Abstract
Despite half a century of research, the biology of dinoflagellates remains enigmatic: they defy many functional and genetic traits attributed to typical eukaryotic cells. Genomic approaches to study dinoflagellates are often stymied due to their large, multi-gigabase genomes. Members of the genus Symbiodinium are photosynthetic endosymbionts of stony corals that provide the foundation of coral reef ecosystems. Their smaller genome sizes provide an opportunity to interrogate evolution and functionality of dinoflagellate genomes and endosymbiosis. We sequenced the genome of the ancestral Symbiodinium microadriaticum and compared it to the genomes of the more derived Symbiodinium minutum and Symbiodinium kawagutii and eukaryote model systems as well as transcriptomes from other dinoflagellates. Comparative analyses of genome and transcriptome protein sets show that all dinoflagellates, not only Symbiodinium, possess significantly more transmembrane transporters involved in the exchange of amino acids, lipids, and glycerol than other eukaryotes. Importantly, we find that only Symbiodinium harbor an extensive transporter repertoire associated with the provisioning of carbon and nitrogen. Analyses of these transporters show species-specific expansions, which provides a genomic basis to explain differential compatibilities to an array of hosts and environments, and highlights the putative importance of gene duplications as an evolutionary mechanism in dinoflagellates and Symbiodinium.
Dinoflagellates are ubiquitous freshwater and marine protists, having great economic and ecological importance1. Over half a century of extensive research into their biology shows that they defy many cellular and genetic traits commonly attributed to eukaryotic cell biology and function2,3. The organization and regulation of genes in particular is different from most other eukaryotes, and include non-canonical intron splicing, the existence of unusual upstream promoter regions for many genes, DNA that contains 5-hydroxymethyluracil (replacing 12–70% of thymidine), and a greater reliance on translational – rather than transcriptional – gene regulation2. Moreover, dinoflagellate genomes have among the highest levels of DNA acquired through horizontal gene transfer (HGT)4. The increased study of dinoflagellate genomics will enhance basic understanding of the evolution and functionality of eukaryotic genomes and may help to further our knowledge about dinoflagellate physiology and ecology.
Indeed, there are a growing number of transcriptomes and expression data available for many dinoflagellates5,6. However, these analyses lack the scaffolding of a reference genome to know how many genes exist, relate how genes are organized and positioned relative to each other, and allow the future application of transgenic approaches for the interpretation of gene function7. The major obstacle that limits the analysis of dinoflagellate genome organization and function is that their genomes are unusually large (~1.5–250 Gb) relative to other eukaryotes8. Until the sequencing of large and highly repetitive genomes is made easier, comparative genomics among dinoflagellates will be limited to those species with the smallest genomes. The genomes of species in the genus Symbiodinium are among the smallest (~1–5 Gb) relative to other dinoflagellates, which corresponds to their small cell size9. For this reason, the first available draft genome of a dinoflagellate was that of Symbiodinium minutum10, and soon followed by Symbiodinium kawagutii11.
Members of the genus Symbiodinium occur often in widespread symbioses with metazoans in the phylum Cnidaria as well as with many other animals and protists12. Their symbioses with reef-building corals create the foundation for one of the most diverse and productive marine ecosystems on the planet – coral reefs. Growing concerns over climate change and reef degradation heighten the need to understand the genomic underpinning of physiological differences among the vast number of Symbiodinium species. The large numbers of available cultures representing numerous closely and distantly related species and strains constitute a critical resource and model system for comparative genomics among dinoflagellates13. The draft genomes of S. minutum and Symbiodinium kawagutii confirmed that the genomic makeup of Symbiodinium is similar to other dinoflagellates, including the presence of spliced leader sequences and non-canonical splice sites, and a prevalence of genes acquired from bacteria10,11. In addition, large contigs from the genome of S. minutum indicated a strong tendency for unidirectionally aligned genes.
The publication of the genomes of S. minutum and S. kawagutii has been accompanied in recent years by a number of studies that have analyzed and compared the transcriptomes among distantly related species14,15,16,17,18,19. Their long evolutionary divergence was reflected in the considerable differences found between their transcriptome profiles14,18. However, the limited availability of genomes prevented making further generalities about the organization and function of Symbiodinium genomes, how this translates into their ability to form environmentally stable symbioses with specific hosts, and whether gene content and the representation of biochemical pathways is a common feature of all Symbiodinium, or even dinoflagellates in general.
To further advance our capacity for comparative genomics and relating transcriptional profiling with genome wide analyses, we sequenced the genome of Symbiodinium microadriaticum20. This species occurs in symbioses with the jellyfish Cassiopea xamachana21. S. microadriaticum is a member of the most ancestral lineage, Clade A, while S. minutum is a representative member of Clade B22 and S. kawagutii of the more derived Clade F11; these lineages shared a common ancestor at least 45–55 MYA23. Accordingly, comparing the genomes of S. microadriaticum, S. minutum, and S. kawagutii provides an opportunity to determine whether gene organization and content is conserved across lineages separated by tens of millions of years. Moreover, it allows for the comparison of their corresponding gene sets to transcriptomes from other dinoflagellates to unequivocally assess which features are shared among dinoflagellates and which are specific to Symbiodinium, potentially revealing distinct characteristics that contribute to their ecological success as intracellular symbionts.
Results
Genome size of S. microadriaticum
The draft genome of S. microadriaticum (strain CCMP2467) encompasses 808 Mbp of the 1,100 Mbp genome (based on k-mer distribution), of which 746 Mbp were assembled into contigs (Supplemental Information, Table S1). A subsequent FACS (Fluorescence-activated cell sorting) analysis estimated the genome size at 1,400 Mbp, indicating that either k-mer based estimates in Symbiodinium might underestimate dinoflagellate genome sizes or that FACS based analyses include extra-nuclear DNA (Supplemental Information, Fig. S1). The scaffold N50 of the assembled genome is 573.5 kbp featuring a contig N50 of 34.9 kbp and encoding for 49,109 genes, of which 24,610 (~50%) show homology to genes from available databases (Table 1, Supplemental Information, Table S1 and Table S2). This compares well with the ~609 Mbp draft genome containing 41,925 genes (contig N50 of 62.7 kbp and scaffold N50 of 125.2 kbp) of S. minutum and the ~935 Mbp genome containing 36,850 genes (contig N50 of 47.1 kbp and scaffold N50 of 380.9 kbp) of Symbiodinium kawagutii. Notably, GC content was considerably higher in S. microadriaticum (50.5%) than in S. minutum (43.5%) and S. kawagutii (45.5%).
Table 1. Genomes of Symbiodinium microadriaticum, Symbiodinium minutum, and Symbiodinium kawagutii.
| Symbiodinium microadriaticum | Symbiodinium minutum | Symbiodinium kawagutii | ||
|---|---|---|---|---|
| Genome | Genome file used | v1.0 | v1.0 (Jun 2013) | Nov 2015 |
| Total scaffold length (bp) | 808,242,489 | 609,476,485 | 935,067,369 | |
| Scaffold N50 (bp) | 573,512 | 125,226 | 380,908 | |
| Total contig length (bp) | 746,043,463 | 603,743,338 | 902,990,605 | |
| Contig N50 (bp) | 34,883 | 62,714 | 47,143 | |
| GC content, N excluded (%) | 50.5 | 43.5 | 45.5 | |
| Genes | Number of genes | 49,109 | 41,925 | 36,850 |
| Mean gene length (bp) | 12,898 | 11,961 | 3,788 | |
| Gene models with EST support (%) | 76.3 | 77.2^ | 72.8* | |
| Exons | Mean coding region length (bp) | 2,389 | 2,027 | 1,041 |
| Number of exons per gene | 21.8 | 19.9 | 4.1 | |
| Mean length (bp) | 109.5 | 101.7 | 255.7 | |
| Total length (Mb) | 117.3 | 85.0 | 38.4 | |
| Introns | Genes with introns (%) | 98.2 | 95.4 | 64.1 |
| Mean length (bp) | 504.7 | 516.6 | 893.4 | |
| Total length (Mb) | 516.1 | 410.1 | 101.2 | |
| Predominant first two nucleotides at donor splice sites | GC/GT/GA | GT/GC/GA | GT/GC | |
| Intergenic | Average length (bp) | 3,633 | 2,253 | 18,035 |
| Unidirectional arrangement of genes | Yes | Yes^ | Yes |
Genome organization of S. microadriaticum
To estimate the completeness of the assembled genome, we analyzed the presence of 458 highly conserved eukaryotic genes24. This analysis was performed on the genomes of S. microadriaticum, S. minutum, and S. kawagutii to ensure similar completeness for all subsequent comparative analyses. We identified 437 (95.4%), 434 (94.8%), and 383 (83.6%) homologs for S. microadriaticum, S. minutum, and S. kawagutii respectively, of which 373 (81.4%) were common between all three species (Dataset S1.1). A strong directionality in gene orientation was observed for S. microadriaticum (featuring an average of 2.32 gene orientation changes per 10-gene window), but was significantly less pronounced (χ2 test, p-value < 2.2 × 10−16) than in S. minutum (0.64 changes), although similar to S. kawagutii (2.11 changes) (Supplemental Information, Fig. S2). Since the Symbiodinium species belong to clades that are evolutionarily distant from each other (45–55 MYA)23, we wanted to assess whether gene order was a conserved feature between the three species. Syntenic blocks of at least five genes with similarities <1e−5 were identified from all three genomes using MCScanX25. These analyses revealed startlingly few and short synteny blocks between S. microadriaticum and S. minutum (349 blocks ≤10 genes), and even fewer regions could be identified in any of the comparisons to S. kawagutii (S. microadriaticum vs. S. kawagutii: 166 blocks ≤10 genes; S. minutum vs. S. kawagutii: 222 blocks ≤10 genes) (Supplemental Information, Table S3, Fig. S3). This lack of syntenic conservation indicates substantial genomic differences between these species. Comparison of the gene densities between the three genomes showed pronounced differences with 61 vs. 68 vs. 39 genes per Mb for S. microadriaticum, S. minutum, and S. kawagutii, respectively. Patterns of canonical and non-canonical splice sites were highly similar between all three species (Supplemental Information, Fig. S4) with the exception of S. kawagutii, which contained some donor sites starting with cytosine instead of the canonical guanine.
