Abstract
Introduction
Spontaneous preterm birth (PTB) and preterm premature rupture of the membranes (pPROM) remain as a major clinical and therapeutic problem for intervention and management. Current strategies, based on our knowledge of pathways of preterm labor, have only been effective, in part, due to major gaps in our existing knowledge of risks and risk specific pathways.
Areas covered
Recent literature has identified physiologic aging of fetal tissues as a potential mechanistic feature of normal parturition. This process is affected by telomere dependent and p38 mitogen activated protein kinase (MAPK) induced senescence activation. Pregnancy associated risk factors can cause pathologic activation of this pathway that can cause oxidative stress induced p38 MAPK activation leading to senescence and premature aging of fetal tissues. Premature aging is associated with sterile inflammation capable of triggering preterm labor or preterm premature rupture of membranes. Preterm activation of p38MAPK can be considered as a key contributor to adverse pregnancies.
Expert Opinion
This review considers p38MAPK activation as a potential target for therapeutic interventions to prevent adverse pregnancy outcomes mediated by stress factors. In this review, we propose multiple strategies to prevent p38MAPK activation and its functional effects.
1. INTRODUCTION
1.1. Preterm birth pathways
Preterm birth prior to 37 weeks gestation is a leading cause of neonatal mortality [1]. It affects approximately 550,000 pregnancies (11.8%) in the United States each year. The most common (60%) phenotype of preterm birth occurs spontaneously with no known causality. Preterm premature rupture of the membranes (pPROM) occurs in about 3%–4% of all pregnancies, including many without identifiable risk factors and is directly antecedent to 40% of all spontaneous preterm birth (PTB).[1,2,3,4] Although PTB with intact membranes and with pPROM have similar etiologies, it is likely that these conditions are resulting from the activation of one or more biochemical pathways [5,6]. Hence, it is now considered a “multifactorial syndrome with heterogeneity in pathophysiology and biomarkers”. The infants who do survive PTB and pPROM are at significant risk for long-term morbidities, including bronchopulmonary dysplasia, asthma, intraventricular hemorrhage, cerebral palsy, delayed development, learning disabilities, and hearing loss. Although the outcome of babies born preterm has improved drastically during the past 40 years, this is largely due to improvements and innovations in neonatal care [7–11]. A better understanding of risk factors, risk associated pathophysiology, biochemical markers and mechanistic factors that activate the preterm labor process are required to develop screening markers and identify intervention targets to reduce the risk of PTB [12,13][1]
This review will introduce the targeting of the p38α/β MAPK stress response pathway for PTB and pPROM intervention based on novel findings of our ongoing studies and current literature. We will review current intervention strategies, introduce a biological basis new target intervention and discuss the biological and pharmaceutical approaches for this intervention.
1.2. Infectious and sterile inflammatory pathways of PTB and pPROM
Current literature identifies inflammation and oxidative stress as the two most significant pathophysiologic factors that initiate a chain of events resulting in PTB and or pPROM [5,14–16]. Current knowledge of the role of inflammation and oxidative stress-associated PTB and pPROM is based on studies of infections of the amniotic cavity [5,17]. Bacteria from cervical-vaginal flora breach the cervix to invade the intrauterine cavity. There, they activate the host immune response, mostly by increasing the production of proinflammatory cytokines (e.g. IL-1β, TNF–α, IL-6), chemokines (e.g. IL-8, CCL-10) and matrix degrading enzymes (MMP1, MMP9)[5,14,15,18,19]. This overwhelms the anti-inflammatory cytokines that are normally produced in the placenta or fetal membranes to favor the survival of the fetal allograft leading to premature decidual activation, cervical ripening, membrane rupture, labor and delivery. Several reports have shown that inflammatory cytokine, chemokine and matrix metalloproteinase activation promote PTB and pPROM due to infection, mostly mediated through NF-κB activation [6,20–28]. The NF-κB stress response pathway is a well-established transcriptional activator of many pro-inflammatory genes associated with PTB and pPROM. Although infections account for only a subset of PTB inflammation does play a key role in PTB and pPROM[29]. In many cases, there is, however, no clear pathogen that can be identified as an inflammation-causative factor thus suggesting an alternative non-inflammatory mechanism of preterm labor initiation pathways that contribute to the failure of current interventions. It is thus likely that other factors such as genetic and epigenetic, behavioral, socio-economic factors, maternal stress, environmental toxins and pollutants may generate, and contribute to the inflammatory responses, in synergy with infection, thus complicating the responses that result in PTB or pPROM [3,30–34].
Although microbial invasion of the amniotic cavity (MIAC) has been established as an important factor associated with PTB and pPROM, sterile intra-amniotic inflammation is thought to be more common than microbial-associated intra-amniotic inflammation in patients with PTB and pPROM [35,36]. Sterile inflammation is mostly contributed by risk factors described above in a non-infectious scenario. In a recent review, Behnia et al have pointed out the mechanistic pathways leading to infectious inflammation and reported sterile inflammation in the absence of MIAC.[37] Infectious inflammation and sterile inflammation produce similar biochemical signature; however, the initiators of the pathways and mediator are distinct and some of the risk factor contributing to sterile inflammation are described in the above paragraph.
1.3. Current interventions and need for novel interventions
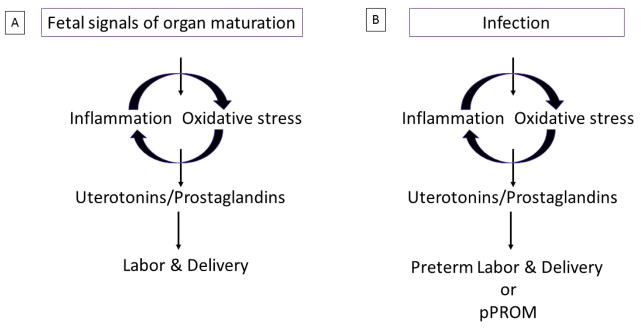
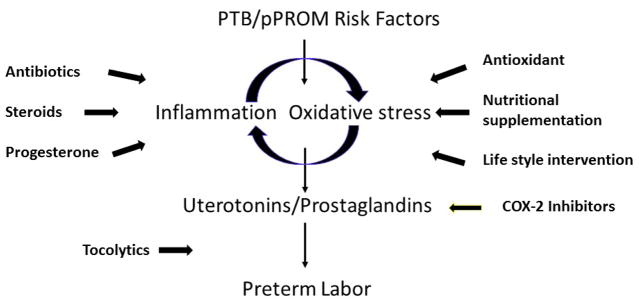
Figure 1A summarizes the pathway by which inflammation and oxidative stress, can trigger maternal contractility and cervical changes producing uterotonic agents such as prostaglandins [6,38,39]. As summarized in Figure 2, many current intervention strategies are designed to control the activation of this pathway. Inflammation and oxidative stress induced by various risk factors of pregnancy have been targeted using a variety agents to control (attenuate) the pathways that eventually lead to preterm deliveries. These include antibiotics, antiinflammatory agents including uterotonic inhibitors, tocolytics, antioxidants, endocrine regulators, in addition to behavioral and life style interventions and providing advanced and personalized in home care. However, recent systematic reviews and meta-analyses show that some existing strategies may prolong the gestational period but no interventions have been effective in reducing the incidence of global PTB rate [40–52]. Prolonging pregnancies are indeed beneficial in that they allow clinicians to administer therapeutic agents such as glucocorticoids thereby accelerating overall fetal growth and maturation processes [53,54]. The lack of consensus among care providers on the best approach for management of PTB is likely due to complexities of PTB pathways, biomarkers and their interactions thus leading to clinical ambiguity in determining management strategies.
