Abstract
The participation of cardiac myosin hinge in contractility was investigated by in vitro motility and ATPase assays and by measurements of sarcomere shortening. The effect on contractile activity was analyzed using an antibody directed against a 20-amino acid peptide within the hinge region of myosin. This antibody bound specifically at the hinge at a distance of 55 nm from the S1/S2 junction, was specific to human, dog, and rat cardiac myosins, did not crossreact with gizzard or skeletal myosin, and had no effect on ATPase activity of purified S1 and myofibrils. However, it completely suppressed the movement of actin filaments in in vitro motility assays and reduced active shortening of sarcomeres of skinned cardiac myocytes by half. Suppression of motion by the anti-hinge antibody may reflect a mechanical constraint imposed by the antibody upon the mobility of the S2 region of myosin. The results suggest that the steps in the mechanochemical energy transduction can be separately influenced through S2.
Full text
PDF
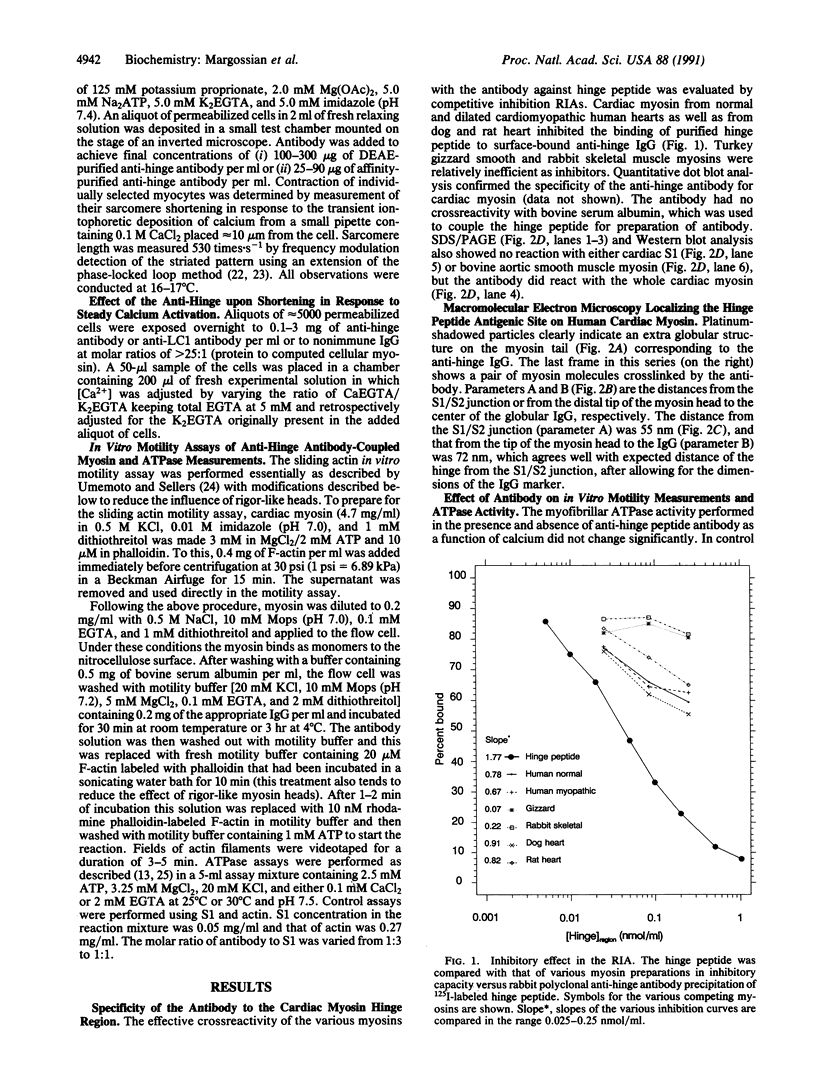
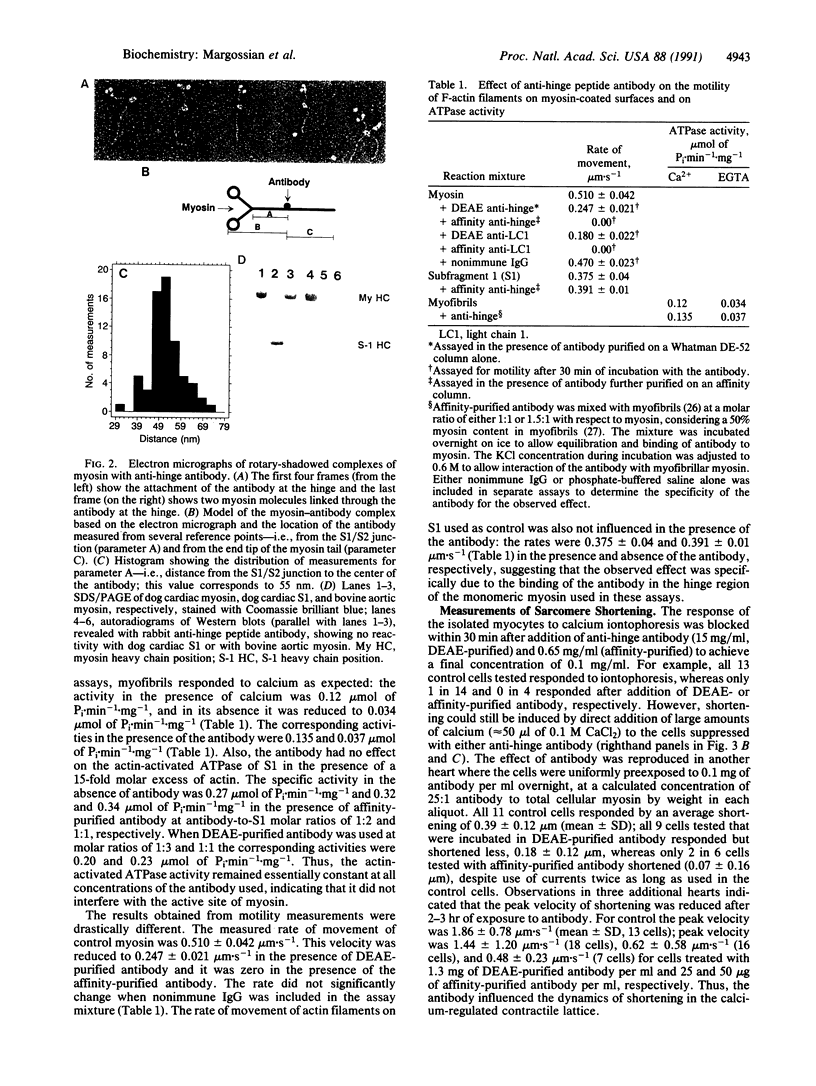
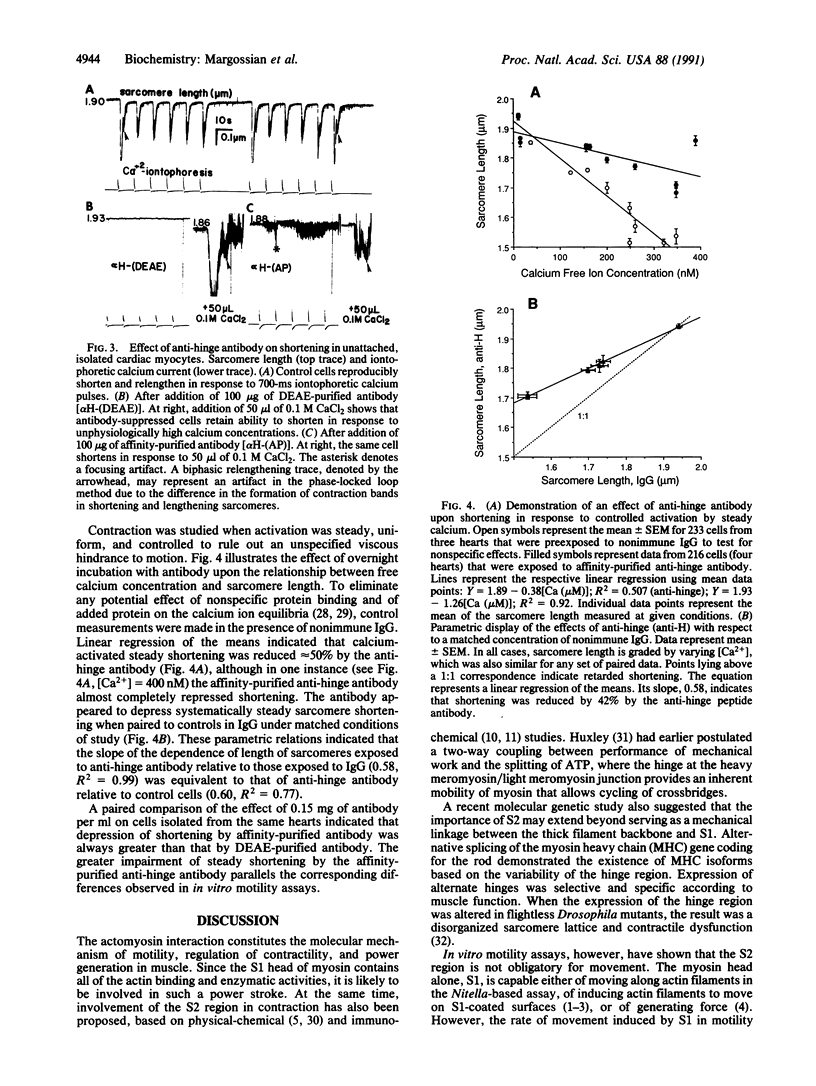

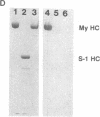
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applegate D., Reisler E. Crossbridge release and alpha-helix-coil transition in myosin and rod minifilaments. J Mol Biol. 1983 Sep 15;169(2):455–468. doi: 10.1016/s0022-2836(83)80061-8. [DOI] [PubMed] [Google Scholar]
- Collier V. L., Kronert W. A., O'Donnell P. T., Edwards K. A., Bernstein S. I. Alternative myosin hinge regions are utilized in a tissue-specific fashion that correlates with muscle contraction speed. Genes Dev. 1990 Jun;4(6):885–895. doi: 10.1101/gad.4.6.885. [DOI] [PubMed] [Google Scholar]
