Abstract
Study Objectives:
This preliminary study investigated the tolerability and efficacy of a novel mattress technology—the Sound-To-Sleep (STS) system—in the treatment of sleep problems in children with autism.
Methods:
After screening, 45 children, ages 2.5 to 12.9 years, were randomized to order of mattress technology use (On-Off vs. Off-On). Treatment conditions (On vs. Off) lasted two weeks with immediate crossover. Tolerability, including study discontinuation and parent-report of mattress tolerance and ease of use, was tracked throughout the study. Efficacy assessments were obtained at baseline, prior to crossover, and end of study and included measures of autism traits, other psychopathology symptoms, sensory abnormalities, communication difficulties, quality of life, sleep diary parameters, and single-blinded actigraphy-derived sleep parameters. Statistical analyses evaluated differences in tolerability and efficacy when the STS system was on versus off.
Results:
STS system use was well tolerated (n = 2, 4.4% dropout) and resulted in parent-reported sleep quality improvements (STS off mean = 4.3, 95% CI = 4.05–4.54 vs. on mean = 4.9, 95%CI = 4.67–5.14). The technology was described by parents as very easy to use and child tolerance was rated as good. Parent-diary outcomes indicated improvements in falling asleep and reduced daytime challenging behavior. Actigraphy-derived sleep parameters indicated improved sleep duration and sleep efficiency. Improvements in child and family quality of life were identified on parent questionnaires.
Conclusions:
A future large sample phase 2 trial of the STS system is warranted and would benefit from extended study duration, an objective primary efficacy outcome, and careful attention to methodological issues that promote compliance with the intervention and study procedures.
Citation:
Frazier TW, Krishna J, Klingemier E, Beukemann M, Nawabit R, Ibrahim S. A randomized, crossover trial of a novel sound-to-sleep mattress technology in children with autism and sleep difficulties. J Clin Sleep Med. 2017;13(1):95–104.
Keywords: autism, sleep, mattress, insomnia
INTRODUCTION
Autism spectrum disorder (ASD) represents an etiologically complex set of neurodevelopmental disorders sharing a common phenotype of qualitatively distinct social communication and interaction deficits and the presence of inflexible repetitive behaviors.1 A number of comorbid psychiatric and medical problems have been documented in ASD, including ADHD, anxiety, and mood symptomatology, as well as gastrointestinal and sleep difficulties.2 Sleep difficulties, including sleep onset delay and decreased sleep duration, are a particularly prevalent and impairing comorbid condition in ASD.3 Parents have reported significant sleep difficulties in up to 80% of children with ASD,4 and actigraphy studies have documented increased prevalence of sleep abnormalities relative to typically developing children,5 with up to 40-minute decreases in sleep duration in ASD.6 Sleep difficulties in children with ASD are impairing to both the child and the family, including significant associations with increased behavior problems, decreased adaptive skill development, and increased family stress.7,8 Sleep abnormalities persist across age and are found in both cognitively impaired and highly intelligent youth with ASD.3 These findings document a substantial need to improve sleep in children with ASD.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Children with autism have significant sleep difficulties that impact the child's functioning and overall caregiver and family life. The present study was conducted to examine whether a novel mattress technology improved sleep in children with autism who also have significant sleep difficulties.
Study Impact: The sound-to-sleep mattress technology was well tolerated and improved some measures of sleep in children with autism. If supported in future trials, this technology may be a safe, effective adjunct or second-line treatment of sleep difficulties in children with autism.
Pharmacologic treatment studies,9 including studies of supplements such as melatonin,10 have indicated significant benefit in improving sleep in children with ASD. However, pharmacologic treatment is not recommended as first-line care,11 parents prefer non-pharmacologic interventions,12 and some pharmacologic approaches frequently used to treat sleep difficulties in ASD carry the potential for significant side effects, such as daytime drowsiness with alpha-adrenergics or weight gain with atypical antipsychotics.13,14 Sleep hygiene and other behavioral treatments have the advantage of low risk for side effects with the ability to directly influence child, parent, or environmental factors that may be perpetuating sleep difficulties.15 While sleep hygiene education in a pamphlet approach may be ineffective,16 more intensive and comprehensive approaches may improve insomnia. Recent empirical studies have supported the potential for behavioral treatments, including parent education17 and parent training in the application of behavioral methods.18 Unfortunately, parents are sometimes difficult to train in behavioral methods or become non-adherent to behavioral interventions, resulting in sub-optimal efficacy.19 Thus, it is necessary to identify alternative treatment approaches with low-risk, high tolerability profiles for improving sleep in children with ASD and parent-reported sleep difficulties.
The Present Study
The literature on treatment of sleep difficulties in ASD has paid little attention to technological approaches to enhancing the child's sleep experience. The present study examines the tolerability and efficacy of a novel mattress-based technology called the Sound-to-Sleep (STS) system in improving sleep in children with autism and parent-reported sleep difficulties. The STS mattress technology embeds vibrations corresponding to a sound source into the mattress, allowing the user to both hear and feel sounds that they choose to play. Relative to behavioral interventions, this technology has several advantages. First, children can choose preferred sounds to play, potentially decreasing resistance to bedtime. Second, the vibrations or combined sound and synced vibrations may tap into sensory experiences that are preferred by children with ASD. The recent revision of diagnostic criteria has included sensory sensitivities and unusual sensory interests as a symptom criterion.1 Differences in sensory experience in ASD have been long documented and, in this case, might be leveraged to improve sleep in some ASD-affected children. Third, the STS system may be easier to faithfully implement relative to behavioral methods. Fourth, the vibrations produced by the STS mattress technology may activate the parasympathetic nervous system, including the vagus nerve which has been shown to respond to tactile input and is crucial for relaxation and promotion of sleep.20 However, given the nature of sensory sensitivities in ASD, it is also possible that the STS system would be intolerable or result in significantly worse sleep. Thus, the present research excluded individuals with substantial sensory abnormalities and utilized a short-term (2-week) crossover design to provide an initial evaluation of tolerability.