Genic composition of Symbiodinium genomes
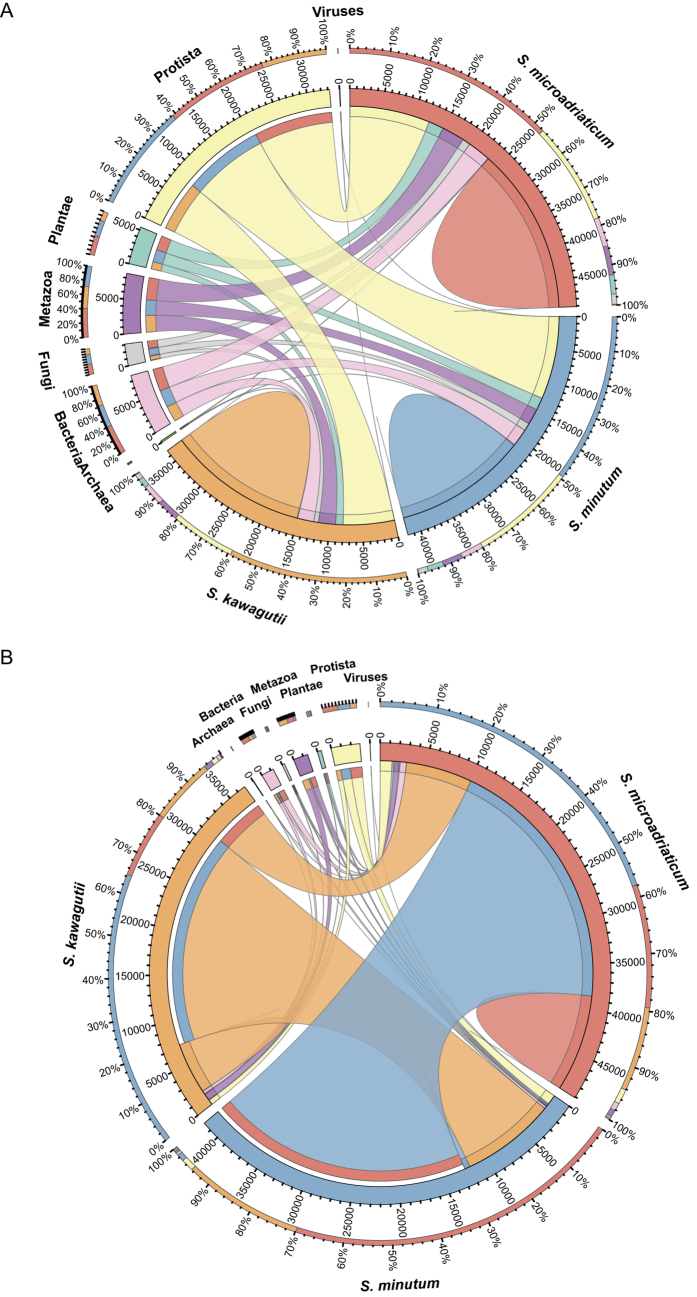
To further understand genic composition of Symbiodinium genomes, we performed a BLASTP analysis using the gene sets encoded in all three genomes. The resulting best hits were categorized and grouped by their putative phylogenetic origin into 7 groups (i.e., Viruses, Bacteria, Archaea, Protista, Viridiplantae, Metazoa, and Fungi) based on their taxonomically best match (Fig. 1A). In addition, and to examine the similarity of all three genomes, we performed the same analysis, but included hits between the Symbiodinium genomes to highlight commonalities and differences in the genic content (Fig. 1B). All genomes showed a highly similar taxonomic distribution of their respective gene sets (Fig. 1A). As expected, the vast majority of the genes matched known proteins from other protists (~25% of all gene models in S. microadriaticum, ~30% in S. minutum, and ~21% in S. kawagutii), followed by bacteria (8% vs. 7% vs. 5%, respectively). All species had a similar amount of genes corresponding to metazoan genes (7% vs. 6% vs. 7%, respectively), while genes with similarities to fungi, archaea, and viruses were generally few and accounted for less than 3%. The inclusion of matches to the other Symbiodinium genomes emphasized the relatedness of the three species (Fig. 1B). Approximately 58% of the S. microadriaticum genes had their best hit in S. minutum, while ~15% had their best match to S. kawagutii genes. Reciprocally, ~70% of the S. minutum genes had their best hit in S. microadriaticum, but only ~24% had a better hit to S. kawagutii, although S. microadriaticum is ancestral to both other species. Interestingly, we found that while the majority of S. minutum genes had their best hit to genes from S. microadriaticum, most of the S. kawagutii genes had their best match to genes from S. minutum (~64%) and considerably less genes hat better hits to S. microadriaticum (~16%). Further, although the majority of genes from all three species had matches at least in one of the other Symbiodinium species, we still found a considerable amount of genes in S. microadriaticum (21%) and S. kawagutii (15%), but not in S. minutum (2%), without significant hits at all, suggesting that these genes might be putatively species- or lineage-specific.
Figure 1. Comparison of genomic composition of the genomes of Symbiodinium microadriaticum, Symbiodinium minutum, and Symbiodinium kawagutii.
Genes were classified by best hits against nr database into seven kingdom/subkingdom-level organismal groups: Archaea, Bacteria, Fungi, Metazoa, Plantae, Protista, and Viruses. Chord diagrams show (A) the fairly even number of matches from both Symbiodinium species to these seven groups respectively, and (B) the large overlap between Symbiodinium species when they were allowed to match against each other.
Functional gene content of Symbiodinium relative to other eukaryotes
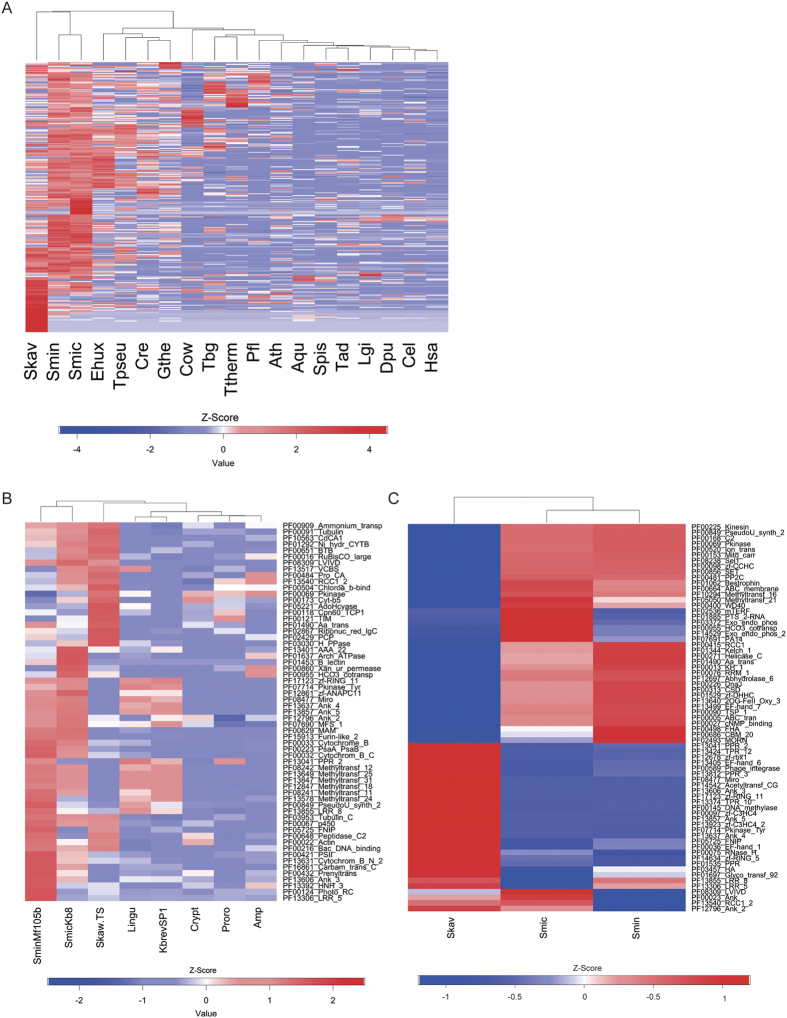
In order to identify specific molecular functions that are enriched in Symbiodinium, we compared the relative frequencies of protein domains obtained from the translated genomic gene sets of S. microadriaticum, S. minutum, and S. kawagutii to reference genomic protein sets from 16 organisms (Fig. 2A, Supplemental Information, Table S4, Dataset S1.2). Our analysis produced 280 significantly enriched protein domains (FDR < 0.001) and emphasized just how distinct Symbiodinium genomes are, even relative to other protist genomes (Fig. 2A, Supplemental Information, Table S5). In line with previous findings11, we found a surprisingly large amount of domains involved in transmembrane transport to be highly enriched in Symbiodinium (20 out of 280). These included transporter domains specific for bicarbonate, ammonium, phosphate, lipids, glycerol, amino acids, choline, sugars, and sulfates (among others), as well as the more general ABC and ion transporters (Supplemental Information, Table S5). We also identified enrichment of carbonic anhydrases in the genomes of S. microadriaticum, S. minutum, and S. kawagutii in comparison to other eukaryotic genomes (Supplemental Information, Table S5).
Figure 2.