- Dreizen P., Gershman L. C. Molecular basis of muscular contraction. Myosin. Trans N Y Acad Sci. 1970 Feb;32(2):170–203. doi: 10.1111/j.2164-0947.1970.tb02052.x. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington W. F., Karr T., Busa W. B., Lovell S. J. Contraction of myofibrils in the presence of antibodies to myosin subfragment 2. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7453–7456. doi: 10.1073/pnas.87.19.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington W. F. On the origin of the contractile force in skeletal muscle. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5066–5070. doi: 10.1073/pnas.76.10.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Hynes T. R., Block S. M., White B. T., Spudich J. A. Movement of myosin fragments in vitro: domains involved in force production. Cell. 1987 Mar 27;48(6):953–963. doi: 10.1016/0092-8674(87)90704-5. [DOI] [PubMed] [Google Scholar]
- Kavinsky C. J., Umeda P. K., Levin J. E., Sinha A. M., Nigro J. M., Jakovcic S., Rabinowitz M. Analysis of cloned mRNA sequences encoding subfragment 2 and part of subfragment 1 of alpha- and beta-myosin heavy chains of rabbit heart. J Biol Chem. 1984 Mar 10;259(5):2775–2781. [PubMed] [Google Scholar]
- Kishino A., Yanagida T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature. 1988 Jul 7;334(6177):74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- Krueger J. W., Forletti D., Wittenberg B. A. Uniform sarcomere shortening behavior in isolated cardiac muscle cells. J Gen Physiol. 1980 Nov;76(5):587–607. doi: 10.1085/jgp.76.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell S., Karr T., Harrington W. F. Suppression of contractile force in muscle fibers by antibody to myosin subfragment 2. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1849–1853. doi: 10.1073/pnas.85.6.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manstein D. J., Ruppel K. M., Spudich J. A. Expression and characterization of a functional myosin head fragment in Dictyostelium discoideum. Science. 