Given the lack of previous studies and uncertainty regarding possible side effects, the primary aim of this preliminary study was to evaluate STS mattress tolerability, defined as any study dropout due to STS mattress use. The secondary aim was to evaluate the efficacy of the STS mattress technology in improving sleep parameters. Efficacy parameters were based on parent-completed sleep diary, blinded actigraphy scoring, and parent questionnaire measures. We focused predominantly on parent report, with parent-reported sleep quality as the primary efficacy outcome, because it was not possible to obtain blinded clinician improvement ratings; we anticipated that actigraphy would be challenging to collect in cognitively impaired children with ASD but did not want to further limit the sample to high functioning children. Specific aspects of parent-reported sleep problems were also explored to determine whether the STS mattress technology may be beneficial for specific facets of sleep problems in children with ASD.
METHODS
Sample
ASD-affected individuals were recruited after diagnostic clinic visits, from local treatment programs, via study fliers, and word of mouth. Institutional IRB approval was obtained for the procedures of the present study. Consent was obtained from parents/caregivers/legally authorized representatives and, where possible, assent was obtained from children where appropriate. The trial procedures were registered with clinicaltrials.gov (NCT02739321).
Inclusion/Exclusion Criteria
Inclusion criteria were: (a) a consensus clinical diagnosis of autism spectrum disorder based on a multidisciplinary evaluation and Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria, (b) age 2.5 years (30 months) to 12.9 years (155 months), (c) stable medication and therapy (physical or occupational therapy, ABA therapy, etc.) four weeks prior to their study participation and throughout the 1-month study period, (d) significant sleep difficulties as indicated by a score of ≥ 41 on the Child Sleep Habits Questionnaire,21 and (e) stable health 4 weeks prior to study participation and throughout the 1-month study period. Exclusion criteria were: (a) extreme hypersensitivity to sound or tactile input as reported by parent using a total raw score resulting in a qualitative description of “definite difference” on the Short Sensory Profile, (b) seizure disorder or a specific neurogenetic disorder (e.g., Fragile X), (c) acute illness or exacerbation of chronic illness during the course of the study, (d) use of anti-allergy medication, or (e) sleep difficulties deemed by the physician to render participation inappropriate (e.g., significant snoring, apnea, parasomnias). Due to the frequency of melatonin use in this population, we elected to include those who had been using melatonin for 1 month prior to study start and continued stable use throughout the study. Any participant who at the discretion of the clinical investigator was not able to comply with study procedures was also excluded. It is important to note that individuals were allowed to change therapy (intensity, schedule, provider, etc.) or engage in a specific intervention to improve sleep (environmental modifications) as long as the therapy change or intervention occurred at least 1 week (1 month for melatonin) prior to the study baseline, sleep problems persisted after the change, and no treatments or environmental changes were made during the 1-month study period.
Measures
Supplement 2 in the supplemental material presents primary and secondary tolerability and efficacy outcomes.
Drop out
The proportion of drop out due to STS mattress technology use was designated as the primary tolerability outcome. Dropout for any reason was also collected as a secondary tolerability outcome.
Sleep diary
Parents completed a sleep diary each day. As part of this diary, parents rated the child's sleep quality using a 7-point Likert scale (1 = extremely poor, 4 = average, 7 = exceptional), and this was considered the primary efficacy outcome. Parents also rated the child's ease of falling asleep and level of challenging behavior/task compliance during the subsequent day as secondary efficacy outcomes. Ratings were made using a 7-point Likert scale with 1 = extremely difficult, 4 = average, 7 = exceptional/exceptionally easy. The child's bedtime, time to fall asleep, number of awakenings, clock times awake during the night (e.g., 00:15–00:45), final wake up time, and time leaving the bed were also recorded. These parameters were scored to generate two additional secondary sleep outcomes: sleep duration and wake after sleep onset (WASO). Sleep duration was defined as the total nocturnal sleep time from sleep onset to final morning awakening minus nocturnal awakenings. WASO was defined as the aggregate duration of all nocturnal awakenings (time out of bed) for that night.
Actigraphy
Actigraphy was collected using a MotionWatch8 actigraphy system (CamNtech), a light weight waterproof watch which uses tri-axial digital accelerometer technology to detect motion. In accordance with standard protocol, participants wore the watch on their non-dominant wrist. However, participants were permitted to try different watch locations during the lead-in period. When subjects were unable to wear the watch for 24 hours, the watch was used during the major sleep period, placed on at bedtime and taken off at wake time. In these instances, parents were instructed to have their children wear the watch beginning one hour prior to bedtime and remove the watch after leaving bed in the morning, each day throughout the study period. Actigraphy data were downloaded from the watch using the CamNTech MotionWare 1.1.15 software and scored by a blinded, highly experienced sleep technologist and reviewed by a blinded, board-certified pediatric sleep medicine physician. Sleep diary information was used in the interpretation of actigraphy data. Sleep duration (in h), sleep efficiency (%), latency to sleep (min), and WASO (in min) were scored for each day and averaged across all available days within each arm. Sleep duration (sleep onset to morning awakening minus night time awakenings) and WASO (wake after sleep onset until final awakening) were defined in the same fashion as for parent diary information. Actigraphy data were considered valid if at least 10 days of complete data were available (per arm) and there were no major intrusions during the study period (e.g., daylight savings time shifts). One participant had significant daytime napping and was excluded from analysis. See Supplement 1 in the supplemental material for additional detail on actigraphy collection, scoring, and analysis. In general, the data collection procedures for individuals with neurodevelopmental disorders outlined by Fawkes, Malow, and colleagues were followed,22 although the specific details were not available to the research team at the time of study initiation.
Social Responsiveness Scale – Second Edition (SRS-2)23
The SRS-2 is a parent-rated 65-item, ordinally scaled (1 = “not true” to 4 = “almost always true”) quantitative assessment of the severity of autism traits. The SRS-2 and its predecessor, the SRS, have been two of the most frequently used quantitative measures of autism symptoms, with very strong measurement properties in healthy and autism-affected samples.24 The total sex-adjusted T-score assessed autism trait levels.
Aberrant Behavior Checklist – Community version (ABC)25
The ABC is a 58-item measure designed to assess 5 psychopathology dimensions in individuals with developmental disabilities: irritability, lethargy, stereotypic behavior, hyperactivity, and inappropriate speech. Items use a 4-point Likert scale (0 = not at all a problem to 3 = the problem is severe). The ABC was completed by the parent with the assistance of the clinician. The total raw score aggregated across the 5 subscales assessed overall psychopathology symptoms levels.
