Abstract
In light of the clinical evidence implicating dopamine in schizophrenia, and the prominent hypotheses put forth regarding alterations in dopaminergic transmission in this disease, molecular imaging has been used to examine multiple aspects of the dopaminergic system. Here we review the imaging methods used and compare the findings across the different molecular targets. Findings have converged to suggest early dysregulation in the striatum, especially in the rostral caudate, manifesting as excess synthesis and release. Recent data showed deficit extending to most cortical regions, and even to other extrastriatal subcortical regions not previously considered to be “hypodopaminergic” in schizophrenia. These findings yield a new topography for the dopaminergic dysregulation in schizophrenia. In this review we discuss the dopaminergic innervation within the individual projection fields to provide a topographical map of this dual dysregulation and explore potential cellular and circuit based mechanisms for brain region-dependent alterations in dopaminergic parameters. This refined knowledge is essential to better guide translational studies and efforts in early drug development.
Keywords: PET imaging, Neuroanatomy, Dopamine, Schizophrenia, Striatum, Cortex
Classifications: Schizophrenia/Psychosis, Accumbens, Cortex, PET, Neurochemical Imaging
I. Historical perspective on dopamine research in schizophrenia
Dopamine (DA) has been a focus of schizophrenia research for decades, yielding two prior conceptual formulations for dopamine’s involvement in schizophrenia. In 1966 Rossum and colleagues proposed a state of excess dopaminergic stimulation in patients with schizophrenia (SZ) (1), substantiated later by the discovery of the D2 receptor binding profiles of antipsychotics and the psychotogenic effects of DA agonists (2–4). This was later reformulated as an imbalance between excess subcortical DA and a deficit in cortical DA, in light of evidence suggesting a prefrontal cortical deficit in schizophrenia and the prominent role of DA in mediating prefrontal-dependent cognitive processes (5, 6). The availability of imaging tools to measure aspects of dopaminergic transmission in vivo allowed testing of these formulations in patients. Improved scanner technology enabled better anatomical resolution. Earlier detection and awareness of the prodromal phase of the disease (7, 8) resulted in testing earlier stages of the illness (9–11), while stress paradigms (12, 13) allowed probing responsiveness of the system to a relevant risk factor for the disease (14, 15), together yielding a replicable set of findings across labs documenting excess presynaptic dopaminergic transmission in the striatum, confirming the original formulation. Furthermore, data from our lab provided new evidence for a cortical DA deficit (16), supporting the second formulation, but also expanding it to multiple extrastriatal regions not previously considered to be “hypodopaminergic” in schizophrenia. A new topographical mapping of DA dysregulation in schizophrenia is the topic of this review. We will describe the imaging methods used to examine dopaminergic indices, and findings in SZ. We will then review dopaminergic innervation and its imaging-relevant targets within individual projection fields to provide a topographical map of the findings and suggest potential mechanisms for brain region-dependent DA dysregulation in schizophrenia. Finally, we discuss future directions.
II. Methodology for imaging the dopamine system
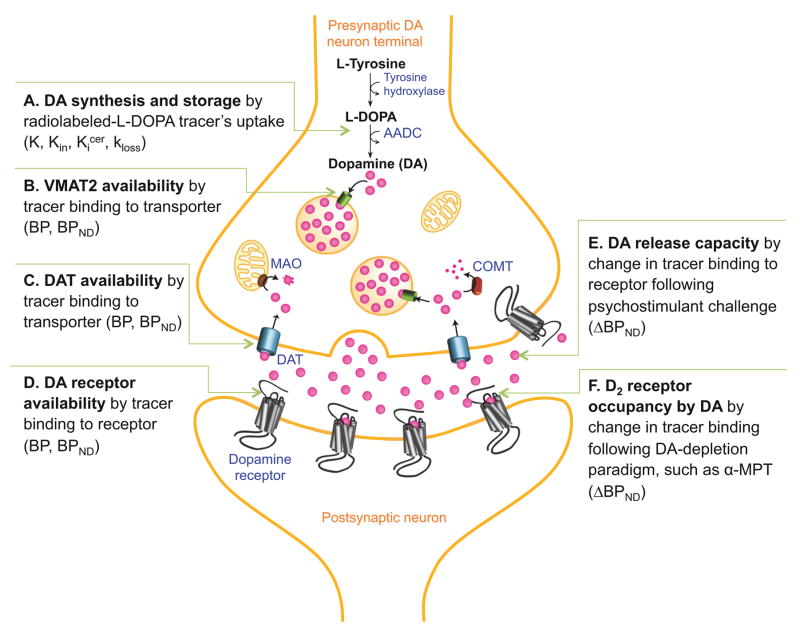
Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) have been used to measure dopamine-related parameters via administration of radioligands that bind to receptors, transporters or other target molecules, or alternatively, trace a metabolic pathway. For radioligands that reversibly bind to receptors, the most commonly derived parameter is the binding potential (BP) (17, 18), which is proportional to BAVAIL/KD, where BAVAIL is the concentration of the target molecule available for binding to the radiotracer and KD is the equilibrium dissociation constant of tracer for the target. There are several versions of BP, depending on which concentration of tracer is used as a reference value. For the frequently used BPND, (Figure 1B–D), the reference is the nondisplaceable compartment, comprised of the sum of the free plus nonspecifically-bound radiotracer in brain; BPND = fND*BAVAIL/KD, where fND is the free fraction of the nondisplaceable radiotracer concentration. BPND is an indicator of target molecule availability, based on the assumption that KD and fND are not different across groups. BAVAIL, as opposed to the total target concentration BMAX, accounts for the masking of some of the targets by the binding of endogenous ligands. BPND is also the ratio of specifically-bound to nondisplaceable radiotracer concentrations at equilibrium, thus representing the associated signal-to-noise ratio (see reference 17 for complete definitions). A BPND lower than 0.5, i.e. signal lower than half of background, is considered too low to provide meaningful information.
Figure 1. Dopaminergic Imaging Targets.
Schematic of imaging methods used to measure aspects of the dopamine (DA) system in vivo. Graphic depicts progression of DA from synthesis (A), storage (B), to release (E,F), then either reuptake by dopamine transporter (DAT, C) or binding to receptor (D). Imaging targets and related paradigms are described in accompanying text.
Tracers with moderate affinity for dopamine-D2-like receptors (D2 and D3, referred to from here on as D2), such as [11C]raclopride and [123I]IBZM, provide reliable BPND in the striatum (Figure 1D). [18F]fallypride has an order of magnitude higher affinity (19, 20) and provides reliable quantification in striatum, thalamus, midbrain, hippocampus, amygdala and temporal cortex. Higher affinity tracers such as [11C]FLB457 (21) or [123I]epidipride (22) can be used to quantify D2 density in cortex, although their equilibration is prohibitively slow for quantification in striatum. Pharmacologically, all of these tracers are antagonists. [11C]-(+)-PHNO is a D3 preferring agonist (23–25). Tracers for D1-like receptors (D1 and D5, hereafter D1) include [11C]NNC112 and [11C]SCH23390 (26, 27). Both have been used to quantify D1 in cortex and striatum, although [11C]SCH23390’s BPND is below 0.5 in cortex.
Tracers for D2 receptors are sensitive to changes in the concentration of DA through competitive interaction. Pharmacological challenges that increase synaptic DA, such as concomitant release and reuptake blockade by amphetamine, or reuptake blockade by methylphenidate, decrease BPND; whereas depletion paradigms that reduce baseline synaptic DA, such as blockade of tyrosine hydroxylase activity with alpha-methylparatyrosine (α-MPT), increase BPND. These effects can be quantified as ΔBPND, the percent change of BPND across conditions (Figure 1E,F). D2 ligand displacement by challenge-induced DA release occurs at the subset of D2 receptors that are in close proximity to DA release sites (28–32). This has led to the postulation that net change in tracer binding at these perisynaptic receptors may comprise the PET “DA release” signal (33), which refers to our PET measurement of intrasynaptic DA levels, either evoked (due to amphetamine administration) or basal (measured with the depletion paradigm).
[18F]DOPA is a substrate for amino acid decarboxylase (AADC), which catalyzes L-Dihydroxyphenylalanine (DOPA) into DA (34). In terminals containing AADC, [18F]DOPA is converted to 6-fluorodopamine ([18F]6-FDA), a substrate for vesicular monoamine transporter 2 (VMAT2), which loads DA into vesicles (Figure 1A). [18F]6-FDA cycles through exocytosis, reuptake through the DA transporter (DAT), and reloading into vesicles. This is generally treated as an irreversible process. The outcome measure is Kin, the steady-state uptake rate constant of the tracer, characterizing [18F]6-FDA formation when the concentration of [18F]DOPA in arterial plasma and in brain are at a hypothetical steady-state. Kin indicates the capacity for DA synthesis. A related outcome measure is Kicer which is the steady state uptake rate (Kin) relative to cerebellum concentration of [18F]DOPA, rather than the arterial plasma concentration, but studies utilizing Kicer require the implicit assumption that concentration of [18F]DOPA in the cerebellum does not differ between groups.