Figure 1.
A schematic representation of term and preterm pathways.
1A: Fetal signals of organ maturation and fetal tissue senescence can cause an inflammatory oxidative stress response in the intrauterine tissues, activating various pro-labor mediators like prostaglandins and endothelins to cause labor associated changes.
1B: These signals are prematurely replaced by maternal-fetal risk exposures to drive the mechanistic pathways to cause spontaneous preterm labor and or pPROM.
Figure 2.
Current intervention or management strategies to reduce the risk of spontaneous preterm birth (PTB) based on specific pathways. (This is not a complete list)
In addition to those in clinical use, several intervention agents (drugs) have recently been reported in the literature based on mechanistic studies and animal model testing. These drugs are primarily inhibitors of inflammatory pathway. Their targets included inhibition of NF-κB [55–58], enhancers of antiinflammatory peroxisome proliferator activated receptor (PPARs) [59], and antioxidant N-acetyl cysteine[60,61], cytokine suppressive anti-inflammatory drugs [62,63], agents to regulate Glycogen synthase kinase 3 (GSK3), an enzyme crucial for inflammation homeostasis [64], surfactant protein-A and PGJ2 to down regulate TLR mediated inflammation [65,66], targeted IL-6[67], and IL-1β signaling [68] to reduce inflammation, over antiinflammatory suppression using IL-10[69–71]. Although many of these (drugs) agents were successful in reducing inflammation or oxidative stress in vitro, some of them were shown to have toxic side effects in animal model trials or in clinical trials, and successful clinical trial outcomes are thus yet to be reported using any of these agents in reducing the risk of PTB.
1.4. Oxidative stress and its association with adverse pregnancy outcome
As the search for novel intervention targets continue, it is clear that many of the agents in use or being tested are focused on inflammatory pathways. As mentioned above, sterile inflammation dominates many cases of PTB including PTB preceded by pPROM [36]. Our investigation of the source of sterile inflammation in non-infectious PTB and pPROM settings have led us to conclude that damage due to oxidative stress dominates many cases of PTB and pPROM in the absence of infection [37,72]. Healthy pregnancy, as with other physiological states, is characterized by a stable redox balance between ROS and antioxidants [16]. There is a paucity of studies specifically addressing the mechanistic role of ROS in PTB and pPROM [73–79]. A recent systematic review of PTB and pPROM biomarkers reveled that oxidative stress pathway markers are hardly investigated for their association with PTB and pPROM [80]. Similarly several clinical trials have been conducted to minimize ROS and improve pregnancy outcome with a marginal success rate [81–83]. Many of these trials were conducted to reduce the risk of preeclampsia, with an underlying ROS pathology with defective placentation. Failure of these trials can be attributed to knowledge gaps in the field where proper basic science studies are needed to identify proper targets for antioxidant intervention or pathways affected by oxidative stress induced damages [72].
1.5. Oxidative stress associated p38 mitogen activated protein kinase (p38MAPK) activation in intrauterine tissues
Failure or minimal impact of antioxidant trials in reducing the risk of PTB and pPROM contributing to adverse outcomes, in a very small subset of high risk pregnant subjects, is suggestive of factors and pathways beyond antioxidant deficiencies. The Menon laboratory has recently been interested in understanding these factors that generate ROS and the mechanistic role of ROS in PTB and pPROM. Using an in vitro model of human fetal membrane tissues, we have reported the activation of a senescence phenotype in response to oxidative stress inducing factors (cigarette smoke, environmental pollutants, damage associate molecular pattern (DAMPs) [84–87].
p38MAPKs are a family of four stress response signaling isoforms (p38α, β, γ, δ) that are evolutionarily conserved serine/threonine kinases (mitogen activated protein kinases) whose functions differ significantly. Although there are four p38MAPK isoforms, the α and β isoforms are the proteins whose activities are associated with normal physiological processes [88,89]Although less is known about these latter isoforms, Risco et al provided a more recent summary of possible roles for these forms[90]. Also gamma can cause cell cycle arrest resulting from gamma irradiation [91] and both gamma and delta can cause senescence induced by oncogenic Ras [92]. p38MAPK activity induces programmed cell death via the apoptosis signal-regulating kinase (ASK1)-signalosome [93,94]. ROS-mediated oxidation of ASK1 activates the p38MAPK and its downstream effectors, phospho-p38MAPK (P-p38), p16Ink4 and p19arf, resulting in cell cycle arrest and senescence. Furthermore, studies by Hsieh et al have shown that ROS generated by dysfunctional electron transport in mitochondria activate the inflammatory ASK1-P-p38MAPK pathway [94,95]. Using an in vitro model of amnion epithelial cell cultures exposed to cigarette smoke extract (oxidative stress inducer), we have reported concurrent activation of the ASK1-associated P-p38α/βMAPK pathway [87]. Our screening of different forms of p38MAPK did not determine the presence of γ and δ subunits in the fetal membrane cells, p38MAPK referred in the rest of this review discusses p38α/β subunits unless otherwise indicated. The ASK1-signalosome, a signaling complex composed of several well-characterized proteins [93,96], [97] can be oxidized by ROS, causing thioredoxin to dissociate from the complex and leading to the phosphorylation of p38MAPK and its downstream effectors, p16ink4 and p19arf. We have observed coordinated ASK1, P-p38MAPK and p19Arf expression following cigarette smoke extract exposure, with a kinetic pattern consistent with oxidative stress. The amnion cells also exhibited a senescent phenotype, known to be a response to ASK1 activation, manifested by senescence associated β-galactosidase staining. We have further demonstrated that ROS signaling eventually leads to telomere shortening, cell cycle arrest and irreversible arrest of cell proliferation. Telomere fragments released as a result of oxidative damage and DAMPs such as high mobility group box (HMGB)1 produced in response senescent associated tissue injury further enhances p38MAPK activation in a feedback loop [98]. Examination of clinical specimens for the activation of these pathways revealed that they are evident in pPROM as well as in a subset of PTB with intact membranes complicated by oxidative stress. [99] Therefore, oxidative stress damage contributes to tissue injury and this injury leads to a vicious cycle of sterile inflammatory activation. Our in vitro studies also revealed that the inflammatory markers increased in response to either oxidative stress or due to tissue injury were down regulated by co-treatment with p38MAPK inhibitor SB203580. The down regulation of sterile inflammation induced by oxidative stress by SB203580 supports the postulate that regulation of the p38MAPK pathway may reduce the risk of oxidative stress damage-induced pathways and sterile inflammation generated by damaged cells.