Pfam domain enrichment in protein sets of Symbiodinium microadriaticum, Symbiodinium minutum, and Symbiodinium kawagutii (A) in comparison to 16 reference eukaryotic genomes, (B) in comparison to other dinoflagellate transcriptomes, (C) among Symbiodinium genomes. Rows indicate distinct Pfam domains. Domain enrichment was estimated via Fisher’s exact test on domain ratios, colors represent z-scores, and samples were clustered by Euclidean distance. Species names providing reference genomes are abbreviated as per the following: Symbiodinium kawagutii (Skav), Symbiodinium minutum (Smin), Symbiodinium microadriaticum (Smic), Emiliania huxleyi (Ehux), Thalassiosira pseudonana (Tpseu), Chlamydomonas reinhardtii (Cre), Guillardia theta (Gthe), Capsaspora owczarzaki (Cow), Trypanosoma brucei gambiense (Tgb), Tetrahymena thermophila (Ttherm), Plasmodium falciparum (Pfl), Arabidopsis thaliana (Ath), Amphimedon queenslandica (Aqu), Stylophora pistillata (Spis), Trichoplax adhaerens (Tad), Lottia gigantea (Lgi), Daphnia pulex (Dpu), Caenorhabditis elegans (Cel), Homo sapiens (Hsa). Species names providing reference transcriptomes are abbreviated as per the following: Symbiodinium microadriaticum strain Kb8 (SmicKb8), Symbiodinium minutum strain Mf1.05b (SminMf1.05b), Symbiodinium kawagutii (Skaw.TS), Karenia brevis strain SP1 (KbrevSP1), Lingulodinium polyedrum (Lingu), Prorocentrum minimum (Proro), Amphidinium carterae (Amp), and Crypthecodinium cohnii (Crypt). A complete list of pfam domains and pfam annotations for all analyses are accessible in the supplement (Dataset S1.2, Dataset S1.3, Dataset S.14, Supplemental Information, Table S5).
Functional gene content of Symbiodinium relative to other dinoflagellates
Due to the absence of other dinoflagellate genomes, we could not rule out whether the observed enrichment of transmembrane transport and other domains is specific to the genus Symbiodinium or a general trait of dinoflagellates (see above). Therefore, we conducted an enrichment analysis using transcriptomic data of the dinoflagellates Karenia brevis26, Lingulodinium polyedrum27, Amphidinium carterae28, Crypthecodinium cohnii28, and Prorocentrum minimum28, and compared these to available transcriptomes from S. microadriaticum (strain KB8)14, S. minutum (strain Mf1.05b)14, and S. kawagutii11 (Supplemental Information, Supplemental Analysis, Fig. S5, Dataset S1.3). This analysis identified 61 protein domains (FDR < 0.001) enriched in at least one of the Symbiodinium species (Fig. 2B, Supplemental Information, Table S5), of which 40 were shared with the previous comparison to (non-dinoflagellate) eukaryotes. We designated these 40 domains as Symbiodinium-specific enriched domains (Supplemental Information, Table S5). The enriched domains confirmed that bicarbonate transporters (PF00955 HCO3_cotransp), carbonic anhydrases (PF00484 Pro_CA), and ammonium transporters (PF00909 Ammonium_transp) are specifically enriched in Symbiodinium when compared to other eukaryotes, including dinoflagellates. We could also confirm that Ankyrin domains (Ank), regulator of chromatin condensation (RCC1) repeat domains, and Methyltransferase domains are specifically enriched in Symbiodinium10,11,16,29, among others (Supplemental Information, Table S5). Importantly, the 40 domains did not contain any of the transmembrane transporters (i.e., lipid, glycerol, phosphate, ion, sulfate, ABC transporters, etc.) that were previously reported to be specifically enriched in Symbiodinium11 with the exception of an amino acid transporter domain (PF01490 Aa_trans) that was highly prevalent in S. minutum and S. kawagutii, but not S. microadriaticum. Further, we did not find enrichment of protein domains associated with oxidative stress in Symbiodinium (e.g., HSP70, HSP90, DnaJ, SOD, APx, catalase, thioredoxin, etc.)14,16,29,30,31,32, suggesting that these domains are a common feature for many dinoflagellates11.
Functional gene content differences between Symbiodinium species
To increase our understanding regarding Symbiodinium species differences, we compared relative domain abundances between S. microadriaticum, S. minutum, and S. kawagutii. This analysis revealed 81 protein domains (FDR < 0.01) with significant differences across the three species (Fig. 2C, Dataset S1.4). Interestingly, we found several transporter domains to be significantly differentially abundant in the three species including bicarbonate and amino acid transporters as well as the more general ABC and ion transporters. Although we did not find specific enrichment of stress related protein domains between Symbiodinium and other dinoflagellates (see above), the number of the stress-associated chaperone domain DnaJ (PF00226 DnaJ) differed significantly between Symbiodinium species (Fig. 2C, Dataset S1.4).
Symbiodinium-specific differences in genes involved in carbon acquisition
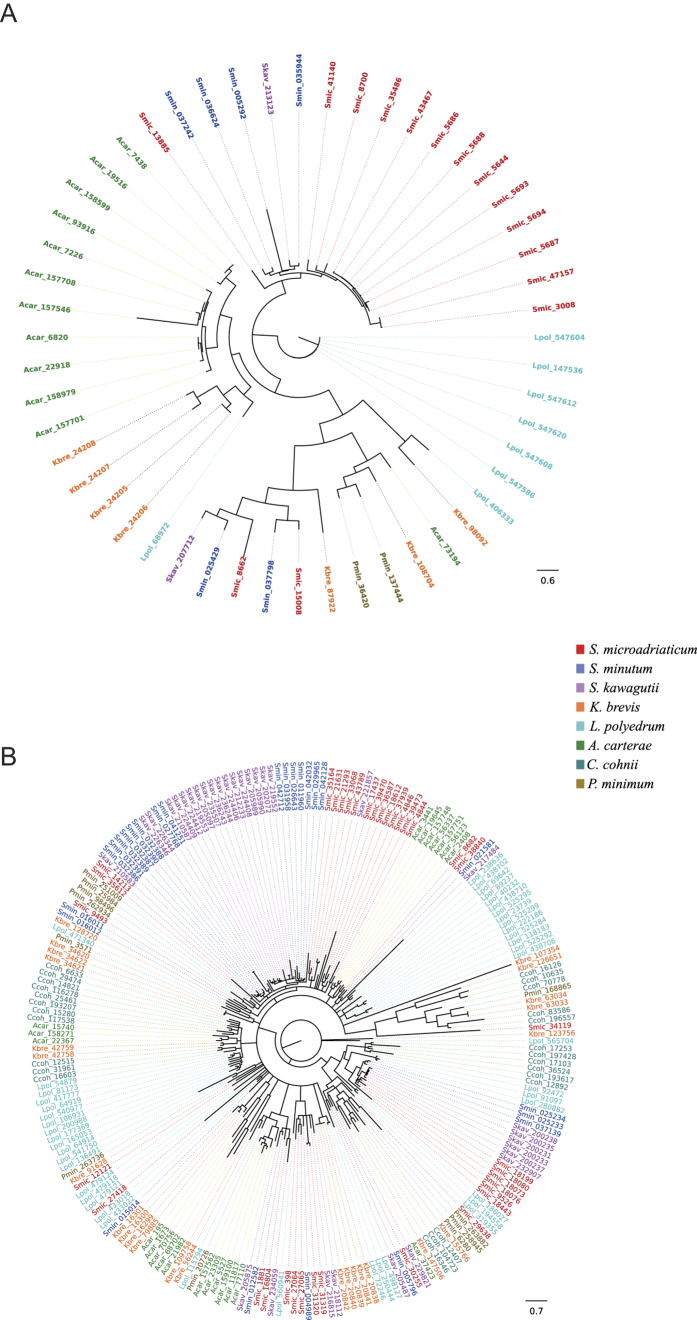
The functional importance of nutrient exchange for the host-algal symbiosis has been broadly shown11,33,34,35. In our enrichment analyses, bicarbonate transporters and carbonic anhydrases were among the Symbiodinium-specific enriched domains (Supplemental Information, Table S5). The proteins containing these domains form integral parts of carbon-concentrating mechanisms (CCMs) that play an important role in the acquisition of inorganic carbon for photosynthesis in many algae36,37,38,39. We found bicarbonate transporter domains (PF00955 HCO3 cotransp) to be highly enriched in Symbiodinium microadriaticum, but significantly less so in S. minutum and S. kawagutii (52 vs. 15 vs. 4, Dataset S.1.2), indicating pronounced differences between the three Symbiodinium species (Dataset S1.4). To further understand species differences, we conducted a phylogenetic analysis of bicarbonate transporters (Fig. 3A, Dataset 1.5, Supplemental Information, File S1). This analysis showed that genes from S. microadriaticum, S. minutum, and S. kawagutii generally clustered together and apart from other dinoflagellate sequences, supporting the phylogenetic relatedness of these species. Interestingly, we observed that bicarbonate transporter genes tended to cluster by species, implicating that several of these genes arose through clade- or species-specific gene duplications (Fig. 3A). We further corroborated this result by phylogenetic analysis of bicarbonate transporters from species in Symbiodinium from Clades A, B, C, and D using transcriptome data from16, where we found that transcripts also tended to cluster by species (Supplemental Information, Fig. S6A). Interestingly, analysis of transcriptomic data derived from Symbiodinium microadriaticum (Clade A1) subjected to nine different experimental treatments (4 °C 4hs, 16 °C 4hs, 34 °C 12hs, 36 °C 4hs, 20 g/L NaCl 4hs, 60g/L NaCl 4hs, dark cycle, dark stress, light cycle)17 showed that the bicarbonate transporters encoded by two genomic genes (Smic15008 and Smic8700) were differentially expressed in response to coral bleaching relevant stressors cold (4 °C) and heat stress (34 °C and 36 °C). In line with the enrichment of bicarbonate transporters, we also found carbonic anhydrase domains (PF00484 Pro_CA) to be overrepresented in all three Symbiodinium species. In contrast to the bicarbonate transporters, however, we found no significant differences in the relative representation of these domains in any of the three Symbiodinium species (Fig. 2C).