Short Sensory Profile (SSP)26
The SSP is a 38-item parent-completed screening questionnaire that measures behaviors related to sensory processing abnormalities and was based on the Sensory Profile. Items are rated on a 5-point Likert scale ranging from “never” to “always.” The total raw score evaluated overall sensory abnormalities.
Child and Family Quality of Life (CFQL)27
The CFQL is a 32-item parent questionnaire that evaluates 7 different aspects of child and family quality of life: child, family, caregiver, financial, partner relationship, external support, and coping quality of life. For the present study, an adapted version was used that decreased the number of total items (26) to reduce rater burden and added one item to each scale that specifically evaluated changes over the past month in quality of life. Items use a 5-point Likert scale ranging from (1 = strongly disagree/decreased substantially to 3 = neutral/ same to 5 = strongly agree/improved substantially). The total raw score (range 26–130) evaluated overall quality of life for the child and family.
Children's Communication Checklist – Second Edition (CCC-2)28
The CCC-2 is a 70-item parent questionnaire that assesses children's communication skills across 10 domains using a 4-point Likert scale (0 = less than once a week/never to 3 = several times a day/always). The composite standard score based on age norms assessed overall communication competency.
Family Inventory of Sleep Habits (FISH)29
The FISH is a 12-item parent-completed questionnaire that assess sleep hygiene-related behaviors in children using a 5-point Likert Scale (1 = never, 2 = occasionally, 3 = sometimes, 4 = usually, 5 = always). Typically, parents rate these behaviors with reference to the past month, but for the present study they were asked to rate for only the previous 2 weeks to correspond to each STS mattress condition. The total raw score assessed sleep hygiene.
Children's Sleep Habits Questionnaire (CSHQ)21
The CSHQ is a 45-item parent-completed questionnaire that measures a variety of sleep-related problems using a 3-point Likert Scale (1 = “rarely – 0 to 1 times per week,” 2 = “sometimes – 2 to 4 times per week,” 3 = “usually – 5 to 7 times per week”). Ordinarily, parents rate these behaviors for the past week, but for the present study they were instructed to rate for the past 2 weeks to correspond to each STS mattress condition. Total raw score evaluated overall sleep difficulties.
Intervention
Sound-To-Sleep (STS) mattress technology
The STS mattress technology embeds resonators into the mattress box spring that convert an audio file into tactile input. A separate control unit connects to the resonators allowing the user to independently adjust both the auditory level and tactile intensity. For the present study, parents, and where possible children, were given the option to play a suggested relaxing audio file or whatever audio file they wished and to adjust the auditory level and tactile intensity as they saw fit. Children were permitted to wear or not wear headphones. If they chose not to wear headphones, they could use external speakers to listen to the audio file. If neither was chosen, the resonators produced low-volume audio that would be heard as ambient noise by the child. The suggested audio file included the copyrighted STS ohm, which is an electronically-generated piece designed to produce a relaxing, peaceful experience. At the end of the study, parents were asked to report whether they used audio with vibration, vibration alone, or alternated between these two uses.
Design and procedure
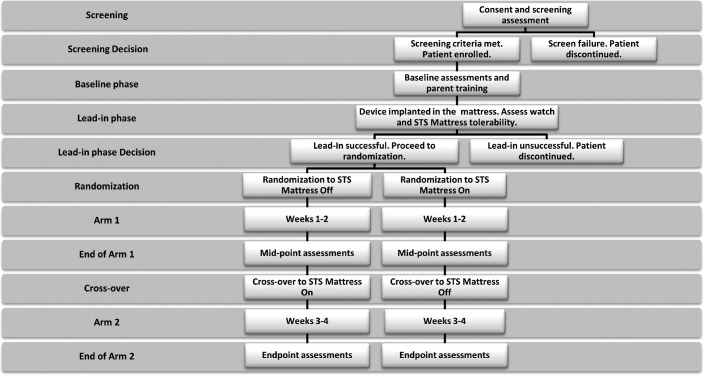
Figure 1 presents study design and key assessment time points. The study used a randomized crossover design with a lead-in mattress technology and actigraphy-watch accommodation period. Each arm included 2 weeks of sleep data collection. Parents, children, and the research coordinator could not a priori be blinded to use of the technology. However, actigraphy data were scored by a sleep technician and reviewed by a pediatric sleep medicine physician, both of whom were blinded to STS mattress condition (on vs. off). The screening visit was used to evaluate inclusion/exclusion criteria and determine study eligibility. If participants were eligible to participate, a baseline visit was scheduled. At this visit, participants were explained daily data collection procedures, practiced applying the watch to their child, and practiced turning on the STS mattress technology. If needed, the research coordinator explained and demonstrated desensitization procedures to promote watch tolerance. Following the baseline visit, participants experienced a lead-in period of 5–7 days that could be extended to up to 2 weeks for children that had initial problems tolerating the addition of STS mattress technology or wearing the actigraphy watch. If the child could not tolerate the watch or STS mattress technology, they were discontinued from the study. If the child achieved success in tolerating the mattress or watch technology, randomization to STS mattress order (on/off vs. off/on) occurred and parents were informed of the order to use. The first treatment arm began the evening of the following day.
Figure 1. Study design and assessment time points.
Actigraphy and sleep diary data were collected each day of the study post-randomization. For days when data were not available, the remaining data points for that period of observation (2 weeks) were averaged. The SSP, FISH, and CSHQ were collected at screening, crossover (mid-point), and end-point. For these measures, screening values served as the patient's baseline scores. The remaining measures were collected at baseline, crossover (mid-point), and end-point. All questionnaires were completed by the same parent at each time point.