[18F]DOPA quantification is complicated by formation in the periphery of the radiolabeled metabolite 3-O-methyl-FDOPA ([18F]OMFD) due to catechol-O-methyl transferase (COMT) activity (35); pretreatment with entacapone can reduce this effect. In addition, the irreversibility of [18F]DOPA uptake is an idealization, as [18F]6-FDA is a substrate for both monoamine oxidase (MAO) and COMT, and metabolites diffuse out of the brain, affecting measurement of Kin. Some models account for this washout with an estimated parameter called kloss (35, 36).
[18F]DOPA Kin can be measured in striatum but extrastriatal Kin is lower and more difficult to measure. In substantia nigra (SN), Kin is approximately half as large as in striatum and, in cortex, too low to be interpretable (37).
Transporters have also been imaged using [11C]DTBZ for VMAT2 (38) (Figure 1B), [11C]PE2I for DAT (Figure 1C) in striatal and extrastriatal regions using PET, and [123I]βCIT (39) for striatal DAT using SPECT.
III. Imaging the dopamine system in schizophrenia
We review here findings from studies that used molecular neuroimaging to investigate the DA system in vivo in schizophrenia - first in striatum, then in extrastriatal regions, with a focus on cortex and midbrain (see Supplemental Table S1).
III.A. Striatum
III.A.1. Presynaptic
Higher striatal [18F]DOPA was first reported in psychosis related to epilepsy and schizophrenia (40). Seven studies replicated this finding in schizophrenia (9, 41–47), while two did not (48, 49), and subsequent meta-analyses confirmed the finding (50, 51). Using D2 radiotracers and a psychostimulant challenge, four studies showed higher release in the striatum of antipsychotic-free (Rx-free) patients compared to healthy controls (HC) (52–55). Excess DA release correlated with transient stimulant-induced worsening of psychotic symptoms in patients, and was observed at disease onset and during exacerbations, but not during periods of remission (56). Furthermore, baseline synaptic DA assessed with a depletion paradigm (57) were enhanced in striatum in schizophrenia (SZ), and were correlated with amphetamine-induced release in a cohort of antipsychotic-naïve (Rx-naïve) patients (58). Using a higher-resolution scanner and more sophisticated region-of-interest (ROI) analysis methods to identify the striatal substructures, we later demonstrated that excess striatal DA was most prominent in the rostral caudate (59). In the associative striatum (AST), which contains the rostral caudate, rostral putamen and post-commissural caudate, the effect size was 0.70, compared to 0.14 in the limbic striatum (LST or VST, ventral striatum), and 0.34 in the sensorimotor striatum (SMST, posterior putamen). This excess does not seem to be related to excess dopaminergic innervation as VMAT2 (60, 61) and DAT (62–72) were normal.
III.A.2. Postsynaptic
Several studies have examined striatal D2 availability. A meta-analysis of 23 studies showed small elevation and greater variability in SZ. When analysis was limited to Rx-naïve patients, SZ and HC did not differ (51), suggesting that D2 increases in striatum in SZ may be due to prior antipsychotic treatment. Striatal D1 availability is also normal in SZ (27, 73–75).
Further support for antipsychotic-induced upregulation of striatal D2 derives from α-MPT studies (57–59), which provide a direct measure of “true” D2 density by unmasking the fraction of receptors bound by endogenous DA. A new analysis of these previously published studies shows that unmasked BPND is higher (by 10–20%) in previously-medicated, but not Rx-naïve patients, compared to HC (Table 1) in striatum (57–59) and in rostral caudate (59). In contrast, in the same cohorts, α-MPT-induced ΔBPNDs showed that striatal DA levels are 65–120% higher in both Rx-naïve and previously-medicated patients compared to HC. This suggests that striatal dopaminergic hyperactivity is present regardless of prior antipsychotic treatment and thus a more reliable index of DA dysregulation than receptor upregulation.
Table 1.
Effect of previous antipsychotic exposure on unmasked BPND Binding potentials from α-MPT depletion studies
| Rx-free v Rx-naïve | Rx-free v HC | Rx-naïve v HC | ||||||
|---|---|---|---|---|---|---|---|---|
| HC | SZ | p | Rx-free | Rx-naïve | p | p | p | |
| [123I]IBZM SPECT: Striatuma | n=18 | n=18 | n=10 | n=8 | ||||
| BPNDBsl | 0.722 ± 0.091 | 0.751 ± 0.103 | 0.38 | 0.779 ± 0.122 | 0.716 ± 0.066 | 0.21 | 0.17 | 0.87 |
| BPNDDpl* | 0.787 ± 0.096 | 0.889 ± 0.124 | 0.009 | 0.930 ± 0.147 | 0.837 ± 0.062 | 0.12 | 0.004** | 0.19 |
| [11C]Raclopride PET: Striatumb | n=18 | n=18 | n=12 | n=6 | ||||
| BPNDBsl | 2.53 ± 0.25 | 2.56 ± 0.52 | 0.83 | 2.71 ± 0.50 | 2.25 ± 0.44 | 0.08 | 0.19 | 0.07 |
| BPNDDpl* | 2.81 ± 0.23 | 2.94 ± 0.54 | 0.35 | 3.12 ± 0.52 | 2.59 ± 0.42 | 0.048 | 0.04** | 0.12 |
| [11C]Raclopride PET: Rostral caudateb | n=18 | n=18 | n=12 | n=6 | ||||
| BPNDBsl | 2.40 ± 0.23 | 2.41 ± 0.45 | 0.89 | 2.54 ± 0.43 | 2.16 ± 0.42 | 0.10 | 0.25 | 0.10 |
| BPNDDpl* | 2.61 ± 0.27 | 2.77 ± 0.49 | 0.25 | 2.91 ± 0.47 | 2.47 ± 0.43 | 0.07 | 0.03** | 0.36 |
Abi-Dargham et al., PNAS 2000 (57)
Kegeles et al., AGP 2010 (59)
Significant one-way ANOVA comparing BPNDDpl for Rx-free, Rx-naïve, and HC (p < 0.05).
Significant post-hoc t test for BPNDDpl, Rx-free compared to HC (but not significant for Rx-naive compared to HC).
Abbreviations: D2 binding potential in the baseline state, partially masked by baseline levels of endogenous dopamine (BPNDBsl); Unmasked D2 binding potential in the dopamine-depleted state (BPNDDpl); Healthy control participants (HC); Participants with schizophrenia (SZ); Antipsychotic-free, previously-medicated patients (Rx-free); Antipsychotic-naïve patients (Rx-naïve)
III.A.3. Clinical correlates of the striatal findings
The striatal dopaminergic hyperactivity in schizophrenia is associated with the psychotic symptoms of the illness. It was shown to extend to physiological conditions under psychosocial stress and to be most enhanced in AST and SMST in Rx-naïve patients and in the prodrome (14). Elevated striatal [18F]DOPA uptake also precedes the onset (76), correlates with greater severity of prodromal symptoms and neuropsychological impairment, predicts conversion and, in both the prodrome and SZ, relates negatively to prefrontal cortical activation during cognitive tasks in (43, 77, but also see 78). It is also predominant in the AST (79, 80).
Furthermore, excess striatal DA predicts treatment response of psychosis to antipsychotics (58) and is higher in antipsychotic-responsive patients (81).
SZ (82) and individuals at clinical high risk for schizophrenia (CHR) (11) with comorbid substance use display a blunted striatal dopamine release. However, despite this presynaptic blunting, D2 receptors remain supersensitive to stimulation, leading to psychosis. This suggests two distinct alterations in psychosis: excess presynaptic release in striatum as well as a functional supersensitivity of striatal D2.
III.B. Cortex
III.B.1. Presynaptic
Using [11C]FLB457 we showed significant blunting of DA release throughout the cortex in SZ. DA release in the dorsolateral prefrontal cortex (DLPFC) was significantly positively associated with working memory-related BOLD activation, suggesting a relationship between blunted release and deficits of frontal cortical function (16). [18F]DOPA (45–48) reports in the cortex are uninterpretable (37).
III.B.2. Postsynaptic
D2 availability in SZ is normal in prefrontal (16, 83–85), occipital (16, 84), parietal (16, 84), entorhinal (86), anterior cingulate (16, 83, 87) (except for (84)), and insular (16, 86) cortices. A meta-analysis (excluding (16)) found no differences in temporal cortex (88). One study reported lower binding in uncus (87) while another did not (16).
Studies of prefrontal cortical D1 availability in SZ yielded inconsistent results of increases (74, 75) and decreases (27), compared to HC (Supplemental Table S1). To reconcile these findings, both D1 tracers were examined in the same subjects (89, 90) and showed similar alteration using either tracer, suggesting cohort-related effects rather than tracer differences. Prior exposure to antipsychotics may explain some of these discrepancies, as higher D1 levels were observed only in Rx-naïve patients, and duration of Rx-free interval positively correlated with higher binding in previously-treated patients (75).