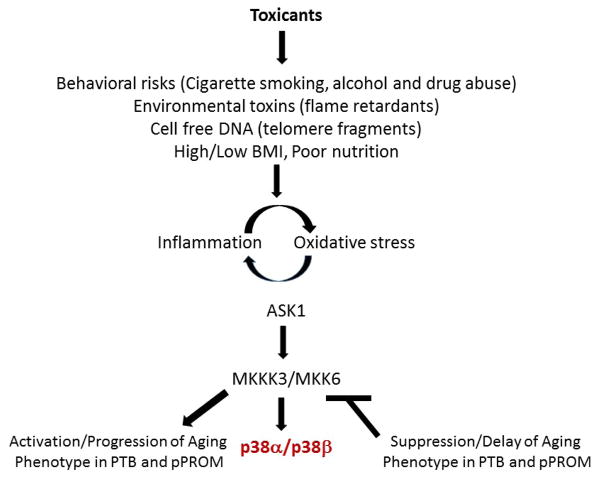
Figure 3, summarizes p38MAPK pathway activation in response to various risk factors. Furthermore, studies have shown that elevated levels of activated p38MAPK are associated with the activation of multiple upstream transcription factors that include the MAPKAPK family, ATF-2, HMGB1, etc., as well as the levels of expression of multiple downstream senescence markers such as tumor suppressor genes of the Cdkn2a locus [97,100,101]. Altogether, our studies have described the physiological progression of an age-associated phenotype that includes the activation of the p38MAPK stress response signal transduction pathway (Figure 3) that plays an important role in the inflammation – oxidative stress mediated induction of the senescence phenotype associated with pPROM and PTB. We thus propose that the p38α/β MAPK stress response pathway is a potential therapeutic target for the control and prevention of pre-term birth. Our results and those of others strongly suggest that the p38MAPK stress response pathway is a major target for the pharmaceutical development of therapeutic agents. In this review we discuss the potential use of small molecule inhibitors of p38MAPK stress response pathway that have been a) designed to impart their therapeutic effects solely on the site of inflammation and b) shown to alleviate the pathophysiological symptoms of diseases of inflammation and oxidative stress of multiple tissues. We present these inhibitors as potential examples and candidates for future development of therapeutic protocols for the delay of the progression of the aging phenotype in pPROM and PTB. Furthermore our goal is to demonstrate that a) the alleviation and/or delay, specifically, of expression of placental pathophysiological symptoms may be achieved by the antedrug and prodrug approach and, b) that the antedrug-prodrug concept be applied to the development of unique therapeutic protocols for the protection of both mother and fetus from toxic systemic effects during the treatment of pPROM and PTB.
Figure 3. Activation of the p38α/β MAPK stress response signaling pathway by exogenous toxicant factors.
Exogenous toxicants such as cigarette smoke extracts, flame retardants and bacterial lipopolysaccharide are major toxicants that activate the inflammation-oxidative stress promoted aging phenotype in PTB and preterm premature rupture of the membranes (pPROM).
2. The importance of the p38MAPK signaling pathway to the development of inhibitors
2.1. Significance of p38MAPK as a target for intervention
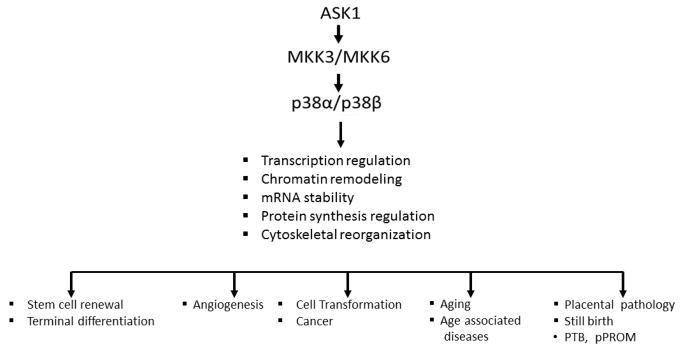
Development of inhibitors of p38MAPK is one of the most intensely studied areas of pharmacological research that focuses on the control of human diseases of inflammation and oxidative stress [102–107]. The aims of these research programs are to target the tissue-specific activity of the α and/or β isoforms for specific therapeutic purposes. The severity and frequency of human diseases of inflammation and oxidative stress makes the intense efforts at the development of ideal inhibitors of p38 a “hot topic” for high potency, low toxicity and long in vivo half-life. The advantage of such inhibitors lies in the fact that p38MAPK activity plays a significant controlling role in many diseases (Figure 4), thereby, targeting a broad range of pathologies [102,104,106–108]. The role of these inhibitors in alleviation and protection against the development of PTB and pPROM is an important part of this extensive research effort.
Figure 4. p38α/βMAPK are targets for pharmaceutical development.
Multiple diseases are associated with chronic inflammation and oxidative stress and elevated p38α/β MAPK activity. The symptoms of each disease listed above are ameliorated by specific inhibitors of p38MAPK.
2.2. Pathophysiological targets of the p38α/β MAPK isoforms of the p38MAPK family of stress response signaling proteins
The level of expression of p38MAPK isoforms (especially the α isoform) plays a key role in activation of the physiological processes of inflammation and oxidative stress. For example, the elevated levels of p38MAPK activity are associated with pathophysiological consequences shown in Figure 4, whereas normal levels of expression promote the physiologically important processes that regulate differentiation, stem cell replication and regenerative capacity (Figure 5). The major functions of these signal transduction proteins thus include linking extracellular signals to the intracellular machinery that regulates multiple normal physiological functions such as differentiation, replication (Figure 5), whereas elevated and sustained activity promote chronic inflammation and associated multiple diseases (Figure 4). The diversity of tissue distribution of the p38α/β MAPK isoforms suggests that their signal transduction targets involve multiple normal physiological functions as well as pathophysiological syndromes. In fact, the sustained over expression of the p38α/β isoforms is a critical physiological factor characteristic of a state of chronic inflammation in aging tissues and the inflammation-oxidative stress (ROS) that are crucial to the promotion of multiple human diseases (Figure 4).
Figure 5. The p38α and β isoforms play an important role in the regulation of multiple normal physiological processes.
Normal levels of expression of p38α/β MAPK promote the physiological processes that regulate differentiation, stem cell replication and cellular renewal in regeneration.