Statistical Analysis
Sample characteristics (age, sex, autism traits, psychopathology symptoms, communication level, and sleep difficulties) were compared using independent samples t-tests or Pearson χ2. To evaluate tolerability, the proportions of children dropping out due to use of the STS mattress technology or for any reason were compared to the hypothesized upper boundary of 30% using one-sample proportion tests.30 The difference in dropout for any reason between STS mattress on vs. STS mattress off conditions was also computed using McNemar paired proportion test.31
To evaluate the remaining tolerability and efficacy outcomes, linear mixed effects regression models were computed with Randomization Group (Off-On vs. On-Off), Time (Weeks 1–2 vs. Weeks 3–4), and their interaction as fixed effects factors and tolerability and efficacy outcomes as dependent variables. The interaction between Randomization Group and Time represents the treatment effect with the expectation of improved outcomes during the weeks when the STS mattress technology was turned on. For parent questionnaire outcomes, baseline was included in the model and post hoc contrasts were examined to compare STS mattress on vs. off conditions. Models were estimated using restricted estimation maximum likelihood and model fit was evaluated using the Bayesian Information Criterion.32 Alternative covariance structures were considered iteratively and final models are presented using unstructured error covariance which yielded better fitting models than alternative covariance structures (e.g., autoregressive, diagonal, compound symmetry). The present application of linear mixed models is consistent with the intent-to-treat principle for analysis of clinical trials and have significant advantages over traditional methods, including tolerance for missing follow-up data and more accurate (less biased) estimation of within-individual change.33
For parent questionnaires showing evidence of treatment efficacy, follow-up exploratory analyses using the same mixed effects regression models were computed with subscales as the dependent variable. For all outcomes showing significant treatment efficacy, exploratory Pearson bivariate correlations investigated the relationships between baseline patient characteristics and improvements in outcome measures with STS mattress use (change scores – STS mattress on vs. off). Exploratory bivariate correlations were also computed among change scores for outcome measures showing significant improvements with STS mattress use.
Analyses were computed in IBM SPSS version 24 (IBM Corp., Armonk, New York). For the primary tolerability and efficacy outcome measures, statistical significance was determined using p < 0.05. For secondary outcomes, a Benjamini-Hochberg false discovery rate correction was applied to control for type 1 error inflation due to multiple testing.34 For all exploratory analyses, type 1 error was maintained at 0.05, as the purpose of these analyses was to identify possible relationships that would require confirmation in future studies. Power to detect a significant treatment effect was estimated using GPower v3.0.35 An analysis of variance model with a moderate repeated measures correlation (r = 0.50) was specified, with the expectation that actual power would be enhanced for mixed effects regression models.36 Power was excellent (1-β ≥ 0.90) for detecting medium treatment effect sizes (Cohen's d ≥ 0.50) for the primary efficacy outcome, based on a total sample size of 45 and a type 1 error rate of α = 0.05. For secondary outcomes, power remained very good (1-β ≥ 0.90) for detecting medium-to-large treatment effects (Cohen's d ≥ 0.65) when a conservative Bonferroni correction is used to control type 1 error (α = 0.05/15 = 0.003).
RESULTS
Sample Description
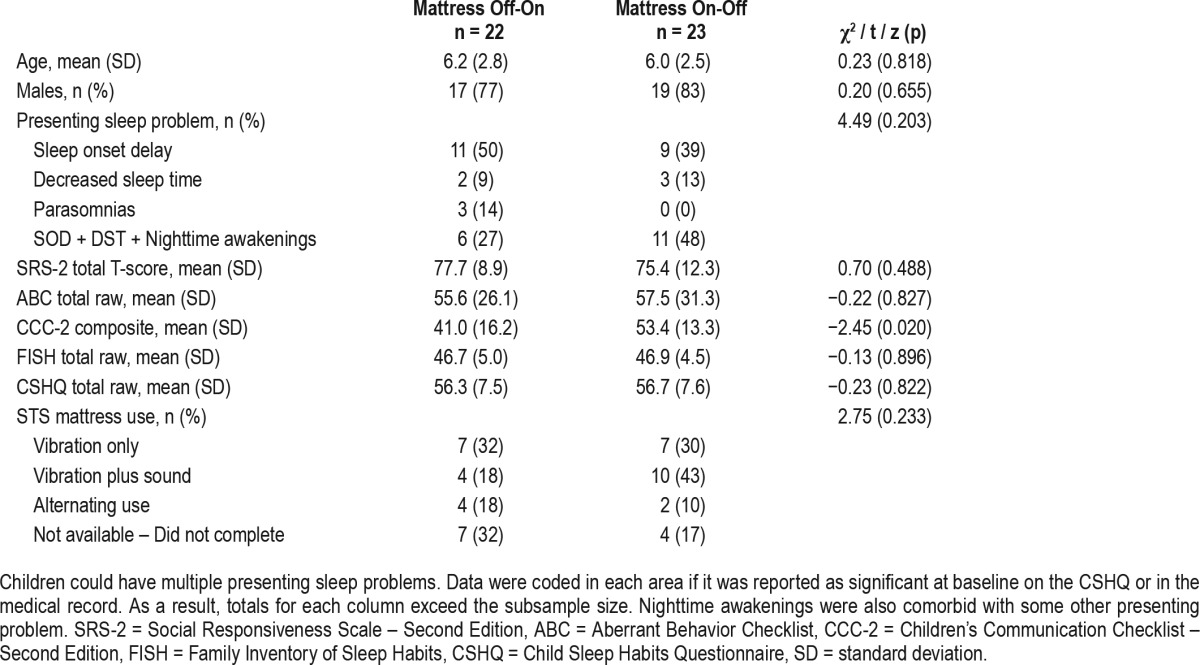
Figure 2 presents participant flow from recruitment and screening to trial completion based on CONSORT recommendations.37 As depicted, 68 patients were screened to participate. Of these, 17 were screen failures and 6 were lead-in failures, resulting in a final randomized, intent-to-treat sample of 45. Of these 45 individuals, 22 were randomized to STS Off-On while 23 were randomized to STS On-Off. There were no significant differences between these groups for age, sex, autism traits, primary baseline sleep problem, questionnaire-measured sleep difficulties, or the way in which the STS mattress system was used (Table 1). Parent-rated communication level was significantly lower in the STS Off-On group, suggesting imperfect randomization. However, post hoc analyses including baseline communication levels as a covariate did not alter the pattern of results. Therefore, we continue to report the a priori proposed analyses.
Figure 2. CONSORT participant flow diagram.