III.C. Extrastriatal subcortical regions and midbrain
III.C.1. Presynaptic
[18F]DOPA uptake in SZ is normal in thalamus (47) and entorhinal cortex (47), but enhanced in amygdala (46) and midbrain (46, 91). In midbrain, one study reported higher [18F]DOPA utilization (K) and turnover (kloss), while Kin was numerically lower (46). Another reported higher Kicer in the midbrain, which correlated with symptom severity in SZ (91) and predicted conversion in CHR (92). We measured significant blunting of amphetamine-induced DA release measured by [11C]FLB457 displacement in extrastriatal subcortical regions including midbrain (16). Thus, for the amygdala and midbrain, PET indices of presynaptic DA synthesis/turnover and amphetamine-evoked DA release seem discrepant. If this discrepancy is indeed true, it may suggest elevated enzymatic activity in the presence of lower cytoplasmic and vesicular pools of DA in midbrain in SZ (see discussion below).
Using [11C]PE2I, one study reported higher DAT in the thalamus but not in SN (72). However, the small sample size and low BPND suggest caution in interpreting this study. VMAT2 was normal in extrastriatal regions (61) except for ventral midbrain, where an increase was reported (93); however as BPND was below 0.5, this finding should also be considered with caution.
III.C.2. Postsynaptic
Of the nine studies in thalamus (16, 84–87, 94–97), only one ((94), which overlaps with (98)) found lower D2 in SZ, and meta-analysis (88) was negative. Likewise, no differences were found in globus pallidus (97), amygdala (16, 86, 87), entorhinal cortex (16, 86) or hippocampus (84, 86, 87). In SN, normal (16, 86, 97, 99), higher (87) and lower (96) D2 were reported; and meta-analysis (88) was negative.
No differences in D1 availability have been observed in extrastriatal subcortical ROIs (see Supplemental Table S1).
III.D. Summary of imaging findings
In summary, three main dopaminergic alterations have emerged in schizophrenia:
DA synthesis and release capacity are increased in the striatum (51).
Although needing replication, DA release capacity in prefrontal cortical and other extrastriatal regions is decreased (16).
There is subregional heterogeneity in the DA dysregulation within the striatum. The rostral caudate and the AST in general, show lower DA release capacity than the SMST in HC (100), but not in SZ due to a prominent increase in the AST (9, 14, 101). Supportive evidence for the prominent role of DA dysregulation in AST also derives from studies in prodrome (9, 14).
Postsynaptic receptors and transporters do not show a reliably detectable altered expression either in the striatum or in extrastriatal regions of the brain in SZ.
IV. Topography and synaptic characteristics of dopaminergic projections
To understand the abnormal PET DA signal in SZ, we will consider the regional anatomical factors that may affect it. Here we review the complex topography and chemical neuroanatomy of DA systems underlying PET indices of basal and evoked DA release.
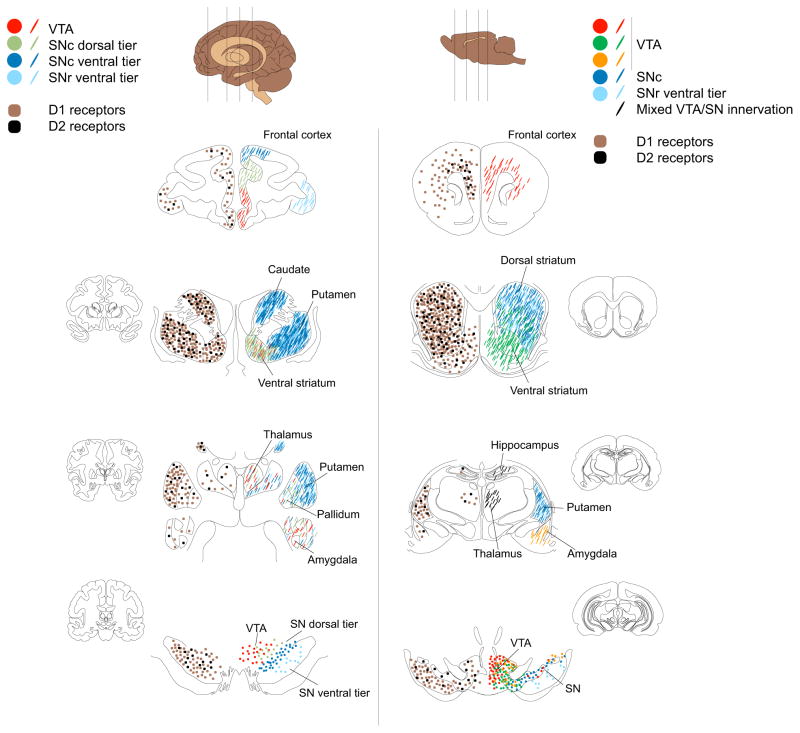
DA projections comprise the retrorubral field (RRF)(A8), substantia nigra (SN)(A9) and ventral tegmental area (VTA)(A10) (102–104) (Figure 2). These have different intrinsic properties and afferents regulating spike activity; synthesis, release or reuptake of DA; and postsynaptic effects (102–105) (Figure 3). “Dorsal tier” DA neurons, a band along the dorsal SN pars compacta (SNc) and contiguous regions of VTA and RRF, project to cerebral cortex, ventromedial striatum, pallidum, “extended amygdala”, and thalamus. The “ventral tier” neurons, including the densocellular region of the SNc and DA cell columns within the pars reticulata (SNr), project to the striatum. The SMST receives a dense projection, with high density of DA release sites (105), accounting for the higher PET DA release signal, and highest levels of DAT, exerting tighter spatiotemporal regulation of DA diffusion compared to other subregions. The VST, innervated by VTA and medial SNc DA neurons, has lower DA release potential and lower levels of DAT and D2 autoreceptors (106, 107). The AST receives a mosaic of dorsal and ventral tier neurons.
Figure 2. Topography of dopaminergic innervation and receptor distribution.
Schematic representation of distributions of dopamine D1 and D2 receptors (left hemispheres) and patterns of dopaminergic innervations (right hemispheres) in select primate (left panel) and rodent (right panel) brain regions. Left hemispheres: Brown and black squares depict D1 and D2 receptors, respectively. Throughout the primate and rodent brain, D1 receptors (D1) are present at a higher density than D2 receptors (D2). The striatum, and in particular the caudate-putamen, has the highest densities of dopamine (DA) receptors. DA receptors are also present in medium-to-low densities in the cortex, pallidum and midbrain. Receptor densities are relatively low in thalamus, amygdala and hippocampus. See text for details. Right hemispheres: Topographical distribution of DA cell bodies (filled circles) and their terminals (lines). In the primate panel, red circles represent DA cell bodies in the VTA with terminals in the cortex, striatum (in particular the ventral part), pallidum, thalamus and amygdala. The VTA dopaminergic cellular organization is better characterized in the rodent where discrete VTA cell groups project to the cortex (red), nucleus accumbens (dark green) and amygdala (orange). In the primate, SN dorsal tier cell group (light green) projects to the cortex and ventral striatum, as well as the pallidum, thalamus and amygdala. The rodent brain in contrast has a low density of these dorsal tier neurons. The SN ventral tier groups (SN compacta densocellular part (dark blue) and fingers (light blue)) project heavily and topographically to caudate-putamen with medium/low innervations of cortex, ventral striatum, thalamus and amygdala. See text for further details.
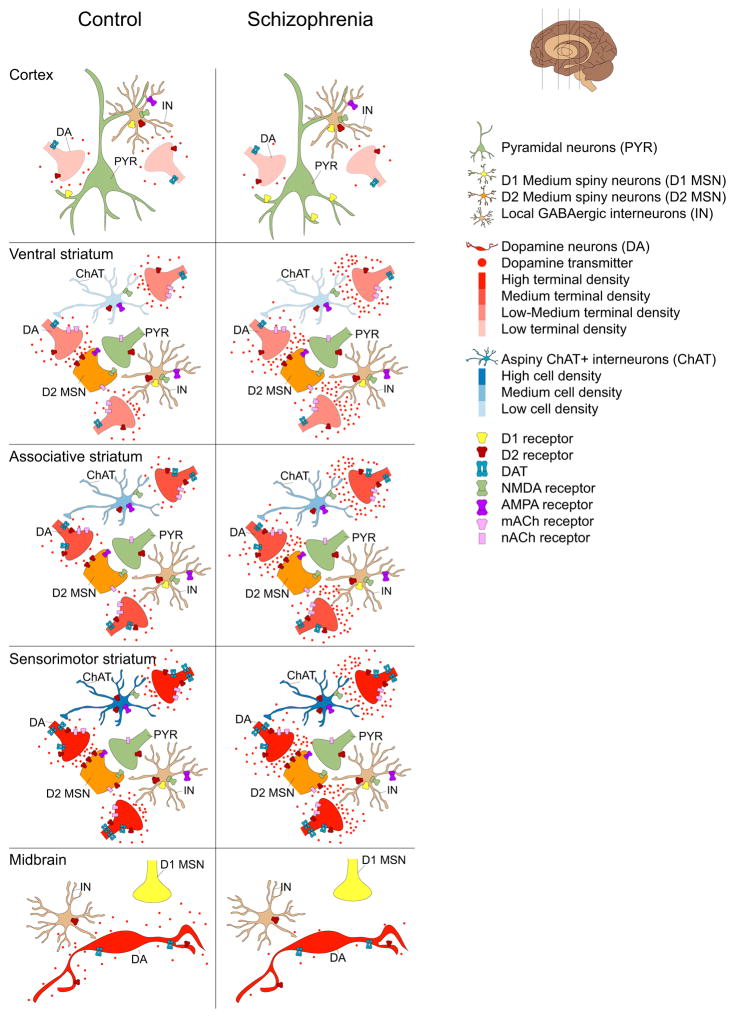
Figure 3. Topography of dopamine release findings in schizophrenia compared to controls.