The state of chronic inflammation is a major contributor to the progression of the aging phenotype associated with multiple diseases that includes adverse pregnancy outcomes.[99,109]. There is a continuous concerted effort by pharmaceutical and academic research teams that focus on the development of potent non-toxic inhibitors of the p38α/β isoforms. The focus of these studies has been especially intense for inhibitors of p38α/β isoforms whose prolonged overexpression is associated with chronic inflammation. However, the critical physiological levels of expression associated with multiple normal physiological functions emphasize the importance of p38MPAK homeostasis and hence development of inhibitors of p38MAPK should not have unfavorable side effects. The intense research activity focusing on the development of inhibitors of p38MAPK isoform activities is based on anti-inflammatory, anti-oxidative stress therapeutics [110,111]. Although many inhibitors have been developed, the similarity of the structures of the α/β isoforms has caused reduced specificity due to the overlapping inhibition of both the α and β isoforms thereby posing major unwanted side effects. This is partially attributed to the fact that these subunits perform multiple physiologic functions in the body besides the pathophysiologic roles they play in specific circumstances such as stress.
2.3. Pathophysiological syndromes whose characteristics are alleviated by inhibitors of the p38MAPK activity
The severity and frequency of human diseases of inflammation and oxidative stress makes the intense efforts at the development of ideal inhibitors of p38α/β a “hot topic” for high potency, low toxicity and long in vivo half-life. The advantage of such inhibitors lies in the fact that the regulation (mostly down regulation) of p38MAPK activities plays a significant controlling role in many diseases (Figure 4), thereby, targeting a broad range of inflammatory disease pathologies [112–114]. We propose that these inhibitors might provide alleviation and protection against the development of PTB and pPROM.
Although the synthesis of inhibitors of p38α/β activity has been a major effort by academic research teams and pharmaceutical companies “unfortunately, many of the clinical trials have failed on the basis of the high levels of systemic toxicity observed in human clinical trials[95,115]. Furthermore, emphasis on the drug delivery procedure, a strategy that targets the drug to a specific tissue and inactivates the drug systemically provides control of systemic toxicity. Thus, in this review we discuss the feasibility of the use of antedrug/prodrug strategy to target specific treatment of fetal membranes experiencing inflammation-oxidative stress challenges and potential consequences of PTB and pPROM. This strategy should enable exploitation of the p38MAPK activities for specific therapeutic benefit. Importantly; however, the strategy of drug delivery must ensure complete protection of the fetus.
Our studies have also shown that p38MAPK are a) activated by oxidative stress generated by mitochondrial dysfunction of electron transport chain complexes I, II and III [95,115]; b) activate the expression of senescence markers [94]; and c) are major stress response signaling proteins that are activated by mitochondrial dysfunction due the Klotho mutation [85,94] and to such environmental factors as flame retardants, cigarette smoke extract and other environmental toxicants that initiate PTB or pPROM [37,84–87]. In this review we identify inhibitors of p38α/β shown to be potent and relatively non-toxic inhibitors that have been synthesized as antedrug and prodrug inhibitors for the therapeutic treatment of inflammation-associated syndromes, and present them as potential drugs for the targeted treatment of PTB and pPROM. Furthermore, we propose that the alleviation of symptoms of p38MAPK-associated diseases such as PTB/pPROM by inhibitors of MKK3 [116]; p38MAPK [117–119] convincingly supports our proposal that inhibition of this major signaling pathway is a logical approach to the development of pharmaceutical protocols. We thus focus on p38MAPK due to its direct regulation of the aging phenotype.
3. The design and mode of administration of inhibitors of p38MAPK for the treatment of PTB and pPROM
3.1. Design and strategy
Recent efforts have focused on the development of drugs that exhibit reduced severe side effects as well as mechanisms that provide potent non-toxic targeted treatment of a specific tissue [117,120,121]. These studies involved the identification of p38α/βMAPK “inhibitors that show prodrug or antedrug properties; The prodrug properties involve an inactive compound that undergoes metabolic transformation-activation in vivo to provide an active drug to a specific targeted tissue. The antedrug properties a) impart their effects solely on the site of inflammation; b) are rapidly metabolically inactivated upon entry into the systemic system thereby limiting unfavorable side effects due to activity in other tissues. Several groups have successfully synthesized such inhibitor(s) that show excellent p38α/β MAPK inhibitory activity; metabolic activation (prodrug) or high selectivity for kinases and rapid metabolism (either metabolic inactivation (antedrug) or metabolic activation (prodrug). We propose that through prodrug/antedrug therapeutics the drugs can be targeted to reach the site of p38MAPK action at feto-maternal interface and then be inactivated before its non-toxic metabolic products reach the fetus.
3.2. Prodrug and antedrug inhibitors of p38MAPK that successfully alleviate diseases of inflammation: The design and administration of prodrug and antedrug inhibitors of the p38MAPK pathway for therapeutics
3.2.1.Prodrugs
Prodrugs are inactivated drugs that undergo metabolic transformation in the targeted tissue (in vivo) to provide the active drug (Figure 6A). This pharmacological design provides an inactive prodrug thereby increasing its therapeutic indices by a) alteration of the physicochemical properties of the drugs e.g., solubility, stability, in vivo bioavailability; and other pharmacokinetic properties, and b) alteration of pharmacodynamics profiles so as to increase duration of pharmacological effects. Thus, based on the prodrug procedures, and upon the identification of an appropriate route of administration, a plan may be developed for the administration of medications for the intervention of high risk subjects for preterm labor and pPROM.
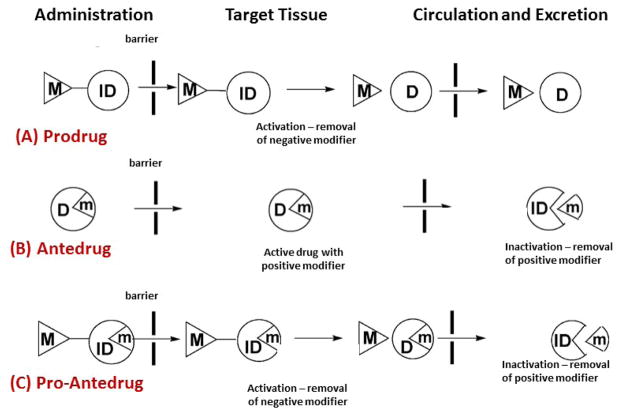
Figure 6. Pro, Ante and Pro-Ante drug models of p38MAPK inhibition.
(A) Prodrug, will undergo bioactivation to remove the negative modifier (M).
(B) Antedrug, the active drug, will undergo metabolic inactivation by removing the positive modifier to eliminate or minimize unwanted systemic effects.