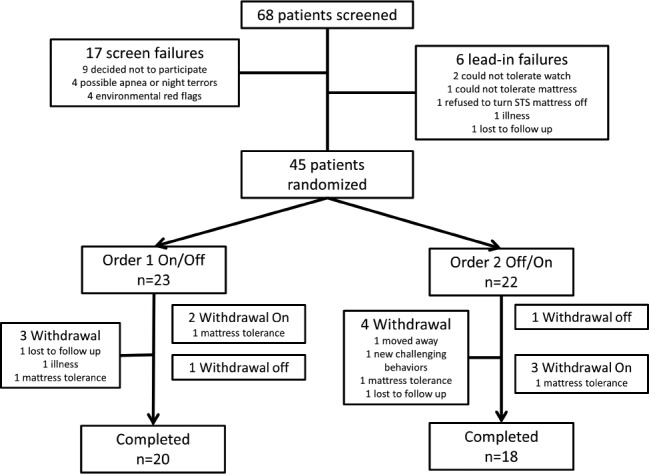
Table 1.
Baseline sample characteristics.
Tolerability
Only 1 lead-in failure resulted from intolerance of the STS mattress system. Additionally, only 2 children (4.4%) dropped out of the study due to STS mattress intolerance, significantly better than the hypothesized benchmark of 30% (z = 3.75, p < 0.001) and also better than a more liberal benchmark of 15% (z = 1.99, p = 0.046). Overall study dropout for any reason was also modest (n = 7, 15.5%) and there was no significant difference in drop-out between the STS mattress on vs. off conditions (on n = 5, 11.1% vs. off n = 2, 4.4%, z = 1.16, p = 0.127). Overall, parents rated their child's tolerance as good to very good (mean = 5.61, SE = 0.25) and the ease of use of the STS mattress technology as very easy (mean = 6.04, SE = 0.15) (Supplement 3 in the supplemental material). There was no difference in these ratings across randomization groups (largest F1,20 = 2.28, p = 0.147). The actigraphy watch was also well tolerated throughout the study (mean = 5.51, SE = 0.13) falling between good and very good tolerance and with no significant difference between on/off and off/on conditions (F1,62 = 1.61, p = 0.210).
Efficacy – Sleep Diary
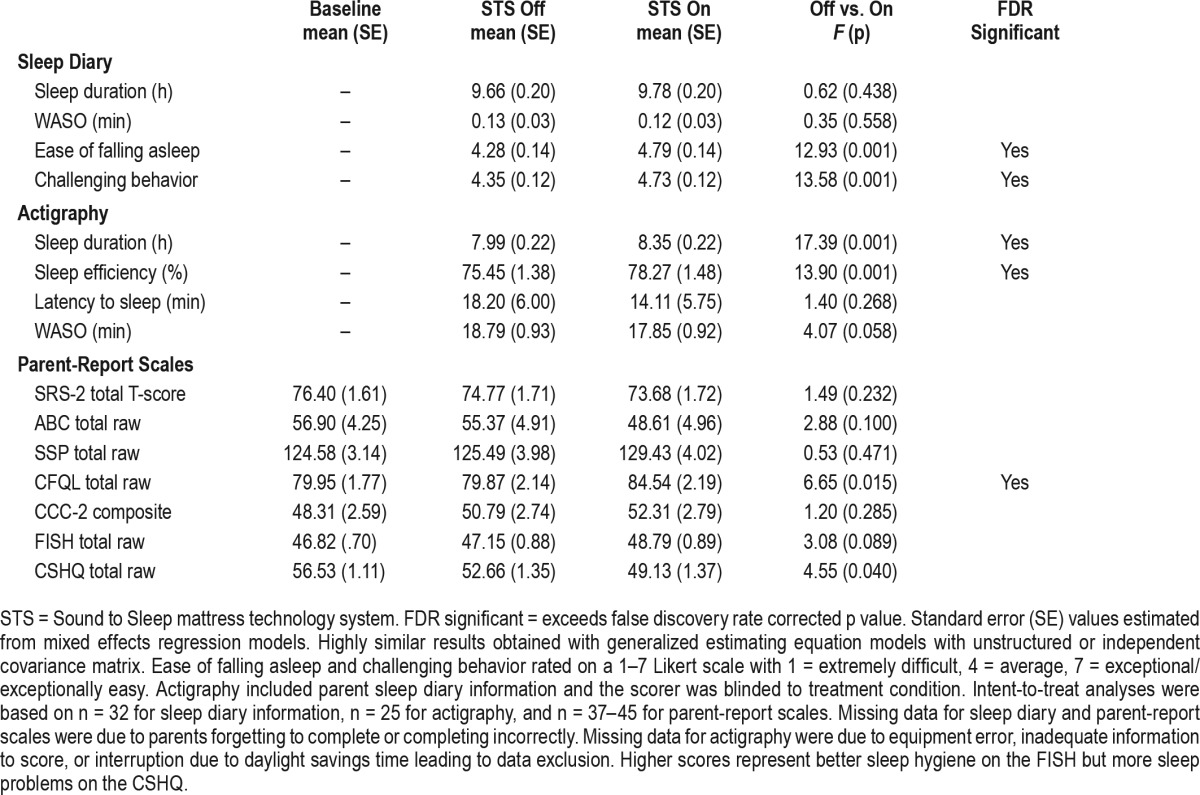
Sleep quality, as rated by parents, was significantly improved when the STS mattress system was on, moving from average-to-good (F1,29 = 12.36, p = 0.001) (Figure 3). Similarly, there was a significant improvement in parent-reported ease of falling asleep and challenging behavior/task compliance during the day in the STS mattress on condition (Table 2). However, there were no significant improvements in sleep duration or WASO per parent report on the sleep diary.
Figure 3. Sleep quality ratings (mean ± 95% confidence interval), separately by randomization group (on/off vs. off/ on) and STS mattress technology condition (on/off).
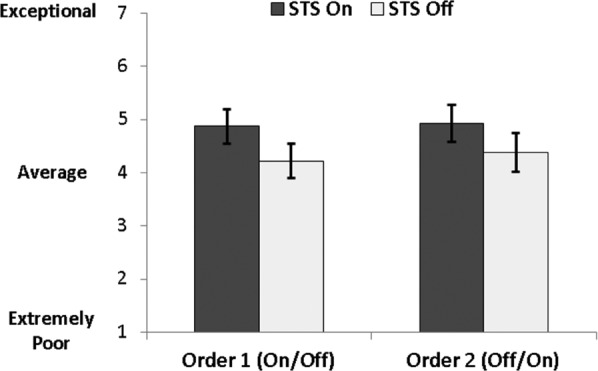
Table 2.