Schematic representations of DA release characteristics in the cortex (top), striatum (middle) and midbrain (bottom) in healthy controls (HC) and patients with schizophrenia (SZ) based on imaging findings in patients. DA neuron cell bodies, terminals and transmitters are depicted in red. Color gradients depict DA terminal densities. Cortex: The cortex receives sparse dopaminergic innervation that is poor in dopamine D2 receptors (D2) and transporter expression. This sculpts D2 displacement measurement, which is low in the cortex. In schizophrenia there is evidence for reduced cortical DA release. See text for details. Striatum: DA and cortical neuron terminals (green) are shown innervating medium spiny neuron spines (orange). Also shown are local cholinergic (blue) and GABAergic (brown) interneuron populations forming the striatal microcircuitry. There is considerable heterogeneity in DA release across striatal regions, e.g. dopaminergic innervation of ventral striatum (VST, also referred to as LST) is relatively sparse and is derived from dorsal tier cell groups that are poor in D2 and DAT. In contrast the sensorimotor striatum (SMST) receives dense dopaminergic inputs mostly from the ventral tier DA neurons that are rich in D2 and DAT. A greater number of synapse sites in the ventral striatum and high levels of D2 and DAT in SMST may account for high D2 displacement in these regions. Compared to VST and SMST, stimulant induced D2 displacement is low in the associative striatum (AST). In schizophrenia, DA release is increased across substriatal divisions due to a prominent increase in the AST. Midbrain: Shown are DA cell bodies, local GABAergic interneurons (brown) and D1 medium spiny neuron terminals (yellow). While there is heterogeneity in the level of expression of D2 receptors and DAT (e.g. dorsal tier and especially medial VTA neurons have low D2 and DAT levels), imaging studies showing subregional analysis of D2 displacement are lacking. However, in SZ there is a reduced stimulant-induced D2 displacement.
The SMST, AST and VST also differ in glutamatergic, cholinergic, and other local (e.g. opioidergic) modulation of DA release, due to neurochemically distinct compartments within each of these subregions, called patch (or striosome) and matrix. These refer to a “mosaic” pattern of grouping of neurons that have differential neurochemical characteristics and specific connections to cortex and other brain regions (Figure 4). In the SMST, the ventral tier DA neurons innervate both the mu opioid receptor and substance P rich ‘patch’ and the enkephalin rich ‘matrix’ compartments; while in the AST, ventral tier innervation is selective to patches. This has implications for DA modulation of cortical afferents, as patches receive projections from limbic (e.g. amygdala) and paralimbic cortical areas (e.g. orbitofrontal cortex); whereas the matrix receives input from other prefrontal cortical regions such as DLPFC.
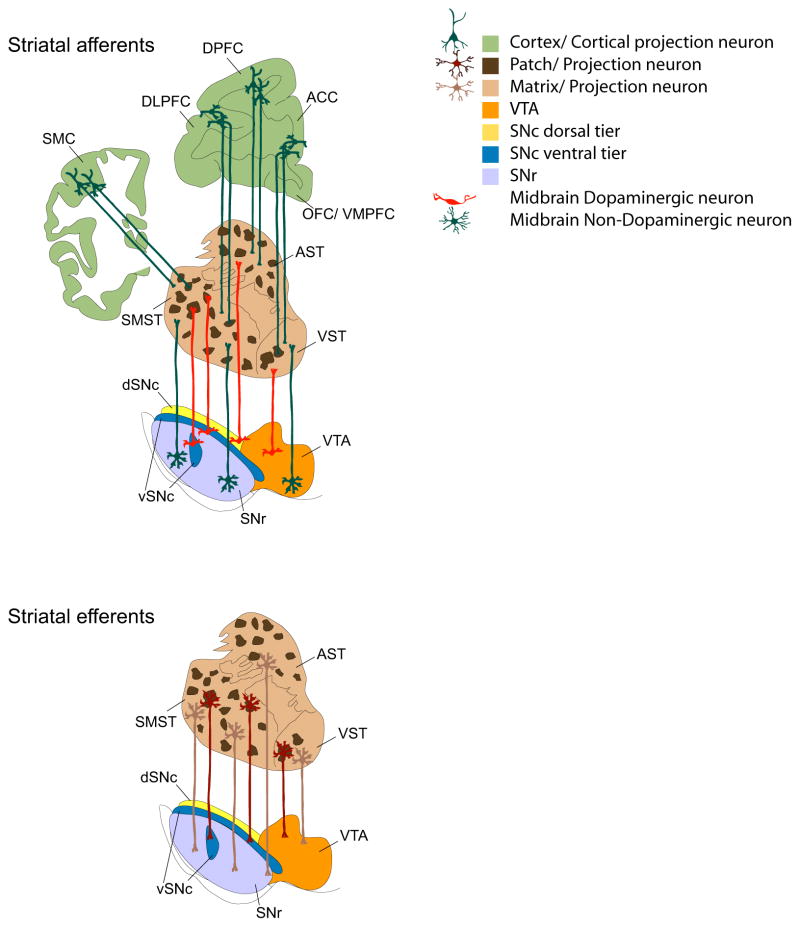
Figure 4. Striatal patch-matrix connectome.
Schematic representation of striatal patch-matrix connectome. Afferents: The cortex topographically projects to the striatum. Within the cortex deeper cortical layers innervate striatal patches (dark brown) whereas the surrounding matrix (light brown) is innervated by superficial cortical layers (light brown). Within the midbrain, the dorsal tier (orange and yellow) innervates the matrix, as do the non-dopaminergic cells (dark green) from the same region. Patch innervation from the midbrain is mostly derived from the ventral tier cell groups (dark blue). Non-dopaminergic (presumably GABAergic) projection neurons within the SNr innervate the striatal matrix complex. Efferents: Striatal patch neurons (maroon) mostly project to ventral tier DA cells. These include both D1 receptor expressing medium spiny neurons and other striatal projection neurons. Striatal projection neurons within the matrix project to both DA and non-dopaminergic populations within the dorsal tier and GABAergic populations in the SNr. See text for further details.
IV.A. Striatal organization
The topography of DA projections interfaces with regional and subcellular localization of DA receptors (Figure 3), which have 5–20-fold higher density in striatum compared to other regions (28, 86, 102–104, 108–111). Post-synaptic D1 and D2 are segregated onto different subpopulations of projection neurons and expressed on striatal interneurons. Cholinergic interneurons express D2-like receptors that mediate fast synaptic events and locally regulate DA release (105, 112). Taken together, ultrastructural and electrophysiological experiments indicate that D2-like receptors are positioned preferentially to mediate DA effects on striatopallidal projection neurons and cholinergic interneurons (28, 113). As with DA inputs, DA receptors and modulators of DA release show distinct patch-matrix distributions in AST and SMST: patches are richer in D1 receptors, lack parvalbumin-expressing interneurons, and show a paucity of cholinergic innervation as indexed by acetylcholinesterase fiber staining (104). Adding to this complexity, neuromodulators differentially affect DA release and projection neuron activity across the patch-matrix organization: e.g. substance P facilitates DA release within patch center, decreases it at patch-matrix border and has no effect in matrix; while enkephalin selectively boosts patch projection output via delta opioid-mediated disinhibitory mechanisms (114, 115).
IV.B. Extrastriatal organization
Extrastriatal regions including cortex are innervated predominantly by the dorsal tier DA system (Figure 2), which is poor in transporter and D2 autoreceptors (102–104). In contrast to low innervation densities in rodents, primates have a dense and extensive cortical DA innervation (116). However, sparse cortical DAT expression suggests a low incidence of DA release sites (107). Moreover, low D2 density and heterogeneous synaptology and DA receptor topography (28) are all consistent with the smaller PET DA release signal in extrastriatal regions. In cortex, D2 are evenly distributed across projection neurons and fast-spiking interneurons (28, 117). Thus, tracer displacement at D2 on fast-spiking interneurons may contribute more to the PET DA release signal in the cortex than in the striatum.
To summarize, spatiotemporal regulation of DA release and localization of D2-like receptors varies considerably across regions and adds complexity to the interpretation of regional and disease-related variation in the PET DA release signal (Figure 3).