Figure 3.
Maximum-likelihood trees (1,000 bootstraps) for (A) bicarbonate transporters (PF00955 HCO3_cotransp) and (B) ammonium transporters (PF00909 Ammonium_transp), present in the genomes of Symbiodinium microadriaticum, Symbiodinium minutum, Symbiodinium kawagutii and the transcriptomes of Karenia brevis, Lingulodinium polyedrum, Amphidinium carterae, Crypthecodinium cohnii, and Prorocentrum minimum. Phylogenetic grouping of bicarbonate and ammonium transporters by species indicates lineage specific duplications in all dinoflagellate species. Only genes and transcripts with transporter domains with e-values > 10−15 and lengths above 150 amino acids were selected for the analysis. Species-specific gene duplications are colored according to species and edges with bootstrap support below 50 are collapsed. Files for phylogenetic tress are provided in the supplement (Supplemental Information, File S1 and File S2).
Symbiodinium-specific differences in genes involved in nitrogen acquisition
Nitrogen is a limiting factor for growth in the oligotrophic environment of tropical seas. Accordingly, many marine organisms acquired the ability to assimilate nitrogen from inorganic sources40,41. In line with that we found ammonium transporters (PF00909 Ammonium_transp) among the Symbiodinium-specific enriched domains (Supplemental Information, Table S5) and highly enriched in all Symbiodinium species. We compared the amount of ammonium transporter domains between Symbiodinium and the genomes of three symbiotic cnidarians, i.e. the scleractinian corals Stylophora pistillata42,43 and Acropora digitifera44 as well as the symbiotic anemone Aiptasia45 (Dataset S1.2). While the cnidarian genomes appear to encode for 18, 7, and 15 ammonium transporter domains respectively, we identified 68, 42, and 46 such domains in the genomes of S. microadriaticum, S. minutum, and S. kawagutii. Phylogenetic analysis of these transporters provided a similar result as for the bicarbonate transporters showing strong clustering of sequences by Symbiodinium species, indicating substantial intra-cladal or even lineage- or species-specific duplications (Fig. 3B, Dataset S1.6, Supplemental Information, File S2). As with the analysis of bicarbonate transporters, Symbiodinium-specific differences in genes involved in nitrogen acquisition could be corroborated by phylogenetic analysis of bicarbonate transporters of species across clades A, B, C, and D using transcriptome data from ref. 16, where it was found that genes clustered by species indicating species-/clade-specific duplication (Supplemental Information, Fig. S6B). Similar to the expression analysis of bicarbonate transporters, we found several ammonium transporters (Smic33068, Smic43789, Smic8682, and Smic37939) to be differentially expressed in response to heat stress (36 °C), but not in any of the other experimental treatments17.
Discussion
A genomic understanding of dinoflagellates has long been elusive due to their large genomes and unusual genome structure10. Our comparative genome and transcriptome analyses incorporating Symbiodinium and dinoflagellate sequence data allowed us to differentiate Symbiodinium-specific and general dinoflagellate-specific traits in order to further understand and pinpoint features that explain the success of dinoflagellates in general and the endosymbiosis lifestyle of Symbiodinium in particular. Of equal importance, we identified pronounced genomic differences between Symbiodinium species that may hold implications for knowledge on their different physiologies and ecologies.
Genome organization
The analysis of the previously published S. minutum and S. kawagutii genomes highlighted several genomic particularities representing presumably Symbiodinium-specific traits. Our comparative analyses confirmed that this is the case for the high abundance of non-canonical splice sites, but it does not necessarily apply for the exceptionally strong tendency towards unidirectionality of gene arrangements in S. minutum for instance. The difference in gene orientation frequency is in line with our overall finding of high genomic dissimilarity, despite a seemingly considerable genic similarity. Although neither of the genome assemblies encompass the entire estimated genome sizes, they showed similar completeness based on the CEG analysis. However, the genome composition analysis highlighted that substantially more S. minutum genes had their best match to the phylogenetically more distant S. microadriaticum, rather than S. kawagutii (70% vs. 24%), while most S. kawagutii genes had their best match to S. minutum (64%). The stark discrepancy of the overall number of gene matches between the S. minutum and S. kawagutii gene sets might indicate accelerated evolution in the S. kawagutii lineage. This is further supported by the observed differences in synteny conservation between the three species representing three clades (Supplemental Information, Table S3) that show that S. minutum is more similar to S. microadriaticum than to S. kawagutii. However, we cannot fully exclude that other factors such as the lower number of genes found in S. kawagutii or differences in genome assembly and gene prediction methods might have also contributed to the patterns observed here.
Enrichment of domains and transporters conducive to an endosymbiotic lifestyle
Our enrichment analysis identified several common domains as well as important differences between Symbiodinium and other dinoflagellates. Arguably, the comparison between Symbiodinium and other dinoflagellates is important if we are to understand which traits potentially represent adaptations conducive to an endosymbiotic lifestyle by means of enriched protein domain analyses. Unfortunately, this analysis is currently limited to the use of transcriptomic data for comparative analyses between dinoflagellates due to the lack of non-Symbiodinium dinoflagellate genomes. It should be noted that using transcriptomic data is not free of caveats and can only provide an approximation, especially considering the high gene duplication rates that potentially impair the assembly of transcripts originating from recently duplicated genes. Resolving such transcripts is likely further affected by our clustering approach, which might combine transcripts originating from different genes into the same locus, thus underestimating the true number of gene copies present in the genome. However, by comparing transcriptomes from sequenced Symbiodinium genomes to other dinoflagellate transcriptomes, we tried to minimize this bias, at least to the extent that we can assess the margin of error. This is highlighted in the correlation analyses of the Symbiodinium genomes and respective transcriptomes, which showed strong and significant, but far from perfect correlations (Supplemental Information, Fig. S5).
In line with previous reports11, we found an unexpected prevalence of transmembrane transporters involved in the translocation of diverse nutrients and ions in Symbiodinium. However, fine-scale comparative analysis with multiple dinoflagellate species revealed that this feature seems to be shared across all dinoflagellates, and hence, does not represent a specific adaptation of Symbiodinium to an endosymbiotic lifestyle as previously suggested11. Accordingly, our findings argue that dinoflagellates in general harbor genomic traits that are conducive to the evolution of symbiotic lifestyles (reviewed in ref. 46). At the same time, our enrichment analysis revealed a surprisingly rich repertoire of Symbiodinium-specific transporter expansions, i.e. bicarbonate- and ammonium-related domains. These transporters are likely to play a fundamental role in symbiotic relationships as they represent key elements for the production of photosynthates, their exchange, and organismal growth.
Inorganic carbon is a key limiting factor of photosynthesis47 and its constant provision in the hyperoxic environment of illuminated coral tissues is highly important for photosynthesis and protection from photodamage48,49. It is therefore not unexpected to find bicarbonate transporters and carbonic anhydrases to be enriched in Symbiodinium. Interestingly though, we found significant differences in the amount of bicarbonate transporters between the three Symbiodinium species analyzed. The phylogenetic analysis of these transporters further suggests that many of these genes arose through independent gene duplications in the respective species (lineages), rather than gene loss.
Significant differences in the capacities of coral host and Symbiodinium to assimilate ammonium were shown in a study by ref. 40. The authors suggested different scenarios as an explanation including the possibility of higher ammonium transporter activity in the symbiont. Our results strongly suggest that the observed differences in ammonium uptake could be caused by the significantly higher number of ammonium transporters present in Symbiodinium. We hypothesize that the increased ability of Symbiodinium in transporting ammonium allows the symbiont to act as an “ammonium sink” within coral host cells, thereby increasing the nitrogen efficiency of the holobiont by creating a strong ammonium gradient that facilitates diffusion of ammonium from the seawater into the host tissue as previously proposed50. It is tempting to assume that this could have evolved as a way to counteract nitrogen limitation by the host (“selfish symbiont”), while ultimately becoming central to the nitrogen efficiency of the holobiont and its increased ability to acquire nitrogen from the environment. Indeed, it has been observed that increases in available ammonium lead to surges in Symbiodinium cell densities within the host tissue51,52,53. Interestingly, it has also been proposed that such nitrogen-induced increases in Symbiodinium cell densities might lead to potential carbon and phosphate limitation due to the increased demand of the growing symbiont population. This, in turn, has been speculated to result in photoinhibition and consequently bleaching of the coral host49,53,54.
What follows is that the observed differences in the number of encoded bicarbonate, ammonium, and other domains might provide a genomic basis to explain species-specific physiological traits that affect symbiosis and host range of different Symbiodinium. Furthermore, these traits might also contribute to the susceptibility of certain host-symbiont combinations to environmental stressors such as temperature and eutrophication.
Pervasive gene duplication in dinoflagellates
The phylogenetic analyses of bicarbonate and ammonium transporters in Symbiodinium and other dinoflagellates (K. brevis, L. polyedrum, A. carterae, C. cohnii, and P. minimum) indicate that these transporters have been duplicated in Symbiodinium in a species-specific manner, with many copies sharing high amino acid similarity within a species. This is in line with previous findings in L. polyedrum and C. cohnii that report on high sequence conservation of genes present in tandem arrays27,55. The high levels of amino acid sequence conservation of bicarbonate and ammonium transporters as well as tandem array genes implicates functional similarity of duplicated gene copies. This raises interesting possibilities if considered in a broader context such as the paucity of transcription factors10,14, a canonical promoter structure2, and steady-state gene expression profiles in response to environmental changes5,6,17,18, but see ref. 56. It has previously been proposed that transcriptional regulation of at least some dinoflagellate genes, such as tandemly arrayed genes, might be controlled through chromatin structure57. Following this line of thought, gene duplication could be a suitable mechanism to increase transcript and consequently protein levels of certain genes. Such a model of transcriptional regulation would also provide an explanation for the observation that transcriptional differences between different Symbiodinium clades appear to be fixed regardless of the environmental condition18.