Secondary efficacy outcomes, separately for baseline and each treatment conditions (STS On/Off).
Efficacy – Actigraphy
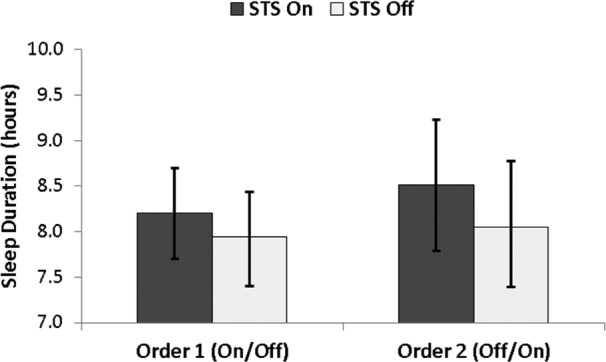
Significant improvements in sleep duration (Figure 4) and sleep efficiency were observed in the STS mattress on condition (Table 2). The improvement in sleep duration in the STS mattress on condition was non-trivial (Δ = 22 minutes). Improvements in sleep latency were not significant and only a weak nonsignificant trend was observed for WASO.
Figure 4. Actigraphy-derived sleep duration (hours; mean ± 95% confidence interval), separately by randomization group (on/off vs. off/on) and STS mattress technology condition (on/off).
Efficacy – Parent Questionnaires
Child and family quality of life was significantly improved in the STS mattress on condition relative to STS mattress off and baseline (Table 2). Follow-up analyses indicated that these improvements were most evident in the areas of child, family, and caregiver quality of life. There were no significant improvements for autism traits, other psychopathology symptoms, sensory abnormalities, or communication level. A weak nonsignificant trend was noted for improvements in sleep hygiene and sleep difficulties during STS mattress on relative to STS mattress off and baseline. Although the effect for the CSHQ did not reach the multiple comparison correction cutoff, exploratory analyses also examined CSHQ subscales. Results indicated a strong effect for sleep duration (F1,31 = 11.71, p = 0.002), but not for any other subscale (largest F1,32 = 3.03, p = 0.091).
Exploratory Efficacy Relationships
Age and sex were uncorrelated with changes in any of the outcome measures showing significant efficacy with STS mattress use. There was a significant relationship between worse sleep hygiene at baseline and greater improvements in child and family quality of life with the STS mattress use (r = −0.46, p = 0.040). No other significant relationships between baseline patient characteristics and changes in efficacy outcomes were observed. For example, baseline sleep problems and sensory function based on the SSP did not predict improvement with the mattress technology. Improvements in sleep efficiency were significantly correlated with improvements in child and family quality of life (r = 0.58, p = 0.014).
DISCUSSION
In this preliminary, small-sample, randomized crossover study, use of the STS mattress technology was well tolerated and resulted in parent-reported improvements in sleep quality. Only a modest proportion of participants dropped out of the study for any reason, and only 3 participants (1 during lead-in) reported problems tolerating the STS mattress technology. Furthermore, the technology was described by parents as very easy to use with good to very good tolerance by their children. Follow-up studies are needed to investigate a longer duration of use and determine whether the high level of tolerability seen in the present study is maintained over time or if a ceiling effect is reached after a period of use. It will also be interesting to observe whether parents continue to consistently use the technology over longer periods and what factors may predict continued usage.
In addition to parent-reported improvements in sleep quality, gains in sleep duration and sleep efficiency were also observed for actigraphy-derived measurements. This pattern suggests that parent-reported efficacy could not be completely explained by unblinded expectations or placebo effects. However, the pattern was not entirely consistent, as parents did not report longer sleep duration in the daily sleep diary, but did report improved sleep duration on the CSHQ. The lack of convergence of parent reports for these domains may be due to the inherent variability of subjective report information. Parent reports are further impacted by the challenge of raising a child with a severe neurodevelopmental disorder and highlight the need to use easier methods to collect sleep information. The possibility of using smartphone apps to trigger data entry may be a particularly easy and reliable solution.38,39 Future larger sample studies are needed to explore enhancements to parent diary and questionnaire collection, to confirm this preliminary efficacy evidence, and to clarify the exact nature of possible sleep improvements with STS mattress use. If the efficacy of the STS mattress technology in improving both sleep onset and maintenance is confirmed in future trials, it may represent a low-risk middle step, after basic sleep hygiene and parent training in behavioral methods have been attempted and before pharmacologic intervention.11
Initial efficacy evidence also extended to parent-reported improvements in ease of falling asleep, challenging behavior/ task compliance during the day, and overall child and family quality of life. Qualitative improvements in parent-reported falling asleep may suggest that, in at least a subset of children, the STS mattress technology provided a method for parents to help their children decrease autonomic activation and calm themselves sufficiently to promote sleep onset. Heightened nighttime activation has been noted in previous studies documenting sleep problems in ASD and is consistent with the general notion of hyperarousal seen in some children with autism. By providing vestibular stimulation, it is possible that the STS mattress technology activates the vagus nerve, promoting broad parasympathetic regulation of the arousal system. Direct evaluation of this possibility and further enhancements to the STS mattress technology, such as using sound-vibration combinations that more efficiently induce relaxation should be explored in future studies. Additionally, if future studies using larger samples confirm a range of positive efficacy outcomes, it may be possible to identify subgroups of responders and baseline characteristics that predict response.
The observation of improvements in challenging behavior/ task compliance and child and family quality of life highlight the importance for improvements in sleep to daily behavior and the overall functioning of the child and family system.8 Intriguingly, exploratory analyses suggested that individuals with poorer sleep hygiene showed the largest improvements in child and family quality of life, although it is possible that this reflects correlated regression to the mean of these measures. Taken together with the above findings regarding ease of falling asleep, these results may suggest that the STS mattress technology is useful for helping children with sleep onset problems get to sleep faster, potentially decreasing parent-child conflict over bedtime and improving parent and family quality of life. While the present results should be considered very preliminary, they highlight the need to evaluate a broader set of efficacy measures in future trials and to explicitly test moderator and mediator models to better understand what factors influence outcome and what factors might be driving broader improvements.