V. Discussion
The literature reviewed here shows that: 1) stimulant-induced presynaptic DA release is decreased in most brain regions in schizophrenia (16), with exception of the striatum where it is enhanced, especially in the rostral caudate (59); 2) in this region, the excess is not observed under conditions of substance abuse despite psychosis (11, 82); 3) alterations in expression levels of receptors and transporters are less reliably observed (51, 88), which does not exclude an alteration in function of these receptors in schizophrenia since even under conditions of low DA tone, as in comorbidity with addiction, blocking striatal D2 remains therapeutic and stimulating striatal D2 is psychotogenic (82); 4) antipsychotic exposure results in upregulation of striatal D2 (51) and may induce down-regulation, or normalization, of cortical D1 (75); and 5) the global nature of the presynaptic DA dysregulation is likely to massively alter information processing in multiple domains and result in the global symptomatology that we observe in SZ, although the specific mechanisms that mediate the formation of abnormal learning (118) and symptoms are currently unknown.
It remains to be seen whether extrastriatal DA deficits occur in the same subjects who display striatal DA upregulation, thus yielding a ‘dual dysregulation’ of DA alteration, as proposed in the reformulation of the DA hypothesis of schizophrenia (5, 6). From this perspective, studies using stimulant challenge and those using [18F]DOPA have provided convergent results in striatum, but not in extrastriatal regions. However, when investigators included metabolism of [18F]6-FDA (kloss) (46) in their model, they observed higher kloss in the amygdala and midbrain in SZ, indicating a possible state of lower intracellular DA tone; excessive washout of DA is consistent with the lower evoked release that we observed. This provides one potential mechanism to reconcile these findings and to support our observation of extrastriatal DA release deficits. The finding of increased Kicer (91) on the other hand, is potentially susceptible to group differences in cerebellar concentration of [18F]DOPA. Additional support to our finding of cortical and midbrain deficit derives from the postmortem observations of reduced tyrosine hydroxylase (TH) (119, 120), however high TH (91) and high (121) or normal (122) TH mRNA have also been reported. More research is needed to understand these discrepancies.
Since one of the main findings in SZ is dysregulation of presynaptic DA function, we have reviewed the multifactorial regulation of DA release and its detection with PET. The AST is of particular interest. In HC, the PET DA release signal in the AST is lower than in the SMST (9, 14, 59); whereas in SZ, it is increased to levels similar to the SMST. We speculate that in the healthy brain, subregional differences may reflect differences in DA innervation, regulation of DA release, and/or distributions of perisynaptic D2-like receptors. The difference in the patch/matrix ratio between the AST and SMST could also reflect and/or contribute to lower spontaneous DA release in AST (105, 123, 124). For example, given the low cholinergic innervation of patches, ACh-augmentation of DA release may be lower in this compartment, and thus relatively lower in the patch-enriched AST. We could postulate that, in schizophrenia, a disruption of brain development leads to abnormal or incomplete development of the AST, consistent with structural imaging studies showing lower caudate volume in early-stage, unmedicated patients with SZ relative to HC (104, 125). A developmental disruption leading to altered differentiation of AST from SMST and/or lower patch/matrix compartmentalization in the AST might lead to abnormalities in the patterning of DA and other inputs to the AST, DA interactions with acetylcholine and other striatal neurotransmitters (104, 105), and DA modulation of cortical inputs to the AST (126). Testing these ideas requires updating the existing postmortem literature (125) with studies applying modern labeling and imaging methods to render the 3-dimensional chemoarchitecture of the striatal complex in healthy humans and patients with schizophrenia. Additional models that consider regional and subregional variation in DA synaptology and modulation of DA release across striatal subcompartments are also needed.
The mechanisms underlying cortical deficits in the PET DA release signal in schizophrenia remain to be determined, but given the distribution of D2 receptors, may involve changes in DA signaling at a variety of neuronal populations including cortical interneurons. The generalized and profound deficits in extrastriatal DA release raise an important therapeutic challenge for the field, as currently approved antipsychotics do not remedy this deficit or the resultant low stimulation of extrastriatal dopaminergic receptors. This generalized deficit is also consistent with the multi-domain functional manifestations of the illness, ranging from deficits in social cognition to executive function and motivation.
While higher DA may be linked to better cognition in a non-schizophrenic brain (127–130), in schizophrenia, higher DA may have a dysfunctional impact either because of its modulatory role on an already abnormal circuitry or because of intrinsic aberrant dynamics of DA cell firing patterns.
While this literature does not provide mechanistic understanding of the dysfunction, it has provided a refined topographical knowledge that can be used in translational studies and in drug development. There is unfortunately limited knowledge at this point regarding the specific alterations in the multiple cellular components that could mediate the altered PET DA signal in schizophrenia. We have reviewed above and discussed a few “suspect” cellular mechanisms. These need to be formally tested in postmortem tissue, in animal models that show DA dysregulation, and in cellular systems such as induced Pluripotent Stem Cells (iPSCs) from patients who show abnormal DA PET signal, to isolate specific components that may be involved. Once those are defined they can be used in drug development as specific targets for novel therapies. Our review highlights the urgent need for this cellular work to be carried out in tandem with imaging in patients.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (NIMH): Sylvio O. Conte Center for the Study of Dopamine Dysfunction in Schizophrenia (P50 MH086404 for AA, MS, and LSK; core 4 for HM) and Training in Schizophrenia and Psychotic Disorders: From Animal Models to Patients (T32 MH018870 for JJW); and the Sidney R. Baer, Jr. Fund (for HM). We also appreciate the assistance of Dr. Xiaoyan Xu.
Footnotes
Conflicts of Interest
Dr. Kegeles has received research support from Amgen.
Dr. Slifstein has received research support from Forest Laboratories, Pierre-Fabre, CHDI, and Otsuka and has provided consultation for Amgen.
Dr. Abi-Dargham has received research support from Takeda and Forest Pharmaceuticals and has served on advisory boards for Roche, Forum, and Otsuka.
Drs. Moore, Chohan, and Weinstein report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rossum V. The significance of dopamine receptor blockade for the mechanism of action of neuroleptic drugs. Arch Int Pharmacodyn Therapy. 1966;160:492–494. [PubMed] [Google Scholar]
- 2.Carlsson A. Antipsychotic drugs, neurotransmitters, and schizophrenia. The American journal of psychiatry. 1978;135:165–173. doi: 10.1176/ajp.135.2.165. [DOI] [PubMed] [Google Scholar]
- 3.Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman JA, Kane JM, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology. 1987;91:415–433. doi: 10.1007/BF00216006. [DOI] [PubMed] [Google Scholar]
- 5.Weinberger DR, Berman KF. Speculation on the meaning of cerebral metabolic hypofrontality in schizophrenia. Schizophrenia Bull. 1988;14:157–168. doi: 10.1093/schbul/14.2.157. [DOI] [PubMed] [Google Scholar]
- 6.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 7.Yung AR, McGorry PD, McFarlane CA, Jackson HJ, Patton GC, Rakkar A. Monitoring and care of young people at incipient risk of psychosis. Schizophrenia bulletin. 1996;22:283–303. doi: 10.1093/schbul/22.2.283. [DOI] [PubMed] [Google Scholar]
- 8.Yung AR, Phillips LJ, McGorry PD, McFarlane CA, Francey S, Harrigan S, et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. The British journal of psychiatry Supplement. 1998;172:14–20. [PubMed] [Google Scholar]
- 9.Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Archives of general psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 10.Suridjan I, Rusjan P, Addington J, Wilson AA, Houle S, Mizrahi R. Dopamine D2 and D3 binding in people at clinical high risk for schizophrenia, antipsychotic-naive patients and healthy controls while performing a cognitive task. Journal of psychiatry & neuroscience: JPN. 2013;38:98–106. doi: 10.1503/jpn.110181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizrahi R, Kenk M, Suridjan I, Boileau I, George TP, McKenzie K, et al. Stress-induced dopamine response in subjects at clinical high risk for schizophrenia with and without concurrent cannabis use. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2014;39:1479–1489. doi: 10.1038/npp.2013.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adler CM, Elman I, Weisenfeld N, Kestler L, Pickar D, Breier A. Effects of acute metabolic stress on striatal dopamine release in healthy volunteers. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2000;22:545–550. doi: 10.1016/S0893-133X(99)00153-0. [DOI] [PubMed] [Google Scholar]