(C) Pro-antedrug, contains both negative and positive modifiers where the negative modifier is removed first at the site of action (as in prodrug) and the positive modifier is removed later in the systemic circulation after its activity (as in antedrug). (Adopted from Khan MO et al (2008)
3.2.2. Antedrugs
Antedrugs are active drug-derivatives designed to undergo biotransformation thereby forming a readily excretable inactive form of the drug upon entry into the systemic circulation (Figure 6B). This design minimizes systemic side effects thereby increasing the therapeutic indices. Incorporation of a metabolically labile functional group into the active drug gives rise to the antedrug, which is active at the application site and on entering into the systemic circulation, is quickly metabolized to inactive molecules that are then eliminated thereby preventing the systemic side effects. Upon further examination of this procedure it may not be a favorable one considering that the activity of the antedrug, if administered orally may be significantly decreased during its transport to the placenta.
This procedure, however, continues to be actively developed for steroidal anti-inflammatory antedrug treatment for bronchial asthma [122], and in developing cytokine-inhibiting antedrugs for treatment of asthma [123,124]. For example, steroid acid esters with intact structures of potent glucocorticoids retain the ante-inflammatory activity of the parent compound but upon entry into the systemic circulation are hydrolyzed to form inactive readily excreteable steroid acids[125].
3.3. The application of antedrug and prodrug pharmacodynamics for treatments of PTB and pPROM
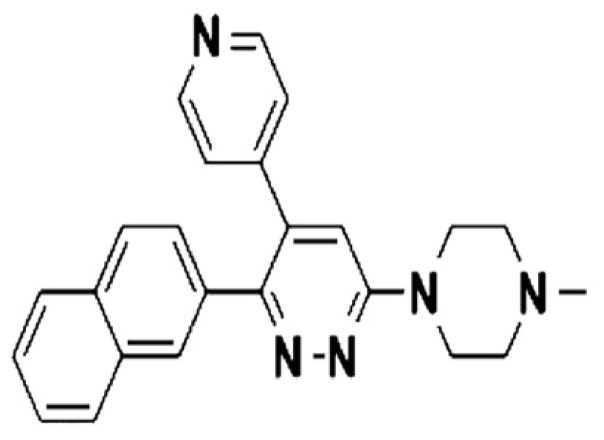
“Stress kinases are of special interest in neurological and neuropsychiatric disorders due to their involvement in synaptic dysfunction and complex disease susceptibility [126].” Clinical and pre-clinical evidence suggests that stress related p38α may be a potential neurotherapeutic target. For example, the p38α specific inhibitor MW01-18-150SRM (MW150) (Figure 7) has been shown to suppress hippocampal-dependent associative and special memory deficits in two distinct synaptic function mouse models [53]. A structural explanation for the target selectivity of MW 150 has been suggested by high resolution crystallographic structure of the p38α/MW150 complex by documenting the binding of the inhibitor to the active site. This inhibitor serves as an excellent example of an isoform-selective p38MAPK inhibitor (or as a kinase inhibitor) capable of attenuating and ameliorating in vivo stress related behaviors. This inhibitor also serves as an excellent candidate for the development of a prodrug or antedrug pharmaceutical protocol that targets a specific inhibitor such as MW150 to a specific tissue, thereby making a major contribution to the treatment of multiple inflammatory diseases. Further it facilitates the ideal opportunity to complement and compare the results of studies of genetic alteration of the expression levels of p38αMAPK with direct in vivo or in vitro inhibition using small molecule inhibitors.
Figure 7. Targeting human central nervous system protein kinases.
An isoform selective p38αMAPK inhibitor that attenuates disease progression in Alzheimer’s disease mouse models. (From Roy et al (2015) ACS Chem. Neurosci., 6, pp 666–680).
3.4. The prodrug strategy: addressing the issue of pH-dependent solubility and absorption
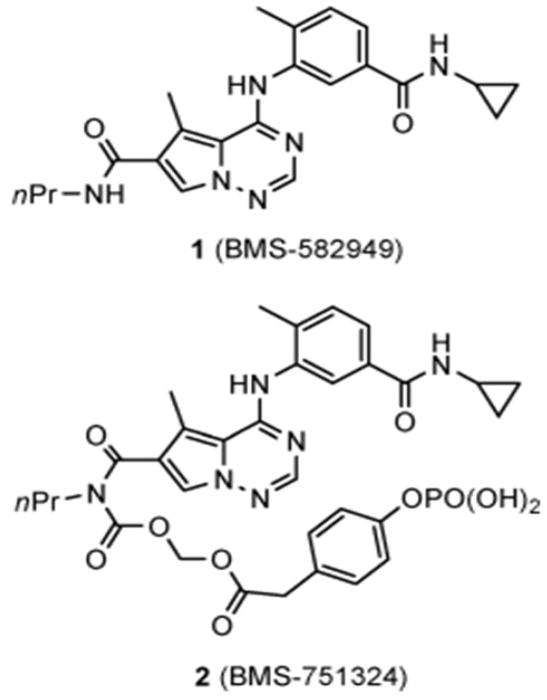
The clinical prodrug BMS-582949 (#1) is a potent inhibitor of p38α used in a phase II trial for the treatment of rheumatoid arthritis [108] (Figure 8). Complications arose, however, when the solubility of BMS-582949 was found to be significantly decreased when administered along with etanercept and infiximab (0.280 mg/mL at pH1.2 to 0.003 mg/mL at pH6.5) which caused a shift to pH 6.5[108]. Similar results were obtained when BMS #1 (Figure 8) was administered to humans with the H2 blocker, famotidine, which caused the stomach pH to increase and the exposure to the drug to decrease by 70%. To address this problem of pH-dependent solubility and absorbtion, i.e., to maintain stability in the pH1-7 range and to be enzymatically bioconverted to a safe parent drug before or during absorption thereby minimizing prodrug systemic ciruclation the drug was modified to most of these criteria. Based on the demonstraation that methylene phosphates are successful derivatives that enhance water solubility, this led to the synthesis of BMS-751324 (Figure 8 (BMS-751324)) a methylene phosphate derivative that met all the criteria listed above. This compoound was advanced to clinical development.
Figure 8.
Structure of p38α inhibitor 1 and its clinical prodrug 2.
The BMS#2 carbamoyl methylene phosphate derivative of BMS#1 resulted in a prodrug that is more stable and more soluble under both acidic and neutral conditions. It provided a significantly improved exposure of parent #1 in vivo compared to the administration of #1 itself. It effectively alleviated the LPS-induced TNFα and adjuvant arthritis in the rat model; did not show pH-dependence for solubility when administered with famotidine and the bioconverted #2 is efficiently inactivated by hydrolysis. These studies suggest that current development of the prodrug approach to pPROM and PTB treatment may provide a more favorable approach because it may be more easily designed for oral administration and targeting of the placenta.