This preliminary study had several limitations. First, the intent-to-treat sample is small and was only adequately powered for medium-sized effects. While this precludes the ability to capture smaller effects, some of which may have clinical significance, in many cases the ability to detect medium-sized effects will capture most clinically relevant sleep improvements. Second, the observation period was short—only two weeks per arm—and a single crossover was used. Future studies would be wise to not only include larger numbers of children but also to extend the window of treatment and possibly even to use multiple crossovers. Multiple crossovers might be particularly attractive for this type of intervention because the intervention is not possible to blind to parents or children and using multiple on/off crossovers may help to decrease initial placebo or expectancy effects. Multiple applications of the STS mattress technology over time may also help to understand the temporal course of the improvements seen in the present study. Do these improvements build or diminish over time? Is there a waxing and waning of improvement? Is a certain dose necessary after which the technology can be weaned? These questions will be crucial for understanding the best application of this novel treatment modality. Multiple crossovers can also increase statistical power, as the patient becomes their own control, and smaller, potentially clinically-relevant effects may be detected. Alternatively, the substantial prevalence of sleep problems in ASD and the methodological rigor of randomized, parallel group designs may favor this approach as a next step in investigating STS mattress tolerability and efficacy. Relative to crossover designs, parallel group trials also have the advantage of allowing for extended observation periods and comparison of STS mattress on vs. off conditions. Third, we did not collect precise information about exactly how the mattress technology was used and parents were given significant discretion. This was done intentionally to maximize external validity by attempting to approximate real-world uses. However, future studies should carefully track sound level, sound type, and length of use each night to determine if these factor influence tolerability or efficacy. Lastly, the a priori focus of this study was on tolerability and parent-reported sleep quality. As a result, the only single-blinded efficacy outcomes, actigraphy-derived measures, were designated as secondary outcomes. This was done intentionally because it is often important to establish safety and tolerability before more rigorously evaluating efficacy and because there were no preexisting data regarding the potential onset and duration of STS mattress efficacy. Parent report, though, is often biased and may not reflect true sleep onset or nighttime and morning awakenings. This is further exacerbated by the lack of blinding of the intervention. Unfortunately, due to the naturalistic nature of this study, video and EEG data were not available to verify and more accurately detect awakenings. Future studies would be wise to adopt a blinded or objective measure as a primary efficacy outcome to more stringently test the value of the STS mattress technology in improving sleep. The present results suggest that shorter duration studies can be adequately powered but that longer duration would be useful for observing the natural course of improvements. Even with these limitations in mind, the present results suggest that a large sample, phase II clinical trial of the STS mattress technology is warranted.
DISCLOSURE STATEMENT
This study was supported by Kugona LLC which produces the Sound-To-Sleep mattress technology examined in the present study. Dr. Frazier has received federal funding or research support from, acted as a consultant to, received travel support from, and/or received a speaker's honorarium from the Cole Family Research Fund, Simons Foundation, Ingalls Foundation, Forest Laboratories, Ecoeos, IntegraGen, Kugona LLC, Shire Development, Bristol-Myers Squibb, National Institutes of Health, and the Brain and Behavior Research Foundation. The other authors have indicated no financial conflicts of interest. Investigational Use: The Sound-To-Sleep mattress technology system was determined to not require an investigational device (IND) use exemption.
ACKNOWLEDGMENTS
The authors thank all of the parents and children who participated in this study.
ABBREVIATIONS
- ABA
Applied Behavior Analysis
- ABC
Aberrant Behavior Checklist
- ASD
autism spectrum disorder
- CCC-2
Children's Communication Checklist – Second Edition
- CFQL-2
Child and Family Quality of Life – Second Edition
- CONSORT
Consolidated Standards of Reporting Trials
- CSHQ
Children's Sleep Habits Questionnaire
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- FISH
Family Inventory of Sleep Habits
- IBM SPSS
International Business Machines Statistical Package for the Social Sciences
- SRS-2
Social Responsiveness Scale – Second Edition
- SSP
Short Sensory Profile
- STS
Sound-To-Sleep
- WASO
wake after sleep onset
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2009;374(9701):1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds AM, Malow BA. Sleep and autism spectrum disorders. Pediatr Clin North Am. 2011;58(3):685–698. doi: 10.1016/j.pcl.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Couturier JL, Speechley KN, Steele M, Norman R, Stringer B, Nicolson R. Parental perception of sleep problems in children of normal intelligence with pervasive developmental disorders: prevalence, severity, and pattern. J Am Acad Child Adolesc Psychiatry. 2005;44(8):815–822. doi: 10.1097/01.chi.0000166377.22651.87. [DOI] [PubMed] [Google Scholar]
- 5.Souders MC, Mason TB, Valladares O, et al. Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep. 2009;32(12):1566–1578. doi: 10.1093/sleep/32.12.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphreys JS, Gringras P, Blair PS, et al. Sleep patterns in children with autistic spectrum disorders: a prospective cohort study. Arch Dis Child. 2014;99(2):114–118. doi: 10.1136/archdischild-2013-304083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sikora DM, Johnson K, Clemons T, Katz T. The relationship between sleep problems and daytime behavior in children of different ages with autism spectrum disorders. Pediatrics. 2012;130(Suppl 2):S83–S90. doi: 10.1542/peds.2012-0900F. [DOI] [PubMed] [Google Scholar]