- 13.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizrahi R, Addington J, Rusjan PM, Suridjan I, Ng A, Boileau I, et al. Increased stress-induced dopamine release in psychosis. Biological psychiatry. 2012;71:561–567. doi: 10.1016/j.biopsych.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Soliman A, O’Driscoll GA, Pruessner J, Holahan AL, Boileau I, Gagnon D, et al. Stress-induced dopamine release in humans at risk of psychosis: a [11C]raclopride PET study. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:2033–2041. doi: 10.1038/sj.npp.1301597. [DOI] [PubMed] [Google Scholar]
- 16.Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA psychiatry. 2015;72:316–324. doi: 10.1001/jamapsychiatry.2014.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 18.Slifstein M, Laruelle M. Models and methods for derivation of in vivo neuroreceptor parameters with PET and SPECT reversible radiotracers. Nucl Med Bio. 2001;28:595–608. doi: 10.1016/s0969-8051(01)00214-1. [DOI] [PubMed] [Google Scholar]
- 19.Slifstein M, Hwang D-R, Huang Y, Guo N, Sudo Y, Narendran R, et al. In vivo affinity of [18F]fallypride for striatal and extrastriatal dopamine D2 receptors in nonhuman plrimates. Psychopharmacology. 2004;175:274–286. doi: 10.1007/s00213-004-1830-x. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee J, Yang ZY, Das MK, Brown T. Fluorinated benzamide neuroleptics--III. Development of (S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3-[18F]fluoropropyl)-2, 3-dimethoxybenzamide as an improved dopamine D-2 receptor tracer. Nuclear Medicine & Biology. 1995;22:283–296. doi: 10.1016/0969-8051(94)00117-3. [DOI] [PubMed] [Google Scholar]
- 21.Halldin C, Farde L, Hogberg T, Mohell N, Hall H, Suhara T, et al. Carbon-11-FLB 457: a radioligand for extrastriatal D2 dopamine receptors. J Nucl Med. 1995;36:1275–1281. [PubMed] [Google Scholar]
- 22.Kessler RM, Mason NS, Votaw JR, De Paulis T, Clanton JA, Ansari MS, et al. Visualization of extrastriatal dopamine D2 receptors in the human brain. Eur J Pharmacol. 1992;223:105–107. doi: 10.1016/0014-2999(92)90825-o. [DOI] [PubMed] [Google Scholar]
- 23.Narendran R, Slifstein M, Guillin O, Hwang YY, Hwang DR, Scher E, et al. The Dopamine (D-2/3) receptor agonist positron emission tomography radiotracer [C-11)-(+)-PHNO is a D-3 receptor preferring agonist in vivo. Synapse. 2006;60:485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- 24.Rabiner E, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, et al. In vivo quantification of regional dopamine-D3 receptor binding potential of (+)PHNO: studies in non-hman primates and transgenic mice. Synapse. 2009;63:782–793. doi: 10.1002/syn.20658. [DOI] [PubMed] [Google Scholar]
- 25.Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D, et al. Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin -9-ol as a potential radiotracer for in vivo Imaging of the dopamine D2 high-affinity state with positron emission tomography. Journal of Medicinal Chemistry. 2005;48:4153–4160. doi: 10.1021/jm050155n. [DOI] [PubMed] [Google Scholar]
- 26.Abi-Dargham A, Martinez D, Mawlawi O, Simpson N, Hwang DR, Slifstein M, et al. Measurement of striatal and extrastriatal dopamine D1 receptor binding potential with [11C]NNC 112 in humans: validation and reproducibility. J Cereb Blood Flow Metab. 2000;20:225–243. doi: 10.1097/00004647-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- 28.Sesack SR. Synaptology of dopamine neurons. In: Di Chiara G, editor. Dopamine in the CNS. Berlin: Springer-Verlag; 2002. pp. 63–120. [Google Scholar]
- 29.Egerton A, Mehta MA, Montgomery AJ, Lappin JM, Howes OD, Reeves SJ, et al. The dopaminergic basis of human behaviors: A review of molecular imaging studies. Neurosci Biobehav Rev. 2009;33:1109–1132. doi: 10.1016/j.neubiorev.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, et al. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience. 1995;65:709–730. doi: 10.1016/0306-4522(94)00536-e. [DOI] [PubMed] [Google Scholar]
- 33.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Cumming P, Gjedde A. Compartmental analysis of dopa decarboxylation in living brain from dynamic positron emission tomograms. Synapse. 1998;29:37–61. doi: 10.1002/(SICI)1098-2396(199805)29:1<37::AID-SYN4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 35.Kumakura Y, Vernaleken I, Grunder G, Bartenstein P, Gjedde A, Cumming P. PET studies of net blood-brain clearance of FDOPA to human brain: age-dependent decline of [18F]fluorodopamine storage capacity. J Cer Blood Flow Metab. 2005;25:807–819. doi: 10.1038/sj.jcbfm.9600079. [DOI] [PubMed] [Google Scholar]
- 36.Sossi V, Doudet D, Holden J. a reversible tracer analysis approach to the study of effective dopamine turnover. J Cer Blood Flow Metab. 2001;21:469–476. doi: 10.1097/00004647-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Cropley VL, Fujita M, Bara-Jimenez W, Brown AK, Zhang XY, Sangare J, et al. Pre- and post-synaptic dopamine imaging and its relation with frontostriatal cognitive function in Parkinson disease: PET studies with [11C]NNC 112 and [18F]FDOPA. Psychiatry Res. 2008;163:171–182. doi: 10.1016/j.pscychresns.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Kish SJ, Robitaille Y, el-Awar M, Clark B, Schut L, Ball MJ, et al. Striatal monoamine neurotransmitters and metabolites in dominantly inherited olivopontocerebellar atrophy. Neurology. 1992;42:1573–1577. doi: 10.1212/wnl.42.8.1573. [DOI] [PubMed] [Google Scholar]
- 39.Laruelle M, Wallace E, Seibyl JP, Baldwin RM, Zea-Ponce Y, Zoghbi SS, et al. Graphical, kinetic and equilibrium analysis of [123-I]b-CIT in vivo binding to dopamine transporters in healthy subjects. J Cereb Blood Flow Metab. 1994;14:982–994. doi: 10.1038/jcbfm.1994.131. [DOI] [PubMed] [Google Scholar]
- 40.Reith J, Benkelfat C, Sherwin A, Yasuhara Y, Kuwabara H, Andermann F, et al. Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:11651–11654. doi: 10.1073/pnas.91.24.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hietala J, Syvalahti E, Vuorio K, Rakkolainen V, Bergman J, Haaparanta M, et al. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 1995;346:1130–1131. doi: 10.1016/s0140-6736(95)91801-9. [DOI] [PubMed] [Google Scholar]
- 42.Hietala J, Syvalahti E, Vilkman H, Vuorio K, Rakkolainen V, Bergman J, et al. Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophrenia research. 1999;35:41–50. doi: 10.1016/s0920-9964(98)00113-3. [DOI] [PubMed] [Google Scholar]
- 43.Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nature neuroscience. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- 44.McGowan S, Lawrence AD, Sales T, Quested D, Grasby P. Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F]fluorodopa study. Archives of general psychiatry. 2004;61:134–142. doi: 10.1001/archpsyc.61.2.134. [DOI] [PubMed] [Google Scholar]
- 45.Lindstrom LH, Gefvert O, Hagberg G, Lundberg T, Bergstrom M, Hartvig P, et al. Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by L-(beta-11C) DOPA and PET. Biological psychiatry. 1999;46:681–688. doi: 10.1016/s0006-3223(99)00109-2. [DOI] [PubMed] [Google Scholar]
- 46.Kumakura Y, Cumming P, Vernaleken I, Buchholz HG, Siessmeier T, Heinz A, et al. Elevated [18F]fluorodopamine turnover in brain of patients with schizophrenia: an [18F]fluorodopa/positron emission tomography study. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:8080–8087. doi: 10.1523/JNEUROSCI.0805-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nozaki S, Kato M, Takano H, Ito H, Takahashi H, Arakawa R, et al. Regional dopamine synthesis in patients with schizophrenia using L-[beta-11C]DOPA PET. Schizophrenia research. 2009;108:78–84. doi: 10.1016/j.schres.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Elkashef AM, Doudet D, Bryant T, Cohen RM, Li SH, Wyatt RJ. 6-(18)F-DOPA PET study in patients with schizophrenia. Positron emission tomography. Psychiatry Res. 2000;100:1–11. doi: 10.1016/s0925-4927(00)00064-0. [DOI] [PubMed] [Google Scholar]
- 49.Dao-Castellana MH, Paillere-Martinot ML, Hantraye P, Attar-Levy D, Remy P, Crouzel C, et al. Presynaptic dopaminergic function in the striatum of schizophrenic patients. Schizophrenia research. 1997;23:167–174. doi: 10.1016/S0920-9964(96)00102-8. [DOI] [PubMed] [Google Scholar]
- 50.Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, part II: meta-analysis of [(18)F/(11)C]-DOPA PET studies. Schizophrenia bulletin. 2013;39:33–42. doi: 10.1093/schbul/sbr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Archives of general psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. The American journal of psychiatry. 1998;155:761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- 54.Pogarell O, Koch W, Karch S, Dehning S, Muller N, Tatsch K, et al. Dopaminergic neurotransmission in patients with schizophrenia in relation to positive and negative symptoms. Pharmacopsychiatry. 2012;45(Suppl 1):S36–41. doi: 10.1055/s-0032-1306313. [DOI] [PubMed] [Google Scholar]