Conclusions
Our comparative analyses of three Symbiodinium species revealed several genomic features that define this genus and are likely to contribute to their success as widespread endosymbionts. Our results corroborate the prevalence of non-canonical splice-sites and a tendency towards unidirectionality of gene orientation in dinoflagellates. We identified a surprisingly large repertoire of proteins involved in molecule transfer in dinoflagellates, but also highlight enrichment of domains involved in the transport of carbon and nitrogen in the Symbiodinium lineage. We also find evidence for substantial differences of these domains between Symbiodinium species, which may provide a genomic basis to explain physiological differences and contribute to host-symbiont specificity. The large amount of intraspecific gene duplications in Symbiodinium putatively represents an alternate mechanism to increase transcript and protein levels in the absence of strong transcriptional control of gene expression.
Methods
Organism and isolation of genomic DNA
S. microadriaticum (CCMP2467) cells were obtained from Bigelow National Center for Marine Algae and Microbiota (NCMA) and spread on an f/2 agar plate enriched with antibiotics as described by ref. 58. Six separate colonies were picked and typed using the ITS2 primer pair described in ref. 59. All six colonies were identified as S. microadriaticum strain CCMP2467 and a single colony was selected as the source for all subsequent experiments. In order to minimize contamination, cultures were frequently treated with a mix of 10 antibiotics60. For DNA extractions, Symbiodinium cells were grown in f/2 medium without silica at 26 °C and 80 μmol quanta m−2 s−1 and harvested in the exponential growth phase (<5 × 105 cells/ml). Briefly, cells were pelleted by centrifugation for 5 min at 3,000 g in a swinging bucket rotor. The cell pellet was washed twice by resuspension in MilliQ water and repeated centrifugation. Subsequently, the cell pellet was snap frozen in liquid nitrogen and transferred to a pre-chilled mortar. Approximately 100 μl of 0.1 mm silica beads were added as grinding aid. The cell pellet was ground to a fine powder. The homogenized cells and beads were transferred to a 50 ml tube and subjected to an RNase treatment for 30 min at 60 °C, followed by a Proteinase K treatment (4 hs at 37 °C) and subsequently extracted using two successive rounds of chloroform extraction, precipitation, and re-extraction with CTAB. The DNA was subjected to two rounds of CTAB extraction to reduce the amount of unwanted polysaccharides in the extraction. The extracted DNA was quantified using a Nanodrop-2000 (Thermo Scientific) and a Qubit (Invitrogen). A 0.8% agarose gel was used to quantify RNA/polysaccharide contamination and to verify that the DNA was of high molecular weight.
Estimation of genome size
For an estimation of the S. microadriaticum genome size, we measured its nuclear DNA content using fluorescence-activated cell sorting (FACS) and the small sea anemone Aiptasia as an internal control of known genome size (2C DNA content: 0.53 pg or ~520 Mbp45). Nuclei extraction and staining for S. microadriaticum and the internal control were performed using the Partec CyStainPI absolute T kit (Partec #05-5023) following the manufacturer’s protocol and the fluorescence signals were measured with a BD FACSCanto II cell analyzer (BD Bioscience) (Supplemental Information, Fig. S1). The reported measurement for S. microadriaticum reflects the 1C genome content as Symbiodinium is reported to be haploid in culture61.
Genome sequencing
A total of 280 Gb sequence data were generated from the 15 sequence libraries with different fragment sizes (8 short insert size libraries ranging from 200 bp to 1,375 bp, 6 long insert size libraries ranging from 3 kb to 10 kb, 1 fosmid library with ~40 kb insert size) (Supplemental Information, Table S6). All sequence libraries were produced using the Illumina TruSeq DNA kits for paired-end or mate-pair libraries according to the manufacturer’s instructions and sequenced on the Illumina HiSeq platform at the KAUST Bioscience Core Facility (KAUST, Thuwal, KSA) (Supplemental Information, Table S6). MP02 and MP03 were produced and sequenced by GATC-Biotech (Konstanz, Germany). An additional fosmid library was generated by Lucigen (Middleton, WI, USA) and a corresponding mate-pair sequencing library based on 255,000 clones was produced and sequenced on 1 lane of the Illumina HiSeq platform at the KAUST Bioscience Core Facility (KAUST, Thuwal, KSA). All raw sequences have been deposited in the NCBI Sequence Read Archive (SRA) as FASTQ and are accessible under the accession number PRJNA292355 (genomic reads).
Genome assembly
Reads with more than 5% ambiguous bases (represented by the letter N) or polyA structure contents were discarded. Furthermore, we removed reads with 60% low-quality bases (base quality <5) for the short insert-size libraries and reads with 30% low-quality bases for the long insert-size libraries. Low-quality ends were trimmed directly. This resulted in 2,728,199,233 filtered raw reads providing ~170x coverage that were used for the assembly process. For the assembly, filtered raw reads were pre-assembled using SOAPdenovo2 (Version r240)62 and filtered raw reads were mapped against the pre-assembly to determine library insert sizes. For the final assembly, filtered raw reads were first assembled using ALLPATHS-LG (release 47998)63 with the default options. The assembly was subjected to two consecutive runs of gap closing using GapCloser (version 1.12-r6)62. This gap-closed genome was scaffolded using SSPACE (version 1.2) and the sequencing data from a 40 kb fosmid library based on 255,000 clones. The resulting scaffolds were further scaffolded using L_RNA_Scaffolder64 and transcriptome data from ref. 17. The resulting assembly was subjected to two further iterations of gap closing using GapCloser. Assembly statistics were calculated as defined in ref. 65. The S. microadriaticum genome is accessible at http://smic.reefgenomics.org66 and at NCBI under the accession number PRJNA292355. The genome assembly, gene models, and protein models described in this study are available for download at http://smic.reefgenomics.org/download. A JBrowse genome browser is available at http://smic.reefgenomics.org/jbrowse.
Identification and removal of contaminating sequences
In order to remove contaminating sequences that were likely to be of bacterial or viral origin, we conducted BLASTN searches against four databases: complete bacterial genomes (ftp://ftp.ncbi.nih.gov/genomes/Bacteria/all.fna.tar.gz), draft bacterial genomes (ftp://ftp.ncbi.nih.gov/genomes/Bacteria_DRAFT/), complete viral genomes (ftp://ftp.ncbi.nih.gov/genomes/Viruses/all.fna.tar.gz) databases from NCBI, and the viral database PhAnToMe (http://phantome.org). As the lengths of the query and hit sequences were up to hundreds of Kb, we used a combination of cutoffs (total bit score >1000, e-value ≤ 10−20) to identify scaffolds with significant similarities to sequences in the databases. In total, 1,851 scaffolds – with a median length of 1,628 bp and a combined length of 3.84 Mbp – had over 50% of their non-N sequences being significantly similar to bacterial or viral sequences. These scaffolds were subsequently removed from the final assembly (Dataset S1.7).
Annotation of repetitive elements
We used RepeatScout67 for de novo annotation of repetitive elements in the genome assembly using an l-mer size of 16 bp. Using the default settings, a total of 5,622 distinct repeat motifs were identified that occurred ≥10 times. Both, the de novo annotated repeat motifs identified by RepeatScout67 and a set of known eukaryotic TEs from RepBase (May 2014 release) were then used to locate and annotate the repetitive elements in the assembled S. microadriaticum genome using RepeatMasker68 (Supplemental Information, Table S7).
Reference transcriptome sequencing and assembly
S. microadriaticum cultures in exponential growth phase were subjected to four different treatments including 4 hs at 36 °C, 4 hs at 16 °C, dark stress (maintained in darkness and harvested at noon), and regular culture conditions (harvested at noon). All treatments were performed in triplicates with the exception of 36 °C, which was performed in duplicate. Cells were harvested as described for the genomic DNA extraction, however, homogenated cells were transferred to microtubes and RNA extracted using the Qiagen Plant RNeasy kit according to the manufacturer’s instructions. RNA samples were quantified using a Qubit (Invitrogen, Carlsbad, CA, USA) and RNA quality was confirmed using a Bioanalyzer RNA NanoChip (Agilent, Santa Clara, CA, USA). Strand-specific RNAseq sequencing libraries were constructed using the NEBNext Ultra Directional RNA Library Prep Kit for Illumina (New England Biolabs, Ipswitch, MA, USA). A total of 3 lanes were sequenced on the Illumina HiSeq platform resulting in 757 million reads. All libraries were trimmed using Trimmomatic41 version 0.32 to remove adapters, primers, and low quality ends (Phred score < 30) from reads; reads shorter than 35 bp were removed. PhiX reads were removed using Bowtie228, possible PCR duplicates were removed with PRINSEQ-lite25 version 0.20.3, then all libraries were merged and error correction was carried out using ErrorCorrectReads.pl (from ALLPATHS-LG). The resulting library was assembled de novo using Trinity (release 20140413)69 with strand-specific parameters (--SS_lib_type RF –min_kmer_cov 5 –normalize_reads). The transcriptome reference assembly contains 58,592 transcripts representing 41,679 putative loci (http://smic.reefgenomics.org/download).
