Abstract
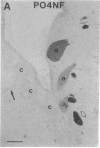
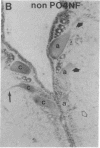
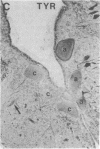
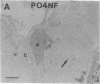
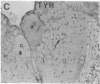
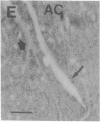
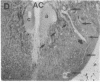
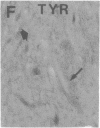
Axotomy of giant lamprey (Petromyzon marinus) central neurons (anterior bulbar cells) close to their somata results in ectopic axon-like sprouting from the dendritic tips. Such sprouts first appear as swellings at the tips of a small subset of dendrites 2-3 weeks after "close" axotomy. We report here that immunocytochemical examination of these swellings reveals a structure and composition that differs from that of conventional growth cones; incipient sprouts contain many highly phosphorylated neurofilaments (NFs), little tubulin, and virtually no stable (acetylated) microtubules (MTs). The dendrites of anterior bulbar cells after close axotomy also show pronounced changes in NF protein and tubulin staining patterns prior to the emergence of sprouts from the dendrites. The amount of tyrosinated tubulin increases greatly; this rise is tightly coupled to the appearance of highly phosphorylated NFs and the loss of nonphosphorylated NFs in the dendrites. Acetylated tubulin is generally reduced after close axotomy and is selectively lost from dendrites that gave rise to sprouts. These changes indicate that an invasion of the dendrites by phosphorylated NFs may be linked to the destabilization of dendritic MTs, and in some dendrites this may lead to a marked loss of stable MTs, which is correlated with the emergence of NF-filled sprouts from the dendritic tips.
Full text
PDF


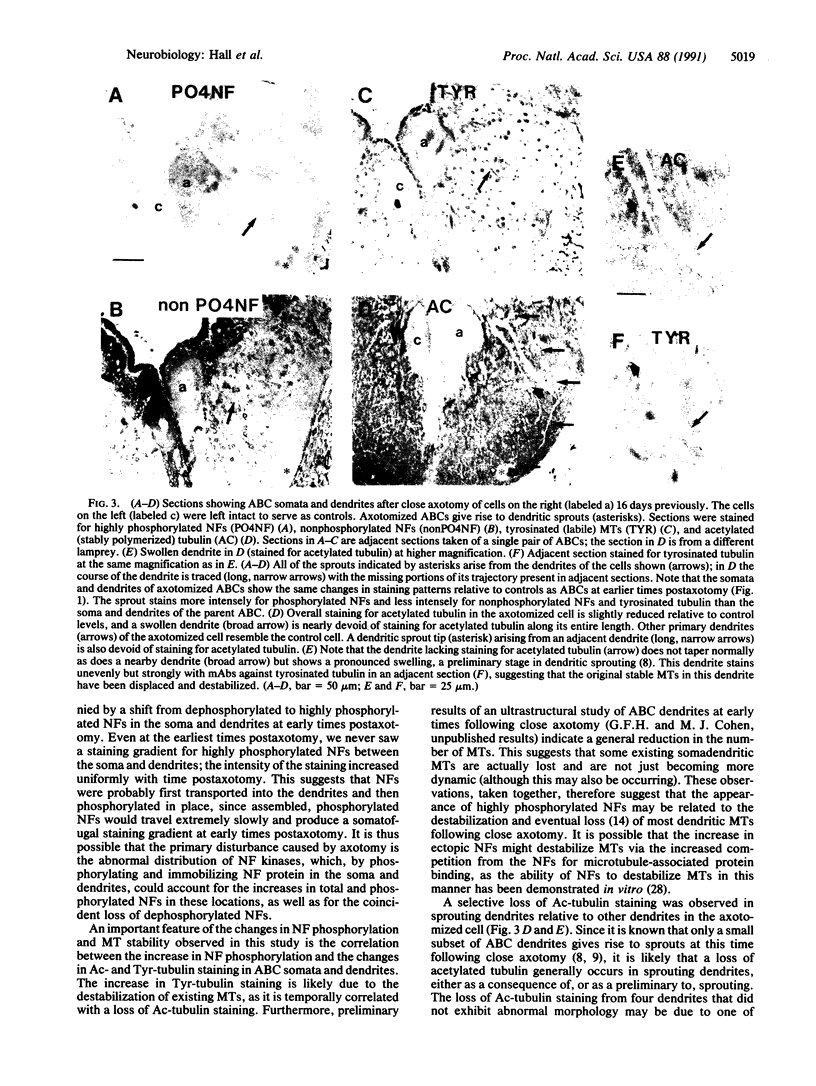

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arce C. A., Hallak M. E., Rodriguez J. A., Barra H. S., Caputto R. Capability of tubulin and microtubules to incorporate and to release tyrosine and phenylalanine and the effect of the incorporation of these amino acids on tubulin assembly. J Neurochem. 1978 Jul;31(1):205–210. doi: 10.1111/j.1471-4159.1978.tb12449.x. [DOI] [PubMed] [Google Scholar]
- Bentley D., Toroian-Raymond A. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 1986 Oct 23;323(6090):712–715. doi: 10.1038/323712a0. [DOI] [PubMed] [Google Scholar]
- Bray D. Mechanical tension produced by nerve cells in tissue culture. J Cell Sci. 1979 Jun;37:391–410. doi: 10.1242/jcs.37.1.391. [DOI] [PubMed] [Google Scholar]
- Carden M. J., Schlaepfer W. W., Lee V. M. The structure, biochemical properties, and immunogenicity of neurofilament peripheral regions are determined by phosphorylation state. J Biol Chem. 1985 Aug 15;260(17):9805–9817. [PubMed] [Google Scholar]
- Cohen M. J., Hall G. F. Control of neuron shape during development and regeneration. Neurochem Pathol. 1986 Dec;5(3):331–343. doi: 10.1007/BF02842942. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Cáceres A. The expression of acetylated microtubules during axonal and dendritic growth in cerebellar macroneurons which develop in vitro. Brain Res Dev Brain Res. 1989 Oct 1;49(2):205–213. doi: 10.1016/0165-3806(89)90022-9. [DOI] [PubMed] [Google Scholar]