- 55.Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biological psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 57.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biological psychiatry. 2009;65:1091–1093. doi: 10.1016/j.biopsych.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 59.Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Archives of general psychiatry. 2010;67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 60.Hitri A, Casanova MF, Kleinman JE, Weinberger DR, Wyatt RJ. Age-related changes in [3H]GBR 12935 binding site density in the prefrontal cortex of controls and schizophrenics. Biological psychiatry. 1995;37:175–182. doi: 10.1016/0006-3223(94)00202-E. [DOI] [PubMed] [Google Scholar]
- 61.Taylor SF, Koeppe RA, Tandon R, Zubieta JK, Frey KA. In vivo measurement of the vesicular monoamine transporter in schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2000;23:667–675. doi: 10.1016/S0893-133X(00)00165-2. [DOI] [PubMed] [Google Scholar]
- 62.Laakso A, Vilkman H, Alakare B, Haaparanta M, Bergman J, Solin O, et al. Striatal dopamine transporter binding in neuroleptic-naive patients with schizophrenia studied with positron emission tomography. The American journal of psychiatry. 2000;157:269–271. doi: 10.1176/appi.ajp.157.2.269. [DOI] [PubMed] [Google Scholar]
- 63.Laruelle M, Abi-Dargham A, van Dyck C, Gil R, D’Souza DC, Krystal J, et al. Dopamine and serotonin transporters in patients with schizophrenia: an imaging study with [(123)I]beta-CIT. Biological psychiatry. 2000;47:371–379. doi: 10.1016/s0006-3223(99)00257-7. [DOI] [PubMed] [Google Scholar]
- 64.Laakso A, Bergman J, Haaparanta M, Vilkman H, Solin O, Syvalahti E, et al. Decreased striatal dopamine transporter binding in vivo in chronic schizophrenia. Schizophrenia research. 2001;52:115–120. doi: 10.1016/s0920-9964(00)00095-5. [DOI] [PubMed] [Google Scholar]
- 65.Lavalaye J, Linszen DH, Booij J, Dingemans PM, Reneman L, Habraken JB, et al. Dopamine transporter density in young patients with schizophrenia assessed with [123]FP-CIT SPECT. Schizophrenia research. 2001;47:59–67. doi: 10.1016/s0920-9964(00)00023-2. [DOI] [PubMed] [Google Scholar]
- 66.Hsiao MC, Lin KJ, Liu CY, Tzen KY, Yen TC. Dopamine transporter change in drug-naive schizophrenia: an imaging study with 99mTc-TRODAT-1. Schizophrenia research. 2003;65:39–46. doi: 10.1016/s0920-9964(03)00006-9. [DOI] [PubMed] [Google Scholar]
- 67.Schmitt GJ, la Fougere C, Dresel S, Frodl T, Hahn K, Moller HJ, et al. Dual-isotope SPECT imaging of striatal dopamine: first episode, drug naive schizophrenic patients. Schizophrenia research. 2008;101:133–141. doi: 10.1016/j.schres.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 68.Mateos JJ, Lomena F, Parellada E, Mireia F, Fernandez-Egea E, Pavia J, et al. Lower striatal dopamine transporter binding in neuroleptic-naive schizophrenic patients is not related to antipsychotic treatment but it suggests an illness trait. Psychopharmacology. 2007;191:805–811. doi: 10.1007/s00213-006-0570-5. [DOI] [PubMed] [Google Scholar]
- 69.Mateos JJ, Lomena F, Parellada E, Font M, Fernandez E, Pavia J, et al. Decreased striatal dopamine transporter binding assessed with [123I] FP-CIT in first-episode schizophrenic patients with and without short-term antipsychotic-induced parkinsonism. Psychopharmacology. 2005;181:401–406. doi: 10.1007/s00213-005-2250-2. [DOI] [PubMed] [Google Scholar]
- 70.Yoder KK, Hutchins GD, Morris ED, Brashear A, Wang C, Shekhar A. Dopamine transporter density in schizophrenic subjects with and without tardive dyskinesia. Schizophrenia research. 2004;71:371–375. doi: 10.1016/j.schres.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 71.Yang YK, Yu L, Yeh TL, Chiu NT, Chen PS, Lee IH, et al. Associated alterations of striatal dopamine D2/D3 receptor and transporter binding in drug-naive patients with schizophrenia: a dual-isotope SPECT study. The American journal of psychiatry. 2004;161:1496–1498. doi: 10.1176/appi.ajp.161.8.1496. [DOI] [PubMed] [Google Scholar]
- 72.Arakawa R, Ichimiya T, Ito H, Takano A, Okumura M, Takahashi H, et al. Increase in thalamic binding of [(11)C]PE2I in patients with schizophrenia: a positron emission tomography study of dopamine transporter. Journal of psychiatric research. 2009;43:1219–1223. doi: 10.1016/j.jpsychires.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Karlsson P, Farde L, Halldin C, Sedvall G. PET study of D(1) dopamine receptor binding in neuroleptic-naive patients with schizophrenia. The American journal of psychiatry. 2002;159:761–767. doi: 10.1176/appi.ajp.159.5.761. [DOI] [PubMed] [Google Scholar]
- 74.Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abi-Dargham A, Xu X, Thompson JL, Gil R, Kegeles LS, Urban N, et al. Increased prefrontal cortical D(1) receptors in drug naive patients with schizophrenia: a PET study with [(1)(1)C]NNC112. Journal of psychopharmacology (Oxford, England) 2012;26:794–805. doi: 10.1177/0269881111409265. [DOI] [PubMed] [Google Scholar]
- 76.Howes OD, Bose SK, Turkheimer F, Valli I, Egerton A, Valmaggia LR, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. The American journal of psychiatry. 2011;168:1311–1317. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fusar-Poli P, Howes OD, Allen P, Broome M, Valli I, Asselin MC, et al. Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Archives of general psychiatry. 2010;67:683–691. doi: 10.1001/archgenpsychiatry.2010.77. [DOI] [PubMed] [Google Scholar]
- 78.Fusar-Poli P, Howes OD, Allen P, Broome M, Valli I, Asselin MC, et al. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Molecular psychiatry. 2011;16:67–75. doi: 10.1038/mp.2009.108. [DOI] [PubMed] [Google Scholar]
- 79.Howes O, Bose S, Turkheimer F, Valli I, Egerton A, Stahl D, et al. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Molecular psychiatry. 2011;16:885–886. doi: 10.1038/mp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Egerton A, Chaddock CA, Winton-Brown TT, Bloomfield MA, Bhattacharyya S, Allen P, et al. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biological psychiatry. 2013;74:106–112. doi: 10.1016/j.biopsych.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 81.Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. The American journal of psychiatry. 2012;169:1203–1210. doi: 10.1176/appi.ajp.2012.12010144. [DOI] [PubMed] [Google Scholar]
- 82.Thompson JL, Urban N, Slifstein M, Xu X, Kegeles LS, Girgis RR, et al. Striatal dopamine release in schizophrenia comorbid with substance dependence. Molecular psychiatry. 2013;18:909–915. doi: 10.1038/mp.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Talvik M, Nordstrom AL, Olsson H, Halldin C, Farde L. Decreased thalamic D2/D3 receptor binding in drug-naive patients with schizophrenia: a PET study with [11C]FLB 457. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2003;6:361–370. doi: 10.1017/S1461145703003699. [DOI] [PubMed] [Google Scholar]
- 84.Suhara T, Okubo Y, Yasuno F, Sudo Y, Inoue M, Ichimiya T, et al. Decreased dopamine D2 receptor binding in the anterior cingulate cortex in schizophrenia. Archives of general psychiatry. 2002;59:25–30. doi: 10.1001/archpsyc.59.1.25. [DOI] [PubMed] [Google Scholar]
- 85.Glenthoj BY, Mackeprang T, Svarer C, Rasmussen H, Pinborg LH, Friberg L, et al. Frontal dopamine D(2/3) receptor binding in drug-naive first-episode schizophrenic patients correlates with positive psychotic symptoms and gender. Biological psychiatry. 2006;60:621–629. doi: 10.1016/j.biopsych.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 86.Kegeles LS, Slifstein M, Xu X, Urban N, Thompson JL, Moadel T, et al. Striatal and extrastriatal dopamine D2/D3 receptors in schizophrenia evaluated with [18F]fallypride positron emission tomography. Biological psychiatry. 2010;68:634–641. doi: 10.1016/j.biopsych.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kessler RM, Woodward ND, Riccardi P, Li R, Ansari MS, Anderson S, et al. Dopamine D2 receptor levels in striatum, thalamus, substantia nigra, limbic regions, and cortex in schizophrenic subjects. Biological psychiatry. 2009;65:1024–1031. doi: 10.1016/j.biopsych.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kambeitz J, Abi-Dargham A, Kapur S, Howes OD. Alterations in cortical and extrastriatal subcortical dopamine function in schizophrenia: systematic review and meta-analysis of imaging studies. The British journal of psychiatry: the journal of mental science. 2014;204:420–429. doi: 10.1192/bjp.bp.113.132308. [DOI] [PubMed] [Google Scholar]
- 89.Kosaka J, Takahashi H, Ito H, Takano A, Fujimura Y, Matsumoto R, et al. Decreased binding of [11C]NNC112 and [11C]SCH23390 in patients with chronic schizophrenia. Life sciences. 2010;86:814–818. doi: 10.1016/j.lfs.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 90.Poels EM, Girgis RR, Thompson JL, Slifstein M, Abi-Dargham A. In vivo binding of the dopamine-1 receptor PET tracers [11C]NNC112 and [11C]SCH23390: a comparison study in individuals with schizophrenia. Psychopharmacology. 2013;228:167–174. doi: 10.1007/s00213-013-3026-8. [DOI] [PubMed] [Google Scholar]