3.5. A Design for the targeting of antedrugs to specific tissues – AKP-001, the p38MAPK antedrug
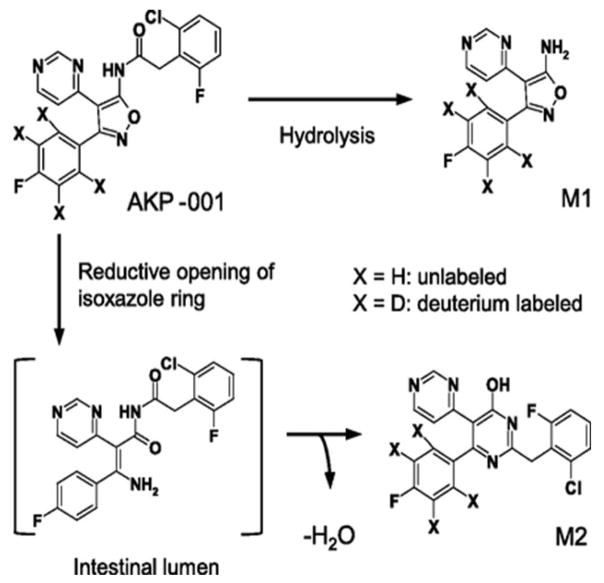
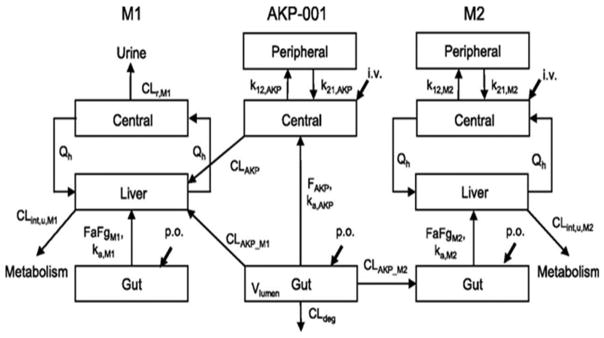
The development of the AKP-001 p38α MAPK inhibitor was designed for local targeting of specific drugs to specific tissues. This study has analyzed the in vivo disposition of a novel p38 MAPK inhibitor, AKP-001, and its metabolites in rats with a simple physiologically based pharmacokinetic mode [121]. AKP-001 (Figure 9) is a novel and potent p38α/β inhibitor designed for its tissue-specific targeting that inhibits the phosphorylation of downstream protein targets. [ICα 50 = 10.9 nM; ICβ 50 =326 nM. AKP-001] [121]. It was synthesized for the treatment of inflammatory bowel disease (IBD), a disease of severe inflammation and oxidative stress, following the principles of ante/soft drug design [121]. The active antedrug is easily administered (orally) to the gut and is rapidly inactivated systemically. It ameliorates experimental colitis induced by dextran sulfates in mice and 2,4,6,-trinitro enzone sufonic in rats [120]. The antedrug design of AKP-001 facilitates its rapid metabolism to an inactive form, M1, through first-pass metabolism in the intestine and liver after oral administration (Figure 9. AKP-001 is also partially metabolized in the normal intestine, producing a second metabolite (M2). [Figure 9]. The synthesis and administration of AKP-001 is an excellent example of an in vitro and in vivo pharmacokinetic model showing metabolic inactivation (M1 and M2), thereby identifying the factors affecting the pharmacokinetic characteristics of a parent active compound (AKP-001) and its metabolic inactivation. The results of this approach showed that AKP-001 is absorbed from the gut and is extensively metabolized by hepatic first-pass metabolism. Although the metabolism of AKP-001 to M1 also occurs in various tissues and biological fluids including the small intestine and plasma its major metabolic conversion occurs in the liver. For example, rat intestinal S9 fractions have been shown to metabolize ~16% of AKP-001 [which accounts for 80% of metabolites] after 15 min. AKP-001 is also hydrolyzed to M1 in rat plasma. This hydrolytic clearance (of AKT-001) in rat extracellular fluid is quite low compared with the total clearance; thus the hydrolytic clearance of AKP-001 and the contribution of plasma hydrolysis is minimal [Figure 10][121].” “In vitro inhibition studies indicate that M1 formation in rat liver S9 fractions is mediated by carboxylesterase, which, in fact was observed in various rat tissues [127]. “The second metabolite, M2, was found in plasma after oral administration of AKP-001, but not after intravenous injection. This indicates that M2 metabolism occurs specifically in the gut which is the target tissue. The metabolism of AKP-001 to M2 was shown to involve a reductive opening of the isoxazole ring; and the formation of M2 from AKP-001 in the GI tract is considered to be mediated by intestinal microflora.
Figure 9. Chemical structures and metabolic pathways of AKP-001 and its metabolites (M1 and M2).
AKP-001, 5-amino-3-(4-fluorophenyl)-4-(4-pyrimidinyl)isoxazole (M1), and 2′-(2-chloro-6-fluorobenzyl)-6′-(4-fluorophenyl)-[4,5′]bipyrimidinyl-4′-ol (M2) are the metabolic products of the (A) hydrolysis and (B) reductive opening of the isoxazole ring of AKP-001, respectively. These in vivo metabolic processes demonstrate the inactivation of this antedrug used for the treatment of inflammatory bowel disease (IBD).
Figure 10. Model scheme of AKP-001 and metabolite (M1 and M2) disposition in rats.
Distinct model structures were developed for the parent compound (AKP-001) and the metabolites (M1 and M2). A conventional two-compartment pharmacokinetic model with an incorporated gut compartment was applied for AKP-001 and was connected with that of M1 by first-pass metabolism and liver metabolism, assuming that hepatic metabolism mainly contributed to systemic formation of M1. For the production of M2, the pharmacokinetic model was linked to gut metabolism. Qh represents the hepatic blood flow. ka represents the absorption rate constant. k12 and k21 are the first-order rate constants for transfer from the central to peripheral and from the peripheral to central compartment, respectively. FaFg represents the intestinal availability. F represents the availability. CLint,u represents the hepatic unbound metabolic clearance. CLAKP represents the systemic clearance of AKP-001. CLAKP_M1 represents the clearance for metabolic conversion of AKP-001 to M1 by first-pass metabolism. CLAKP_M2 represents the metabolic clearance of AKP-001 to M2 in the gut compartment. CLdeg represents the clearance for metabolic conversion from AKP-001 to other metabolites. CLr represents the renal clearance. Subscripts: AKP, AKP-001; M1, metabolite M1; M2, metabolite M2.