- Goldstein M. E., Cooper H. S., Bruce J., Carden M. J., Lee V. M., Schlaepfer W. W. Phosphorylation of neurofilament proteins and chromatolysis following transection of rat sciatic nerve. J Neurosci. 1987 May;7(5):1586–1594. doi: 10.1523/JNEUROSCI.07-05-01586.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. F., Cohen M. J. Dendritic amputation redistributes sprouting evoked by axotomy in lamprey central neurons. J Neurosci. 1988 Oct;8(10):3598–3606. doi: 10.1523/JNEUROSCI.08-10-03598.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. F., Cohen M. J. Extensive dendritic sprouting induced by close axotomy of central neurons in the lamprey. Science. 1983 Nov 4;222(4623):518–521. doi: 10.1126/science.6623092. [DOI] [PubMed] [Google Scholar]
- Hall G. F., Cohen M. J. The pattern of dendritic sprouting and retraction induced by axotomy of lamprey central neurons. J Neurosci. 1988 Oct;8(10):3584–3597. doi: 10.1523/JNEUROSCI.08-10-03584.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. F., Poulos A., Cohen M. J. Sprouts emerging from the dendrites of axotomized lamprey central neurons have axonlike ultrastructure. J Neurosci. 1989 Feb;9(2):588–599. doi: 10.1523/JNEUROSCI.09-02-00588.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. N., Lasek R. J. Axonal transport of the cytoskeleton in regenerating motor neurons: constancy and change. Brain Res. 1980 Dec 8;202(2):317–333. doi: 10.1016/0006-8993(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hoffman P. N., Thompson G. W., Griffin J. W., Price D. L. Changes in neurofilament transport coincide temporally with alterations in the caliber of axons in regenerating motor fibers. J Cell Biol. 1985 Oct;101(4):1332–1340. doi: 10.1083/jcb.101.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliatsos V. E., Applegate M. D., Kitt C. A., Walker L. C., DeLong M. R., Price D. L. Aberrant phosphorylation of neurofilaments accompanies transmitter-related changes in rat septal neurons following transection of the fimbria-fornix. Brain Res. 1989 Mar 20;482(2):205–218. doi: 10.1016/0006-8993(89)91183-9. [DOI] [PubMed] [Google Scholar]
- Kreis T. E. Microtubules containing detyrosinated tubulin are less dynamic. EMBO J. 1987 Sep;6(9):2597–2606. doi: 10.1002/j.1460-2075.1987.tb02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Llinás R. Alterations of synaptic action in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):823–838. doi: 10.1113/jphysiol.1970.sp009244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasek R. J., Brady S. T. The axon: a prototype for studying expressional cytoplasm. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):113–124. doi: 10.1101/sqb.1982.046.01.015. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W. Structural similarities and differences between neurofilament proteins from five different species as revealed using monoclonal antibodies. J Neurosci. 1986 Aug;6(8):2179–2186. doi: 10.1523/JNEUROSCI.06-08-02179.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Trojanowski J. Q. Novel monoclonal antibodies provide evidence for the in situ existence of a nonphosphorylated form of the largest neurofilament subunit. J Neurosci. 1986 Mar;6(3):850–858. doi: 10.1523/JNEUROSCI.06-03-00850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier J. F., Liem R. K., Shelanski M. L. Interactions between neurofilaments and microtubule-associated proteins: a possible mechanism for intraorganellar bridging. J Cell Biol. 1982 Dec;95(3):982–986. doi: 10.1083/jcb.95.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau P. C. Cell-substratum adhesion of neurite growth cones, and its role in neurite elongation. Exp Cell Res. 1979 Nov;124(1):127–138. doi: 10.1016/0014-4827(79)90263-5. [DOI] [PubMed] [Google Scholar]
