunol. 1989 Feb 1;142(3):1022–1026. [PubMed] [Google Scholar]
- Seidman M. M., Bredberg A., Seetharam S., Kraemer K. H. Multiple point mutations in a shuttle vector propagated in human cells: evidence for an error-prone DNA polymerase activity. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4944–4948. doi: 10.1073/pnas.84.14.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman M. M., Dixon K., Razzaque A., Zagursky R. J., Berman M. L. A shuttle vector plasmid for studying carcinogen-induced point mutations in mammalian cells. Gene. 1985;38(1-3):233–237. doi: 10.1016/0378-1119(85)90222-7. [DOI] [PubMed] [Google Scholar]
- Sharpe M. J., Neuberger M., Pannell R., Surani M. A., Milstein C. Lack of somatic mutation in a kappa light chain transgene. Eur J Immunol. 1990 Jun;20(6):1379–1385. doi: 10.1002/eji.1830200625. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Weiss S., Wu G. E. Somatic point mutations in unrearranged immunoglobulin gene segments encoding the variable region of lambda light chains. EMBO J. 1987 Apr;6(4):927–932. doi: 10.1002/j.1460-2075.1987.tb04840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Shawi R., Kinnaird J., Burke J., Bishop J. O. Expression of a foreign gene in a line of transgenic mice is modulated by a chromosomal position effect. Mol Cell Biol. 1990 Mar;10(3):1192–1198. doi: 10.1128/mcb.10.3.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]