Preliminary attempts to extrapolate the pharmacokinetic data from AKP-001 and its metabolites (M1 and M2) from rats to humans have suggested that the metabolic activity of AK-001 in human liver S9 fractions is approximately 3-fold higher than in rats; it is almost completely inhibited by bis(4-nitro phenyl)-phosphate, thus suggesting that the intrinsic hepatic clearance of AKP-001 in humans is higher than in rats. Furthermore, since carboxyesterase is also expressed in various human tissues, such as liver and intestine[128], it is expected that systemic exposure to AKP-001 after oral administration in humans would be quite low owing to extensive first pass metabolism. Importantly this avoids the undesirable side effects arising from systemic inhibition of p38α/βMAPK. The use of the AKP-001 antedrug for targeting placenta and membranes may require major modifications, e.g., to a prodrug form in order to target the placenta and membranes via oral administration.
The studies with the antedrug/soft drug approach for targeted delivery to the intestine (for inflammatory bowel disease [IBD]) suggest that this procedure may be applied to other tissues afflicted with inflammatory syndromes. This technique may thus be designed to exert a specific effect at the drug administration site, and then be rapidly metabolized to inactive compounds upon circulation [124,129]. One of the most successful therapeutic agents for IBD was shown to be highly metabolized to inactive forms in the liver [130]. This technique minimizes systemic off-target effects of the parent drug and thus raises the question as to whether it can be designed for PTB or pPROM, i.e., can the drug be targeted to the placenta or fetal membranes cells considering its rapid degradation (or metabolism to an inactive form) by maternal tissues. e.g., the liver, prior to its transport to the placenta? This would offset or minimize the effect of the parent drug after it has worked on the main target tissue, but raises the question of the degree of involvement of the placental tissues.
Many of the p38MAPK inhibitors have not been exhaustively tested for the management of inflammatory diseases, including inflammatory bowel diseases and rheumatoid arthritis because clinical trials have shown that systemic inhibition of p38MAPK cause adverse effects including abnormal liver function, dizziness and skin rashes [131].The recent development of low toxicity, potent inhibitors is an encouraging advance for their use as antedrug and prodrug therapeutics.
3.6. Cytokine inhibiting antedrugs
The severity of allergic asthma is associated with the influx of leukocytes (eosinophils, lymphocytes and monocytes) and their production of proinflammatory cytokines has encouraged the design of cytokine-inhibiting antedrugs with strong broncho-dilating effects in an animal model of asthma [123]. The triazinylphenylalkylthiazolecarboxylic acid esters are a group of pharmaceuticals designed to act as lung-specific antedrugs and inhibitors of the production of IL-5, a primary eosinophil-activating and proinflammatory cytokine. As a drug that qualifies to be an antedrug, it was shown that the hydroxypropyl ester has high metabolic stability (t1/2 = 240 min) in human lung S9 fraction (the target tissue), and rapid conversion (t1/2 = 15 min) into a pharmacologically inactive carboxylic acid by human liver preparations. Since allergic inflammation is a chronic lung disease characterized by air flow obstruction, airway inflammation [132] and multiple cytokine production (IL-4. IL-5, MCP1, MCP-2, MCP-3), the suppression of these proallergic cytokines poses a viable approach for the treatment of asthma [123,133]. The results of these experiments suggest a potential successful use of this procedure in the suppression of proinflammatory cytokines of PTB and pPROM.
4. Significance
Inflammation-oxidative stress mediated activation of the p38MAPK pathway activates the p16Ink4a and p19Arf senescence-associated genes of the Cdkn2a tumor suppressor locus [97,134,135]. Furthermore, ROS generated by electron transport chain dysfunction activates the aging phenotype via the p38α/β senescence pathway [97]. The significance of our discussion is unique and of the utmost importance in that our approach a) identifies a direct interaction of inflamnation-ROS with the complex p38MAPK protein-protein interactions that initiate the signling processes that link these challenges to the development of the placntal aging phenotype and PTB/pPROM of the amniotic cells; b) elucidates, in a stepwise manner, the mechanism(s) that link inflammtion-oxidative stress challenges to the ROS-responsive signaling pathways associated with the development of PTB and pPROM. and c) provide an understanding of the molecular interactions that impact the progression and potential prognosis of placental pathogenesis. Furthermore, we identify molecular interactions that are potential sites for the development of intervension protocols for the treatment of PTB and pPROM.
An important question on the relevance of the aging marker expression to PTB and pPROM asks whether inflammation and oxidative stress cause rather than correlate with the development of the PTB and pPROM. Demonstration that the decreased expresion of p38α/β and its targeted activation of aging phenotype markers addresses this question in that; a) inhibition of p38α/β decreases the expression of markers of aging; b) the attenuation of p38α/β in a haploinsuficient p38α+/− mouse delays the aging of pancreatic β-cells and skeletal muscle progenitor cells [134,136]. The p38MAPK inhibitors provide an experimental linkage of the molecular mechanisms activated by inflammtion-oxidative stress to the molecular mechanisms of initiation of PTB and pPROM. We are thus presenting a model which directly addresses physiological responses to inflammation and/or oxidative stress i.e., and the consequences of misregulation of a major biological redox controling system. This approach dissects, in a step-wise manner, the mechanism by which an inflammation-ROS-Sensory complex serves as a center for the distribution of signals that activate stress-response pathways and the development of PTB and pPROM [95,137].
4.1. Potential for beneficial translation of scientific knowledge to clinical practice
The importance of inhibitors of p38α/β is clearly suggested by the urgent need for specific, potent, low toxicity inhibitors that provide promising results in the alleviation of inflammatory diseases in animal models and human cohorts and the very large amount of funds provided by government and private agencies to support this line of research. We thus strongly encourage the development and use of p38α/β-specific inhibitors to achieve a favorable delay and/or attenuation of symptoms of PTB and pPROM. Our genetic animal model, the haploinsufficient p38α+/− mouse, suggests that treatment of wild type models with inhibitors of p38MAPK would significantly delay the progression of the PTB and pPROM by alleviating the symptoms of inflammation and oxidative stress [117–119].
Our proposed approach to the therapeutic treatment of PTB/pPROM provides the foundations for the pursuit of the use of inhibitors for the clinical treatment of pathologic pregnncies involving PTB and pPROM. Chronically increased p38αβ activity and its enhancement of the aging phenotype in PTB and pPROM can thus be attributed to elevated inflammation and oxidative stress. Our model further suggests that sequestration of p38MAPK with the p38α inhibitors, PH787904 or AKP-001, provides examples for the development of clinical pharmacological treatment for PTB and pPROM [85,135,138,139].