- 91.Howes OD, Williams M, Ibrahim K, Leung G, Egerton A, McGuire PK, et al. Midbrain dopamine function in schizophrenia and depression: a post-mortem and positron emission tomographic imaging study. Brain: a journal of neurology. 2013;136:3242–3251. doi: 10.1093/brain/awt264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Allen P, Luigjes J, Howes OD, Egerton A, Hirao K, Valli I, et al. Transition to psychosis associated with prefrontal and subcortical dysfunction in ultra high-risk individuals. Schizophrenia bulletin. 2012;38:1268–1276. doi: 10.1093/schbul/sbr194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zubieta JK, Taylor SF, Huguelet P, Koeppe RA, Kilbourn MR, Frey KA. Vesicular monoamine transporter concentrations in bipolar disorder type I, schizophrenia, and healthy subjects. Biological psychiatry. 2001;49:110–116. doi: 10.1016/s0006-3223(00)00981-1. [DOI] [PubMed] [Google Scholar]
- 94.Lehrer DS, Christian BT, Kirbas C, Chiang M, Sidhu S, Short H, et al. 18F-fallypride binding potential in patients with schizophrenia compared to healthy controls. Schizophrenia research. 2010;122:43–52. doi: 10.1016/j.schres.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Talvik M, Nordstrom AL, Okubo Y, Olsson H, Borg J, Halldin C, et al. Dopamine D2 receptor binding in drug-naive patients with schizophrenia examined with raclopride-C11 and positron emission tomography. Psychiatry Res. 2006;148:165–173. doi: 10.1016/j.pscychresns.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 96.Tuppurainen H, Kuikka JT, Laakso MP, Viinamaki H, Husso M, Tiihonen J. Midbrain dopamine D2/3 receptor binding in schizophrenia. European archives of psychiatry and clinical neuroscience. 2006;256:382–387. doi: 10.1007/s00406-006-0649-3. [DOI] [PubMed] [Google Scholar]
- 97.Graff-Guerrero A, Mizrahi R, Agid O, Marcon H, Barsoum P, Rusjan P, et al. The dopamine D2 receptors in high-affinity state and D3 receptors in schizophrenia: a clinical [11C]-(+)-PHNO PET study. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:1078–1086. doi: 10.1038/npp.2008.199. [DOI] [PubMed] [Google Scholar]
- 98.Buchsbaum MS, Christian BT, Lehrer DS, Narayanan TK, Shi B, Mantil J, et al. D2/D3 dopamine receptor binding with [F-18]fallypride in thalamus and cortex of patients with schizophrenia. Schizophrenia research. 2006;85:232–244. doi: 10.1016/j.schres.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 99.Yasuno F, Suhara T, Okubo Y, Sudo Y, Inoue M, Ichimiya T, et al. Low dopamine d(2) receptor binding in subregions of the thalamus in schizophrenia. The American journal of psychiatry. 2004;161:1016–1022. doi: 10.1176/appi.ajp.161.6.1016. [DOI] [PubMed] [Google Scholar]
- 100.Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 101.Kegeles LS, Abi-Dargham A, Frankle W, et al. INcreased synaptic dopamine function in associative regions of the striatum in schizophrenia. Archives of general psychiatry. 2010;67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 102.Haber SN, Knutson B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- 104.Bentivoglio M, Morelli M. Chapter I The organization and circuits of mesencephalic dopaminergic neurons and the distribution of dopamine receptors in the brain. Handbook of Chemical Neuroanatomy. 2005:1–107. [Google Scholar]
- 105.Rice ME, Patel Jc, Cragg SJ, Cragg SJ. Dopamine release in the basal ganglia. Neuroscience. 2011;198:112–137. doi: 10.1016/j.neuroscience.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 107.Haber SN, Ryoo H, Cox C, Lu W. Subsets of midbrain dopaminergic neurons in monkeys are distinguished by different levels of mRNA for the dopamine transporter: comparison with the mRNA for the D2 receptor, tyrosine hydroxylase and calbindin immunoreactivity. The Journal of comparative neurology. 1995;362:400–410. doi: 10.1002/cne.903620308. [DOI] [PubMed] [Google Scholar]
- 108.Cropley VL, Innis RB, Nathan PJ, Brown AK, Sangare JL, Lerner A, et al. Small effect of dopamine release and no effect of dopamine depletion on [18F]fallypride binding in healthy humans. Synapse. 2008;62:399–408. doi: 10.1002/syn.20506. [DOI] [PubMed] [Google Scholar]
- 109.Kegeles LS, Slifstein M, Frankle WG, Xu X, Hackett E, Bae SA, et al. Dose-occupancy study of striatal and extrastriatal dopamine D2 receptors by aripiprazole in schizophrenia with PET and [18F]fallypride. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:3111–3125. doi: 10.1038/npp.2008.33. [DOI] [PubMed] [Google Scholar]
- 110.Riccardi P, Baldwin R, Salomon R, Anderson S, Ansari MS, Li R, et al. Estimation of baseline dopamine D2 receptor occupancy in striatum and extrastriatal regions in humans with positron emission tomography with [18F] fallypride. Biological psychiatry. 2008;63:241–244. doi: 10.1016/j.biopsych.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 111.Slifstein M, Kegeles LS, Xu X, Thompson JL, Urban N, Castrillon J, et al. Striatal and extrastriatal dopamine release measured with PET and [(18)F] fallypride. Synapse. 2010;64:350–362. doi: 10.1002/syn.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chuhma N, Mingote S, Moore H, Rayport S. Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling. Neuron. 2014;81:901–912. doi: 10.1016/j.neuron.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gonzales KK, Smith Y. Cholinergic interneurons in the dorsal and ventral striatum: anatomical and functional considerations in normal and diseased conditions. Annals of the New York Academy of Sciences. 2015;1349:1–45. doi: 10.1111/nyas.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Banghart MR, Neufeld SQ, Wong NC, Sabatini BL. Enkephalin Disinhibits Mu Opioid Receptor-Rich Striatal Patches via Delta Opioid Receptors. Neuron. 2015;88:1227–1239. doi: 10.1016/j.neuron.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brimblecombe KR, Cragg SJ. Substance P Weights Striatal Dopamine Transmission Differently within the Striosome-Matrix Axis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:9017–9023. doi: 10.1523/JNEUROSCI.0870-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends in neurosciences. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- 117.Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 119.Rice MW, Roberts RC, Melendez-Ferro M, Perez-Costas E. Mapping dopaminergic deficiencies in the substantia nigra/ventral tegmental area in schizophrenia. Brain structure & function. 2014 doi: 10.1007/s00429-014-0901-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Akil M, Edgar CL, Pierri JN, Casali S, Lewis DA. Decreased density of tyrosine hydroxylase-immunoreactive axons in the entorhinal cortex of schizophrenic subjects. Biological psychiatry. 2000;47:361–370. doi: 10.1016/s0006-3223(99)00282-6. [DOI] [PubMed] [Google Scholar]
- 121.Mueller HT, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of the ionotropic glutamate receptor subunits and NMDA receptor-associated intracellular proteins in the substantia nigra in schizophrenia. Brain Res Mol Brain Res. 2004;121:60–69. doi: 10.1016/j.molbrainres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 122.Ichinose H, Ohye T, Fujita K, Pantucek F, Lange K, Riederer P, et al. Quantification of mRNA of tyrosine hydroxylase and aromatic L-amino acid decarboxylase in the substantia nigra in Parkinson’s disease and schizophrenia. J Neural Transm Park Dis Dement Sect. 1994;8:149–158. doi: 10.1007/BF02250926. [DOI] [PubMed] [Google Scholar]
- 123.Kemel ML, Desban M, Glowinski J, Gauchy C. Distinct presynaptic control of dopamine release in striosomal and matrix areas of the cat caudate nucleus. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:9006–9010. doi: 10.1073/pnas.86.22.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Graybiel AM, Ragsdale CW., Jr Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proceedings of the National Academy of Sciences of the United States of America. 1978;75:5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Perez-Costas E, Melendez-Ferro M, Roberts RC. Basal ganglia pathology in schizophrenia: Dopamine connections and anomalies. Journal of neurochemistry. 2010;113:287–302. doi: 10.1111/j.1471-4159.2010.06604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Crittenden JR, Graybiel AM. Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat. 2011;5:59. doi: 10.3389/fnana.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vernaleken I, Buchholz HG, Kumakura Y, Siessmeier T, Stoeter P, Bartenstein P, et al. ‘Prefrontal’ cognitive performance of healthy subjects positively correlates with cerebral FDOPA influx: an exploratory [18F]-fluoro-L-DOPA-PET investigation. Human brain mapping. 2007;28:931–939. doi: 10.1002/hbm.20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cools R, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Working memory capacity predicts dopamine synthesis capacity in the human striatum. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:1208–1212. doi: 10.1523/JNEUROSCI.4475-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Deserno L, Huys QJ, Boehme R, Buchert R, Heinze HJ, Grace AA, et al. Ventral striatal dopamine reflects behavioral and neural signatures of model-based control during sequential decision making. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:1595–1600. doi: 10.1073/pnas.1417219112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.