1989 Nov 3;246(4930):656–658. doi: 10.1126/science.2530629. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Chantler P. D., Sellers J. R., Malhotra A., Stafford W. F., Slayter H. S. Proteolytic susceptibility of both isolated and bound light chains from various myosins to myopathic hamster protease. J Biol Chem. 1984 Nov 10;259(21):13534–13540. [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Substructure of the myosin molecule. IV. Interactions of myosin and its subfragments with adenosine triphosphate and F-actin. J Mol Biol. 1973 Mar 5;74(3):313–330. doi: 10.1016/0022-2836(73)90376-8. [DOI] [PubMed] [Google Scholar]
- Margossian S. S. Reversible dissociation of dog cardiac myosin regulatory light chain 2 and its influence on ATP hydrolysis. J Biol Chem. 1985 Nov 5;260(25):13747–13754. [PubMed] [Google Scholar]
- McNally E. M., Kraft R., Bravo-Zehnder M., Taylor D. A., Leinwand L. A. Full-length rat alpha and beta cardiac myosin heavy chain sequences. Comparisons suggest a molecular basis for functional differences. J Mol Biol. 1989 Dec 5;210(3):665–671. doi: 10.1016/0022-2836(89)90141-1. [DOI] [PubMed] [Google Scholar]
- Myers J., Tirosh R., Jacobson R. C., Pollack G. H. Phase-locked loop measurement of sarcomere length with high time resolution. IEEE Trans Biomed Eng. 1982 Jun;29(6):463–466. doi: 10.1109/TBME.1982.324975. [DOI] [PubMed] [Google Scholar]
- Saglio S. D., Slayter H. S. Use of a radioimmunoassay to quantify thrombospondin. Blood. 1982 Jan;59(1):162–166. [PubMed] [Google Scholar]
- Sellers J. R., Kachar B. Polarity and velocity of sliding filaments: control of direction by actin and of speed by myosin. Science. 1990 Jul 27;249(4967):406–408. doi: 10.1126/science.2377894. [DOI] [PubMed] [Google Scholar]
- Slayter H. S. High-resolution metal replication of macromolecules. Ultramicroscopy. 1976 Sep-Oct;1(4):341–357. doi: 10.1016/0304-3991(76)90050-4. [DOI] [PubMed] [Google Scholar]