Gene models
Gene models in the S. microadriaticum genome were built using the transcriptome assembly and ab initio prediction based on selected and refined gene structure models that were annotated with PASA70. First, all transcripts of the reference transcriptome (n = 58,592 transcripts, representing 41,679 distinct gene loci) were mapped to the genome assembly using PASA, yielding 22,827 annotated full-length gene structure models. In order to account for putative non-canonical splice sites in the gene structure models, we modified the source code of PASA (http://smic.reefgenomics.org/download). From the set of 22,827 gene structure models, we applied several stringency criteria to filter and obtain a bona fide set of gene structure models that could be used as a training gene set for ab initio gene prediction using AUGUSTUS (version 3.0.2)71,72,73. The subsequently applied filter steps were: (1) removal of incomplete genes without start and stop codon; (2) removal of genes with less than 3 exons; (3) removal of genes with ambiguous 5′ or 3′ untranslated regions (UTRs); (4) removal of redundant protein sequences in protein clusters as indicated by BLASTP (e-value < 10−10), only the longest one was retained in every protein cluster; (5) removal of genes with repeat sequences as indicated by BLASTN alignments to a repeat library generated by RepeatScout (see above). The final set of bona fide gene structure models contained 2,957 gene models on which AUGUSTUS was trained in order to predict genes models in the genome assembly using the default training pipeline. To improve the prediction accuracy, we also generated “hints” as supplementary evidence of gene presence and location by mapping all transcripts of the reference transcriptome to the genome assembly using BLAT and the respective scripts provided in AUGUSTUS. For the final ab initio gene prediction, the source code of AUGUSTUS was also slightly modified to account for non-canonical exon-intron boundaries (http://smic.reefgenomics.org/download). Finally, the set of ab initio genomic gene models was further refined using PASA as described in ref. 45.
Genome completeness analysis
Genome completeness analyses for all three Symbiodinium genomes were initially performed using the CEGMA software24, which analyzes the genome to determine the presence of 248 highly conserved eukaryotic genes (CEGs), and resulted in low completeness (<50%). Due to the old evolutionary origin of dinoflagellates that likely predates the split of the three multicellular kingdoms (~1.5–1.9 billion years)74,75,76,77, we downloaded the complete list of 458 CEGs used by CEGMA and performed a TBLASTX search against our genomes to identify potential homologs that did not pass the rigorous criteria used by CEGMA. Using a minimum cutoff value of 1e-5, we identified 437 (95.4%), 434 (94.8%), and 383 (83.6%) homologs, of which 9 were only found in S. microadriaticum, 6 only in S. minutum, and 2 only in S. kawagutii (Dataset S1.1).
Gene orientation analysis
Gene orientation in S. microadriaticum was analyzed using a 10-gene window and a 10-gene step. The numbers of orientation changes between every two adjacent genes were counted in every window. For comparative purposes, we performed this analysis on the previously published S. minutum and S. kawagutii genomes as well as on the genomes of Plasmodium falciparum, Trypanosoma brucei, Tetrahymena thermophila, Arabidopsis thaliana, and Homo sapiens. A χ2 test was performed to compare the degree of unidirectionality between S. microadriaticum, S. minutum, S. kawagutii, and T. brucei, the latter two showed significantly stronger unidirectionality than S. microadriaticum and S. kawagutii.
Symbiodinium genome composition
In order to determine the putative origins of Symbiodinium genes, we performed BLASTP searches of gene models from S. microadriaticum, S. minutum, and S. kawagutii against the NCBI nr database (June 2014). We selected for all hits with e-value ≤ 10−5 and then extracted the species that had had the best hit against the Symbiodinium gene models. Full taxonomic hierarchy for these organisms were obtained via a Python script that queried Encyclopedia of Life (http://eol.org)78 and parsed the resulting JSON reply from the server. Based on the resulting hierarchies, we tallied the number of best hits into seven kingdom/subkingdom-level organismal groups: Archaea, Bacteria, Fungi, Metazoa, Plantae, Protista, and Viruses. For the analysis that allowed for intra-genus matches, we performed pairwise BLASTP searches of S. microadriaticum, S. minutum, and S. kawagutii gene models. For each gene model, if the search produced a higher bitscore (bitscores were used instead of e-values as they are independent of database size) than the corresponding best hit against nr, the best match for that gene model was changed to that of Symbiodinium. Chord diagrams were drawn using Circos79.
Functional gene annotation and protein domain enrichment analyses
The final set of predicted proteins was annotated against UniProt (i.e., SwissProt and TrEMBL) and the NCBI nr database to derive gene-based annotations (Dataset S1.8). GO terms were assigned to the gene models as described in ref. 42. Briefly, BLASTP searches of all genomic protein models were successively carried out against SwissProt and TrEMBL databases (June 2014 release). GO terms associated with SwissProt and TrEMBL hits were subsequently obtained from UniProt-GOA (July 2014 release)80. If the best-scoring hit of the BLASTP search did not yield any GO annotation, further hits (up to 20 hits, e-value ≤ 10−5) were considered, and the best-scoring hit with available GO annotation was used. If none of the SwissProt hits had GO terms associated with them, the TrEMBL database was queried using the same approach. Using this procedure, 22,340 genes (45.5% of the 49,109 gene models) were annotated and had at least one GO term associated with them. Of these, 17,275 had GO annotations via SwissProt, while the remaining 5,065 were retrieved from TrEMBL. In total, 15,989 genes featured e-values < 10−10, and 9,871 genes had e-values < 10−20, indicating that the majority of the annotations were based on high-confidence alignments to the SwissProt and TrEMBL databases. Proteins that had no matches to either database were subjected to an additional search against the NCBI nr database (e-value ≤ 10−5). An additional 2,270 proteins were annotated this way. 24,499 proteins (49.9%) had no hits to any of the three databases – the large fraction of unannotated genes is most likely due to the dearth of dinoflagellate genes in any of these three databases. This procedure was repeated for the S. minutum and S. kawagutii gene models to eliminate potential biases stemming from the use of different annotation pipelines (Dataset S1.9 and Dataset S1.10).
In order to identify enrichments of protein domains and associated molecular functions encoded in the genomic protein sets of S. microadriaticum, S. minutum, and S. kawagutii, we also annotated the genomic proteins sets of 16 eukaryote reference genomes including Plasmodium falciparum, Tetrahymena thermophila, Guillardia theta, Emiliania huxleyi, Thalassiosira pseudonana, Trypanosoma brucei gambiense, Chlamydomonas reinhardtii, and Capsaspora owczarzaki as well as the higher eukaryotes Arabidopsis thaliana, Trichoplax adhaerens, Amphimedon queenslandica, Stylophora pistillata, Lottia gigantea, Caenorhabditis elegans, Daphnia pulex, and Homo sapiens (Supplemental Information, Table S4) against the Pfam database (version 27) using HMMER81 (version 3.1b1) (Dataset S1.2). Domain counts were normalized to the total domain count in each species and expanded protein domain families in S. microadriaticum, S. minutum, and S. kawagutii were identified with a Fisher’s exact test comparing in-group counts (i.e. S. microadriaticum, S. minutum, or S. kawagutii) to average counts in the outgroups (all other species). Obtained p-values were corrected with the Benjamini-Hochberg FDR correction82, and z-scores were calculated according to the formula z = (x − u)/s, whereby ‘x’ is the domain ratio, ‘u’ the mean of all domain ratios over all species, and ‘s’ the standard deviation across domain ratios over all species considered. The z-scores from the domain enirchment analyses were visualized using R’s heatmap.2 function (from package gplots)83.
We repeated the same analysis using transcriptome data available for S. microadriaticum strain KB814, S. minutum Mf1.05b14, S. kawagutii11, and the dinoflagellates K. brevis26, L. polyedrum27, Amphidinium carterae28, Crypthecodinium cohnii28, and Prorocentrum minimum28 (Dataset S1.3) in order to determine whether the above identified domains were generally enriched in dinoflagellates or unique to Symbiodinium. To remove putative isoforms that would artificially inflate the number of domains, we first clustered the transcripts using CD-HIT-EST84. For each transcriptome we clustered the transcripts at 90% nucleotide identity resulting in 58,707 transcripts for S. microadriaticum KB8, 52,240 for S. minutum Mf1.05b, 47,281 for S. kawagutii, 83,135 for K. brevis SP1, 116,534 for L. polyedrum, 29,340 for Amphidinium carterae, 33,155 for Crypthecodinium cohnii, and 52,466 for Prorocentrum minimum. For the within-Symbiodinium comparison of enriched protein domains and associated molecular functions, we performed a Fisher’s exact test on the domain annotated genomic protein sets of S. microadriaticum, S. minutum and S. kawagutii using a FDR cutoff of <0.01 (Dataset S1.4).
Phylogenetic analysis of bicarbonate and ammonium transporters
For the phylogenetic analysis of bicarbonate transporters (PF00955 HCO3_cotransp) and ammonium transporters (PF00909 Ammonium_transp), we extracted protein sequences from all putative genes encoded in the genomes of S. microadriaticum, S. minutum, and S. kawagutii. Extracted sequences were validated for their putative function by aligning them against a set of reference sequences from bacteria, protists, plants, and animals to verify the presence of conserved amino acid positions. In addition, we extracted homologous sequences from the transcriptomes of Symbiodinium species from Clade A, B, C, and D16 as well as from the dinoflagellates K. brevis SP1, L. polyedrum, Amphidinium carterae, Crypthecodinium cohnii, and Prorocentrum minimum. For phylogenetic reconstruction, we aligned the respective sequences using MUSCLE85 and trimmed the resulting alignments using trimAl v1.4.186, employing the -automated1 function optimized for maximum-likelihood phylogenetic trees. The best evolutionary model for each of the trimmed alignments was empirically tested using ProtTest387 – LG + G was the most suitable model for PF00955 HCO3_cotransp sequences. The model BLOSUM62 + G was found to perform best for sequences harboring PF00909 Ammonium_transp domain. The alignments were subsequently constructed using RAxML v8.2.088 with 1,000 bootstraps (-x 12345 -p 12345 -N 1000 -f a).
Availability of supporting data
The genome assembly, gene models, and protein models described in this study are available for download at http://smic.reefgenomics.org/download. A JBrowse genome browser is available at http://smic.reefgenomics.org/jbrowse. Customized PASA and AUGUSTUS scripts for gene calling are available at http://smic.reefgenomics.org/download. A BLAST server for the Symbiodinium microadriaticum genome is available at http://smic.reefgenomics.org/blast/. All data reported in the manuscript are deposited at NCBI under the accession number PRJNA292355.