- 8.Doo S, Wing YK. Sleep problems of children with pervasive developmental disorders: correlation with parental stress. Dev Med Child Neurol. 2006;48(8):650–655. doi: 10.1017/S001216220600137X. [DOI] [PubMed] [Google Scholar]
- 9.Blackmer AB, Feinstein JA. Management of sleep disorders in children with neurodevelopmental disorders: a review. Pharmacotherapy. 2016;36(1):84–98. doi: 10.1002/phar.1686. [DOI] [PubMed] [Google Scholar]
- 10.Rossignol DA, Frye RE. Melatonin in autism spectrum disorders: a systematic review and meta-analysis. Dev Med Child Neurol. 2011;53(9):783–792. doi: 10.1111/j.1469-8749.2011.03980.x. [DOI] [PubMed] [Google Scholar]
- 11.Malow BA, Byars K, Johnson K, et al. A practice pathway for the identification, evaluation, and management of insomnia in children and adolescents with autism spectrum disorders. Pediatrics. 2012;130(Suppl 2):S106–S124. doi: 10.1542/peds.2012-0900I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gail Williams P, Sears LL, Allard A. Sleep problems in children with autism. J Sleep Res. 2004;13(3):265–268. doi: 10.1111/j.1365-2869.2004.00405.x. [DOI] [PubMed] [Google Scholar]
- 13.Malow BA, Katz T, Reynolds AM, et al. Sleep difficulties and medications in children with autism spectrum disorders: a registry study. Pediatrics. 2016;137(Suppl 2):S98–S104. doi: 10.1542/peds.2015-2851H. [DOI] [PubMed] [Google Scholar]
- 14.Leskovec TJ, Rowles BM, Findling RL. Pharmacological treatment options for autism spectrum disorders in children and adolescents. Harv Rev Psychiatry. 2008;16(2):97–112. doi: 10.1080/10673220802075852. [DOI] [PubMed] [Google Scholar]
- 15.Vriend JL, Corkum PV, Moon EC, Smith IM. Behavioral interventions for sleep problems in children with autism spectrum disorders: current findings and future directions. J Pediatr Psychol. 2011;36(9):1017–1029. doi: 10.1093/jpepsy/jsr044. [DOI] [PubMed] [Google Scholar]
- 16.Adkins KW, Molloy C, Weiss SK, et al. Effects of a standardized pamphlet on insomnia in children with autism spectrum disorders. Pediatrics. 2012;130(Suppl 2):S139–S144. doi: 10.1542/peds.2012-0900K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malow BA, Adkins KW, Reynolds A, et al. Parent-based sleep education for children with autism spectrum disorders. J Autism Dev Disord. 2014;44(1):216–228. doi: 10.1007/s10803-013-1866-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson CR, Turner KS, Foldes E, Brooks MM, Kronk R, Wiggs L. Behavioral parent training to address sleep disturbances in young children with autism spectrum disorder: a pilot trial. Sleep Med. 2013;14(10):995–1004. doi: 10.1016/j.sleep.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardan AY, Gengoux GW, Berquist KL, et al. A randomized controlled trial of Pivotal Response Treatment Group for parents of children with autism. J Child Psychol Psychiatry. 2015;56(8):884–892. doi: 10.1111/jcpp.12354. [DOI] [PubMed] [Google Scholar]
- 20.Feldman R, Singer M, Zagoory O. Touch attenuates infants' physiological reactivity to stress. Dev Sci. 2010;13(2):271–278. doi: 10.1111/j.1467-7687.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 21.Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051. [PubMed] [Google Scholar]
- 22.Fawkes DB, Malow BA, Weiss SK, et al. Conducting actigraphy research in children with neurodevelopmental disorders--a practical approach. Behav Sleep Med. 2014;13(3):181–196. doi: 10.1080/15402002.2013.854245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Constantino JN, Gruber CP. The Social Responsiveness Scale Manual. 2nd ed. Los Angeles, CA: Western Psychological Services; 2012. [Google Scholar]
- 24.Frazier TW, Ratliff KR, Gruber C, Zhang Y, Law PA, Constantino JN. Confirmatory factor analytic structure and measurement invariance of quantitative autistic traits measured by the Social Responsiveness Scale-2. Autism. 2014;18(1):31–44. doi: 10.1177/1362361313500382. [DOI] [PubMed] [Google Scholar]
- 25.Aman MG, Singh NN, Stewart AW, Field CJ. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic. 1985;89(5):485–491. [PubMed] [Google Scholar]
- 26.McIntosh DN, Miller LJ, Shyu V, Dunn W. Overview of the Short Sensory Profile (SSP) In: Dunn W, editor. The Sensory Profile. San Antonio, TX: The Psychological Corporation; 1999. pp. 59–73. [Google Scholar]
- 27.Markowitz LA, Reyes C, Embacher RA, Speer LL, Roizen N, Frazier TW. Development and psychometric evaluation of a psychosocial quality-of-life questionnaire for individuals with autism and related developmental disorders. Autism. 2016;20(7):832–844. doi: 10.1177/1362361315611382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bishop DVM. Children's Communication Checklist-2 Manual. San Antonio, TX: Pearson; 2006. US ed. [Google Scholar]
- 29.Malow BA, Crowe C, Henderson L, et al. A sleep habits questionnaire for children with autism spectrum disorders. J Child Neurol. 2009;24(1):19–24. doi: 10.1177/0883073808321044. [DOI] [PubMed] [Google Scholar]
- 30.Altman DG. Practical Statistics for Medical Research. London, UK: Chapman and Hall; 1991. [Google Scholar]
- 31.McNemar Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 1947;12(2):153–157. doi: 10.1007/BF02295996. [DOI] [PubMed] [Google Scholar]
- 32.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6(2):461–464. [Google Scholar]
- 33.Bryk AS, Raudenbush SW. Hierarchical Linear Models: Applications and Data Analysis Methods. Newbury Park, CA: Sage; 1992. [Google Scholar]
- 34.Benjamini Y, Hochberg T. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat. 2000;25(1):60–83. [Google Scholar]
- 35.Erdfelder E, Faul F, Buchner A. GPOWER: a general power analysis program. Behav Res Methods Instrum Comput. 1996;28(1):1–11. [Google Scholar]
- 36.Borenstein M, Hedges LV, Rothstein H, Cohen J, Schoenfeld D SPSS. IBM SPSS SamplePower v3.0.1. Somers, NY: IBM Corp.; 2011. [Google Scholar]
- 37.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Clin Oral Investig. 2003;7:2–7. doi: 10.1007/s00784-002-0188-x. [DOI] [PubMed] [Google Scholar]
- 38.Rofey DL, Hull EE, Phillips J, Vogt K, Silk JS, Dahl RE. Utilizing ecological momentary assessment in pediatric obesity to quantify behavior, emotion, and sleep. Obesity (Silver Spring) 2010;18(6):1270–1272. doi: 10.1038/oby.2009.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ehlers DK, Huberty J, Buman M, Hooker S, Todd M, de Vreede GJ. A Novel inexpensive use of smartphone technology for ecological momentary assessment in middle-aged women. J Phys Act Health. 2016;13(3):262–268. doi: 10.1123/jpah.2015-0059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.