- Slayter H. S. Secretion of thrombospondin from human blood platelets. Methods Enzymol. 1989;169:251–268. doi: 10.1016/0076-6879(89)69066-0. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Pang D. C., Briggs F. N. The purification of cardiac myofibrils with Triton X-100. Biochim Biophys Acta. 1971 Aug 6;245(1):259–262. doi: 10.1016/0005-2728(71)90033-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima Y. Y., Kron S. J., McNally E. M., Niebling K. R., Toyoshima C., Spudich J. A. Myosin subfragment-1 is sufficient to move actin filaments in vitro. Nature. 1987 Aug 6;328(6130):536–539. doi: 10.1038/328536a0. [DOI] [PubMed] [Google Scholar]
- Ueno H., Harrington W. F. Cross-linking within the thick filaments of muscle and its effect on contractile force. Biochemistry. 1987 Jun 16;26(12):3589–3596. doi: 10.1021/bi00386a051. [DOI] [PubMed] [Google Scholar]
- Ueno H., Harrington W. F. Local melting in the subfragment-2 region of myosin in activated muscle and its correlation with contractile force. J Mol Biol. 1986 Jul 5;190(1):69–82. doi: 10.1016/0022-2836(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Umemoto S., Sellers J. R. Characterization of in vitro motility assays using smooth muscle and cytoplasmic myosins. J Biol Chem. 1990 Sep 5;265(25):14864–14869. [PubMed] [Google Scholar]
- Walker M., Trinick J. Electron microscope study of the effect of temperature on the length of the tail of the myosin molecule. J Mol Biol. 1986 Dec 5;192(3):661–667. doi: 10.1016/0022-2836(86)90283-4. [DOI] [PubMed] [Google Scholar]
- Walter G., Scheidtmann K. H., Carbone A., Laudano A. P., Doolittle R. F. Antibodies specific for the carboxy- and amino-terminal regions of simian virus 40 large tumor antigen. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5197–5200. doi: 10.1073/pnas.77.9.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]