- Letourneau P. C. Differences in the organization of actin in the growth cones compared with the neurites of cultured neurons from chick embryos. J Cell Biol. 1983 Oct;97(4):963–973. doi: 10.1083/jcb.97.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau P. C. Possible roles for cell-to-substratum adhesion in neuronal morphogenesis. Dev Biol. 1975 May;44(1):77–91. doi: 10.1016/0012-1606(75)90378-4. [DOI] [PubMed] [Google Scholar]
- Letourneau P. C., Shattuck T. A., Ressler A. H. "Pull" and "push" in neurite elongation: observations on the effects of different concentrations of cytochalasin B and taxol. Cell Motil Cytoskeleton. 1987;8(3):193–209. doi: 10.1002/cm.970080302. [DOI] [PubMed] [Google Scholar]
- Lewis S. E., Nixon R. A. Multiple phosphorylated variants of the high molecular mass subunit of neurofilaments in axons of retinal cell neurons: characterization and evidence for their differential association with stationary and moving neurofilaments. J Cell Biol. 1988 Dec;107(6 Pt 2):2689–2701. doi: 10.1083/jcb.107.6.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman A. R. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- Lindå H., Risling M., Cullheim S. 'Dendraxons' in regenerating motoneurons in the cat: do dendrites generate new axons after central axotomy? Brain Res. 1985 Dec 9;358(1-2):329–333. doi: 10.1016/0006-8993(85)90978-3. [DOI] [PubMed] [Google Scholar]
- Mackler S. A., Yin H. S., Selzer M. E. Determinants of directional specificity in the regeneration of lamprey spinal axons. J Neurosci. 1986 Jun;6(6):1814–1821. doi: 10.1523/JNEUROSCI.06-06-01814.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L., Letourneau P. C. Growth of neurites without filopodial or lamellipodial activity in the presence of cytochalasin B. J Cell Biol. 1984 Dec;99(6):2041–2047. doi: 10.1083/jcb.99.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A., Vaughn J. E. Microtubules and filaments in the axons and astrocytes of early postnatal rat optic nerves. J Cell Biol. 1967 Jan;32(1):113–119. doi: 10.1083/jcb.32.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasure S. J., Selzer M. E., Lee V. M. Lamprey neurofilaments combine in one subunit the features of each mammalian NF triplet protein but are highly phosphorylated only in large axons. J Neurosci. 1989 Feb;9(2):698–709. doi: 10.1523/JNEUROSCI.09-02-00698.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer E., Cohen M. J. Regeneration of an identified central neuron in the cricket. I. Control of sprouting from soma, dendrites, and axon. J Neurosci. 1983 Sep;3(9):1835–1847. doi: 10.1523/JNEUROSCI.03-09-01835.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B. J., Reese T. S. Cytoplasmic structure in rapid-frozen axons. J Cell Biol. 1982 Sep;94(3):667–669. doi: 10.1083/jcb.94.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E., Asai D. J., Bulinski J. C., Kirschner M. Posttranslational modification and microtubule stability. J Cell Biol. 1987 Nov;105(5):2167–2177. doi: 10.1083/jcb.105.5.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner B. E., Watson W. E. Retraction and expansion of the dendritic tree of motor neurones of adult rats induced in vivo. Nature. 1971 Sep 24;233(5317):273–275. doi: 10.1038/233273a0. [DOI] [PubMed] [Google Scholar]