Additional Information
How to cite this article: Aranda, M. et al. Genomes of coral dinoflagellate symbionts highlight evolutionary adaptations conducive to a symbiotic lifestyle. Sci. Rep. 6, 39734; doi: 10.1038/srep39734 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank Matt E. Oates and Julian Gough for protein set annotation to Superfamily, and Adrian C. Carr for preliminary genome assembly. Research reported in this publication was supported by the King Abdullah University of Science and Technology (KAUST) and by an AEA2 grant to V.B.B., G.M., T.R., and C.R.V.
Footnotes
Author Contributions M.A. and C.R.V. conceived and designed the research. M.A., Y.L., Y.J.L., S.B., C.R.V. conducted the experiments and O.S., M.W., J.P., H.A., S. Bou, V.B.B., H.K., and J.B. contributed new reagents or analytic tools. M.A., Y.L., Y.J.L., S.B., O.S., M.W., J.P., H.A., S. Bou., V.B.B., T. Ryu, T. Rav., T.B., G.M., H.K., J.B., T.C.L., and C.R.V. analyzed data. M.A., Y.L., Y.J.L., S.B., T.C.L., and C.R.V. wrote the manuscript.
References
- Taylor F. J. R. The biology of dinoflagellates. (1987).
- Lin S. Genomic understanding of dinoflagellates. Res. Microbiol. 162, 551–569, doi: 10.1016/j.resmic.2011.04.006 (2011). [DOI] [PubMed] [Google Scholar]
- Hackett J. D., Anderson D. M., Erdner D. L. & Bhattacharya D. Dinoflagellates: a remarkable evolutionary experiment. Am. J. Bot. 91, 1523–1534, doi: 10.3732/ajb.91.10.1523 (2004). [DOI] [PubMed] [Google Scholar]
- Wisecaver J. H., Brosnahan M. L. & Hackett J. D. Horizontal gene transfer is a significant driver of gene innovation in dinoflagellates. Genome Biol. Evol. 5, 2368–2381, doi: 10.1093/gbe/evt179 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa A. et al. Transcriptome profiling of a toxic dinoflagellate reveals a gene-rich protist and a potential impact on gene expression due to bacterial presence. PLoS One 5, e9688 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdner D. & Anderson D. Global transcriptional profiling of the toxic dinoflagellate Alexandrium fundyense using Massively Parallel Signature Sequencing. BMC Genomics 7, 88 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P., Esvelt K. M. & Church G. M. Cas9 as a versatile tool for engineering biology. Nat. Methods 10, 957–963, doi: 10.1038/nmeth.2649 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y. & Lin S. Distinct gene number-genome size relationships for eukaryotes and non-eukaryotes: gene content estimation for dinoflagellate genomes. PLoS One 4, e6978, doi: 10.1371/journal.pone.0006978 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaJeunesse T. C., Lambert G., Andersen R. A., Coffroth M. A. & Galbraith D. W. Symbiodinium (Pyrrhophyta) genome sizes (DNA content) are smallest among dinoflagellates. J. Phycol. 41, 880–886 (2005). [Google Scholar]
- Shoguchi E. et al. Draft assembly of the Symbiodinium minutum nuclear genome reveals dinoflagellate gene structure. Curr. Biol. 23, 1399–1408, doi: 10.1016/j.cub.2013.05.062 (2013). [DOI] [PubMed] [Google Scholar]
- Lin S. et al. The Symbiodinium kawagutii genome illuminates dinoflagellate gene expression and coral symbiosis. Science 350, 691–694, doi: 10.1126/science.aad0408 (2015). [DOI] [PubMed] [Google Scholar]
- Trench R. K. Microalgal-invertebrate symbioses: A review. Endocyt. Cell Res. 9, 135–175 (1993). [Google Scholar]
- Parkinson J. E. et al. Gene Expression Variation Resolves Species and Individual Strains among Coral-Associated Dinoflagellates within the Genus Symbiodinium. Genome Biology and Evolution 8, 665–680, doi: 10.1093/gbe/evw019 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer T. et al. Symbiodinium transcriptomes: genome insights into the dinoflagellate symbionts of reef-building corals. PLoS One 7, e35269, doi: 10.1371/journal.pone.0035269 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladner J. T., Barshis D. J. & Palumbi S. R. Protein evolution in two co-occurring types of Symbiodinium: an exploration into the genetic basis of thermal tolerance in Symbiodinium clade D. BMC Evol. Biol. 12, 217, doi: 10.1186/1471-2148-12-217 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosic N. et al. Unfolding the secrets of coral-algal symbiosis. ISME J 9, 844–856, doi: 10.1038/ismej.2014.182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten S. et al. Integrating microRNA and mRNA expression profiling in Symbiodinium microadriaticum, a dinoflagellate symbiont of reef-building corals. BMC Genomics 14, 704 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barshis, D. J., Ladner J. T., Oliver T. A. & Palumbi S. R. Lineage-specific transcriptional profiles of Symbiodinium spp. unaltered by heat stress in a coral host. Mol. Biol. Evol. 31, 1343–1352, doi: 10.1093/molbev/msu107 (2014). [DOI] [PubMed] [Google Scholar]
- Voolstra C. R. et al. Evolutionary analysis of orthologous cDNA sequences from cultured and symbiotic dinoflagellate symbionts of reef-building corals (Dinophyceae: Symbiodinium). Comparative biochemistry and physiology. Part D, Genomics & proteomics 4, 67–74, doi: 10.1016/j.cbd.2008.11.001 (2009). [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Jeong H. J., Kang N. S., Jang T. Y., Jang S. H. & Lajeunesse T. C. Symbiodinium tridacnidorum sp nov., a dinoflagellate common to Indo-Pacific giant clams, and a revised morphological description of Symbiodinium microadriaticum Freudenthal, emended Trench & Blank. European Journal of Phycology, 50(2), 155–172 (2015). [Google Scholar]
- Holland B. S., Dawson M. N., Crow G. L. & Hofmann D. K. Global phylogeography of Cassiopea (Scyphozoa: Rhizostomeae): molecular evidence for cryptic species and multiple invasions of the Hawaiian Islands. Mar. Biol. 145, 1119–1128, doi: 10.1007/s00227-004-1409-4 (2004). [DOI] [Google Scholar]
- Lajeunesse T. C., Parkinson J. E. & Reimer J. D. A genetics-based description of Symbiodinium minutum sp. nov. and S. psygmophilum sp. nov. (Dinophyceae), two dinoflagellates symbiotic with cnidaria. J. Phycol. 48, 1380–1391, doi: 10.1111/j.1529-8817.2012.01217.x (2012). [DOI] [PubMed] [Google Scholar]
- Pochon X., Montoya-Burgos J. I., Stadelmann B. & Pawlowski J. Molecular phylogeny, evolutionary rates, and divergence timing of the symbiotic dinoflagellate genus Symbiodinium. Mol Phylogenet Evol 38, 20–30, doi: 10.1016/j.ympev.2005.04.028 (2006). [DOI] [PubMed] [Google Scholar]
- Parra G., Bradnam K., Ning Z., Keane T. & Korf I. Assessing the gene space in draft genomes. Nucleic Acids Res. 37, 289–297, doi: 10.1093/nar/gkn916 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieder R. & Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 27, 863–864, doi: 10.1093/bioinformatics/btr026 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D., Pepper A. & Campbell L. De novo assembly and characterization of the transcriptome of the toxic dinoflagellate Karenia brevis. BMC Genomics 15, 888 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin M. et al. Dinoflagellate tandem array gene transcripts are highly conserved and not polycistronic. Proc. Natl. Acad. Sci. USA 109, 15793–15798, doi: 10.1073/pnas.1206683109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B. & Salzberg S. L. Fast gapped-read alignment with Bowtie 2. Nat Meth 9, 357–359, doi: 10.1038/nmeth.1923 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang T., Nelson W., Rodriguez J., Tolleter D. & Grossman A. R. Symbiodinium transcriptome and global responses of cells to immediate changes in light intensity when grown under autotrophic or mixotrophic conditions. The Plant Journal 82, 67–80, doi: 10.1111/tpj.12789 (2015). [DOI] [PubMed] [Google Scholar]
- Downs C. A. et al. Oxidative stress and seasonal coral bleaching. Free Radical Biol. Med. 33, 533–543 (2002). [DOI] [PubMed] [Google Scholar]
- Weis V. M. Cellular mechanisms of Cnidarian bleaching: stress causes the collapse of symbiosis. J. Exp. Biol. 211, 3059 (2008). [DOI] [PubMed] [Google Scholar]
- Rosic N. N., Pernice M., Dove S., Dunn S. & Hoegh-Guldberg O. Gene expression profiles of cytosolic heat shock proteins Hsp70 and Hsp90 from symbiotic dinoflagellates in response to thermal stress: possible implications for coral bleaching. Cell Stress Chaperon. 16, 69–80, doi: 10.1007/s12192-010-0222-x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy S. K., Allemand D. & Weis V. M. Cell Biology of Cnidarian-Dinoflagellate Symbiosis. Microbiol. Mol. Biol. Rev. 76, 229–261, doi: 10.1128/mmbr.05014-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E. & Weis V. M. Study of Cnidarian-Algal Symbiosis in the “Omics” Age. Biol. Bull. 223, 44–65 (2012). [DOI] [PubMed] [Google Scholar]
- Gordon B. R. & Leggat W. Symbiodinium—Invertebrate Symbioses and the Role of Metabolomics. Mar. Drugs 8, 2546–2568, doi: 10.3390/md8102546 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggat W., Badger M. R. & Yellowlees D. Evidence for an inorganic carbon-concentrating mechanism in the symbiotic dinoflagellate Symbiodinium sp. Plant Physiol. 121, 1247–1256 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano M., Beardall J. & Raven J. A. CO2 concentrating mechanisms in algae: Mechanisms, Environmental Modulation, and Evolution. Annu. Rev. Plant Biol. 56, 99–131, doi: 10.1146/annurev.arplant.56.032604.144052 (2005). [DOI] [PubMed] [Google Scholar]
- Nakajima K., Tanaka A. & Matsuda Y. SLC4 family transporters in a marine diatom directly pump bicarbonate from seawater. Proc. Natl. Acad. Sci. USA 110, 1767–1772, doi: 10.1073/pnas.1216234110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley C., Schmidt G. & Hopkinson B. Thermal responses of Symbiodinium photosynthetic carbon assimilation. Coral Reefs 33, 501–512, doi: 10.1007/s00338-014-1130-9 (2014). [DOI] [Google Scholar]
- Pernice M. et al. A single-cell view of ammonium assimilation in coral-dinoflagellate symbiosis. ISME J 6, 1314–1324, http://www.nature.com/ismej/journal/v6/n7/suppinfo/ismej2011196s1.html (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A. M., Lohse M. & Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120, doi: 10.1093/bioinformatics/btu170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Y. J. et al. Identification of microRNAs in the coral Stylophora pistillata. Plos One 9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D. et al. Comparative genomics explains the evolutionary success of reef-forming corals. eLife 5, e13288, doi: 10.7554/eLife.13288 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinzato C. et al. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 476, 320–323, http://www.nature.com/nature/journal/v476/n7360/abs/nature10249.html - supplementary-information (2011). [DOI] [PubMed] [Google Scholar]
- Baumgarten S. et al. The genome of Aiptasia, a sea anemone model for coral symbiosis. Proc. Natl. Acad. Sci. USA 112, 11893–11898, doi: 10.1073/pnas.1513318112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez F. A quantitative review of the lifestyle, habitat and trophic diversity of dinoflagellates (Dinoflagellata, Alveolata). Syst. Biodivers. 10, 267–275, doi: 10.1080/14772000.2012.721021 (2012). [DOI] [Google Scholar]
- Smith E. L. Limiting Factors in Photosynthesis: Light and Carbon Dioxide. J. Gen. Physiol. 22, 21–35 (1938). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. et al. Influence of Co2 Concentrating Mechanism on Photoinhibition in Synechococcus sp. PCC7942 (Cyanophyceae). Phycologia 47, 588–598, doi: 10.2216/07-44.1 (2008). [DOI] [Google Scholar]
- Wooldridge S. A. Breakdown of the coral-algae symbiosis: towards formalising a linkage between warm-water bleaching thresholds and the growth rate of the intracellular zooxanthellae. Biogeosciences 10, 1647–1658, doi: 10.5194/bg-10-1647-2013 (2013). [DOI] [Google Scholar]
- D’Elia C. F., Domotor S. L. & Webb K. L. Nutrient uptake kinetics of freshly isolated zooxanthellae. Mar. Biol. 75, 157–167, doi: 10.1007/BF00405998 (1983). [DOI] [Google Scholar]
- Muscatine L., Falkowski P. G., Dubinsky Z., Cook P. A. & McCloskey L. R. The Effect of External Nutrient Resources on the Population Dynamics of Zooxanthellae in a Reef Coral. “Proc. R. Soc. Lond., Ser. B: Biol. Sci.” 236, 311–324, doi: 10.1098/rspb.1989.0025 (1989). [DOI] [Google Scholar]
- Fabricius K. E. Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar. Pollut. Bull. 50, 125–146, doi: 10.1016/j.marpolbul.2004.11.028 (2005). [DOI] [PubMed] [Google Scholar]
- Wiedenmann J. et al. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nature Clim. Change 3, 160–164, http://www.nature.com/nclimate/journal/v3/n2/abs/nclimate1661.html - supplementary-information (2013). [Google Scholar]
- Rädecker N., Pogoreutz C., Voolstra C. R., Wiedenmann J. & Wild C. Nitrogen cycling in corals: the key to understanding holobiont functioning? Trends Microbiol. 23, 490–497, 10.1016/j.tim.2015.03.008 (2015). [DOI] [PubMed] [Google Scholar]
- Mendez G. S., Delwiche C. F., Apt K. E. & Lippmeier J. C. Dinoflagellate Gene Structure and Intron Splice Sites in a Genomic Tandem Array. J. Eukaryot. Microbiol. 62, 679–687, doi: 10.1111/jeu.12230 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. A. et al. Sex, Scavengers, and Chaperones: Transcriptome Secrets of Divergent Symbiodinium Thermal Tolerances. Molecular Biology and Evolution, doi: 10.1093/molbev/msw119 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisecaver J. H. & Hackett J. D. Dinoflagellate Genome Evolution. Annu. Rev. Microbiol. 65, 369–387, doi: 10.1146/annurev-micro-090110-102841 (2011). [DOI] [PubMed] [Google Scholar]
- Soffer N., Gibbs P. & Baker A. Practical applications of contaminant-free Symbiodinium cultures grown on solid media. Proc. 11th International Coral Reef Symposium, 159–163 (2008). [Google Scholar]
- LaJeunesse T. C. & Trench R. K. Biogeography of two species of Symbiodinium (Freudenthal) inhabiting the intertidal sea anemone Anthopleura elegantissima (Brandt). Biol. Bull. 199, 126–134 (2000). [DOI] [PubMed] [Google Scholar]
- Polne-Fuller M. A novel technique for preparation of axenic cultures of Symbiodinium (Pyrrophyta) through selective digestion by amoeba. J. Phycol. 27, 552–554, doi: 10.1111/j.0022-3646.1991.00552.x (1991). [DOI] [Google Scholar]
- Santos S. R. & Coffroth M. A. Molecular Genetic Evidence that Dinoflagellates Belonging to the Genus Symbiodinium Freudenthal Are Haploid. Bio. Bull. 204, 10–20 (2003). [DOI] [PubMed] [Google Scholar]
- Luo R. et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. GigaScience 1, 18 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnerre S. et al. High-quality draft assemblies of mammalian genomes from massively parallel sequence data. Proc. Natl. Acad. Sci. USA 108, 1513–1518, doi: 10.1073/pnas.1017351108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W. et al. L_RNA_scaffolder: scaffolding genomes with transcripts. BMC Genomics 14, 604 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradnam K. R. et al. Assemblathon 2: evaluating de novo methods of genome assembly in three vertebrate species. GigaScience 2, 10–10, doi: 10.1186/2047-217X-2-10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Y. J., Aranda M. & C. R. V. reefgenomics.org-a repository for marine genomics data. Database Accepted (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A. L., Jones N. C. & Pevzner P. A. De novo identification of repeat families in large genomes. Bioinformatics 21, i351–i358, doi: 10.1093/bioinformatics/bti1018 (2005). [DOI] [PubMed] [Google Scholar]
- Smit A. F. A., Hubley R. & Green P. J. RepeatMasker Open-3.0, http://www.repeatmasker.org (1996–2010).
- Haas B. J. et al. De novo transcript sequence reconstruction from RNA-Seq: reference generation and analysis with Trinity. Nat. Protoc. 8, 10.1038/nprot.2013.1084, doi: 10.1038/nprot.2013.084 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B. J. et al. Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucleic Acids Res. 31, 5654–5666 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke M. & Waack S. Gene prediction with a hidden Markov model and a new intron submodel. Bioinformatics 19 Suppl 2, ii215–225 (2003). [DOI] [PubMed] [Google Scholar]
- Stanke M. et al. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 34, W435–439, doi: 10.1093/nar/gkl200 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke M., Steinkamp R., Waack S. & Morgenstern B. AUGUSTUS: a web server for gene finding in eukaryotes. Nucleic Acids Res. 32, W309–312, doi: 10.1093/nar/gkh379 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Xu P. & Glazko G. Estimation of divergence times from multiprotein sequences for a few mammalian species and several distantly related organisms. Proc. Natl. Acad. Sci. USA 98, 2497-2502 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges S. B. & Kumar S. The timetree of life. (Oxford University Press, 2009). [Google Scholar]
- Hedges S., Blair J., Venturi M. & Shoe J. A molecular timescale of eukaryote evolution and the rise of complex multicellular life. BMC Evol. Biol. 4, 2 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfrey L. W., Lahr D. J. G., Knoll A. H. & Katz L. A. Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proc. Natl. Acad. Sci. USA 108, 13624–13629, doi: 10.1073/pnas.1110633108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr C. S. et al. The Encyclopedia of Life v2: Providing Global Access to Knowledge About Life on Earth. Biodivers Data J. e1079, doi: 10.3897/BDJ.2.e1079 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywinski M. et al. Circos: An information aesthetic for comparative genomics. Genome Res. 19, 1639–1645, doi: 10.1101/gr.092759.109 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmer E. et al. The UniProt-GO Annotation database in 2011. Nucleic Acids Res. 40, D565–D570 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry J., Finn R. D., Eddy S. R., Bateman A. & Punta M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Research 41, e121, doi: 10.1093/nar/gkt263 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y. & Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B, Statistical methodology 57, 289–300 (1995). [Google Scholar]
- Warnes G. R. et al. gplots: Various R programming tools for plotting data. R package version 2 (2009). [Google Scholar]
- Li W. & Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22, 1658–1659 (2006). [DOI] [PubMed] [Google Scholar]
- Edgar R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797, doi: 10.1093/nar/gkh340 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella-Gutierrez S., Silla-Martinez J. M. & Gabaldon T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973, doi: 10.1093/bioinformatics/btp348 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D., Taboada G. L., Doallo R. & Posada D. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27, 1164–1165, doi: 10.1093/bioinformatics/btr088 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313, doi: 10.1093/bioinformatics/btu033 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.