5. Expert opinion and conclusion
5.1. The key problem identified and addressed
PTB and pPROM are two major complications of pregnancy. Due to complex etiologies, pathways and biomarkers, proper early diagnosis of high risk pregnancies are rather difficult and hence interventions are administered very late when labor associated changes are already in progress. Tocolytic interventions prolong pregnancies in a subset of subjects. This approach; however, has not reduced the incidences of neonatal morbidities and mortalities due to PTB and pPROM. Similarly, advanced screening and diagnostic strategies for high risk pregnancies or interventions using progestational agents have not yeilded expected reduction in rate of global PTB. Therefore it is timely to discuss novel intervention strategies based on specific pathways that contribute to majority of PTB and pPROM. The pathway that is understudied in PTB and pPROM involve oxidative stress induced by various non infectious risk factors. Intraamniotic infections can also contribute to oxidative stress associated pathways but infection associated oxidative stress pathways are distnct than other risk factor induced pathways.64 We have now reported that oxidative stress in response to various non infectious risk factors accelarate intrauterine tissue aging to cause either premature activation of labor or cause rupture of membranes. Senescence, a mechanism associated with aging is prematurely activated by oxidative stress and damage that accompany oxidative stress. Cell and organelle injuries and damage to cellular elements, especially DNA, lead to activation of p38MAPK dependent pathway resulting in it’s activation and downstream cascade of events ending in senescence. Senescence, an irreversible sterile inflammatory state is a major pathologic feature of aging related syndromes. We propose that a major subset of PTB and pPROM belong to this pathological category. Therefore, controling p38MAPK activation and function can be one of the targets for intervention to reduce the risk of PTB and pPROM.
5.2. Innovative research achivements
Our studies have advanced a novel mechanism for the activation of pathophysiological processes that promote the development of the aging phenotype in PTB and pPROM and the use of antedrug-prodrug strategy to control and treat symptoms of systemic toxicity. Furthermore, we propose that the elevated and sustained endogenous levels of p38MAPK in abnormal pregnencies are key causal factors in the development of PTB and pPROM. We now propose that an innovative design of high-throughput screening of small-molecule libraries should identify unique potent, non-toxic inhibitors of p38MAPK that will attenuate or delay the symptoms of PTB and pPROM as well as elucidate novel biochemical mechanisms to provide understanding of the role of inflammation and oxidative stress in abnormal pregencies and the molecular linking of extracellular exposure to toxicants to the intracellular inflammation-ROS responsive machinery and the treatment of these syndromes.
5.3. Future implications
Because of their implications in the progression of multiple diseases of inflammation and oxidative stress, there is a need for the development of tissue-specific delivery methods that can target the treatment of a specific syndrome thereby avoiding systemic toxicity to multiple tissues. The intense research activity thus focusing on the development of inhibitors of p38MAPK activity (mainly p38α and β), because of their similarity in composition and structure is based on tissue-targeted anti-inflammatory, anti-oxidative stress therapeutics.
5.4 Challenges and goals
The simple physiologically based pharmacokinetic (PBPK) models presented in this review have described the pharmacokinetics of inhibition of p38MAPK by both antedrug and prodrug designs. The simulation indicated that low oral bioavailability is the predominant factor influencing the wide safety margin and that the drugs (AKP-001) satisfy criteria for tissue specific-targeting antedrug or prodrugs. These PBPK models can be applied to the prediction of safety margins and efficacy in clinical situations. The development of both antedrug and prodrug protocols have been successful for treatment of multiple-tissue inflammatory diseases. We propose that this design could guide the development of antedrug and prodrug protocols for placental syndromes including pPROM. We note here that the placental pathologies are linked to the progression of inflammation and that the activity of P-p38MAPK plays a key role in its progression. There are, therefore, many examples of the design of antedrugs and prodrugs that can be applied to placental protocols. The major problem that is still to be addressed is the identification of a safe route of administration.
Future research to test the efficacy should involve testing the drugs and designs in various animal models including non-human primates that resembles human pregnancies and have pathophysiologic pathways of adverse pregnancy events similar to that of humans.
Article highlights.
Human parturition at term is associated with telomere dependent and oxidative stress induced p38MAPK α/β (two dominant isoforms of p38 expressed in fetal tissues) mediated senescence of fetal tissues.
p38MAPK α/β mediated fetal tissue senescence generates signals (sterile inflammation) that can trigger term parturition.
Preterm parturition is associated to premature activation of p38MAPK α/β mediated senescence activation and signaling due to excessive oxidative stress and inflammation in response to various risk factors.
Risk factors such as behavioral (cigarette smoking, alcohol, drug abuse), environmental pollutants, malnutrition, high or low BMI, tissue damage products (in response to oxidative stress due to infection or other risk exposures) can cause preterm p38MAPK activation accelerating premature fetal tissue senescence and untimely signaling of initiation labor (preterm labor). This condition is more dominant in the subset of preterm birth complicated by preterm premature rupture of the membranes (pPROM).
Inhibition of p38MAPK α/β has revered oxidative stress induced senescence and sterile inflammation in pregnant animal models and in in vitro fetal membrane tissue/cell culture models.
Therapeutic interventions to slow down p38MAPK mediated senescence may be beneficial to reduce preterm labor risk in major subset of women with intrauterine oxidative stress due to various risk factors.
We present intervention strategies and a plan to control the activation and/or activity of the p38MAPK α/β stress response pathway specifically for in vivo treatment of intrauterine tissues.
The aims of this review are to present protocols that target specific p38α/β activities as a form of specific intervention of PTB and pPROM.
We discuss the development and use of antedrug and prodrug strategy to apply specific treatment of intrauterine tissues experiencing inflammation-oxidative stress induced p38MAPK α/β linked pathophysiology.
Acknowledgments
Funding
This review was supported by a NIH/NICHD grant (1R03HD08635401) and an Innovative Catalyst Grant from March of Dimes Center of Ohio to R Menon.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Reference annotations
* Of interest
** Of considerable interest
- **1.Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PF. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31–38. doi: 10.2471/BLT.08.062554. This is the very first estimation of global preterm birth rate and reports countrywdie breakdown of preterm birth rate and its consequences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero R, Yeo L, Miranda J, Hassan SS, Conde-Agudelo A, Chaiworapongsa T. A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. J Perinat Med. 2013;41:27–44. doi: 10.1515/jpm-2012-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **3.Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences, and Prevention. Washington DC: National Academies Press; 2007. IOM report estimated US preterm birth rate increase, determined economic impact of preterm birth, discuss causality, pathways, biomarkers and prevention strategies. [PubMed] [Google Scholar]
- 4.Mercer BM, Lewis R. Preterm labor and preterm premature rupture of the membranes. Diagnosis and management. Infect Dis Clin North Am. 1997;11:177–201. doi: 10.1016/s0891-5520(05)70348-2. [DOI] [PubMed] [Google Scholar]
- **5.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. A classic review of risk factors, their manifestiaon and pathways leading to preterm birth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **6.Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand. 2008;87:590–600. doi: 10.1080/00016340802005126. This review describes complexities of preterm birth pathways and biomarker netoworking. Review also provides explanation for disparity in preterm birth rate among different ethnic groups. [DOI] [PubMed] [Google Scholar]
- 7.McCrea HJ, Ment LR. The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clin Perinatol. 2008;35:777–92. doi: 10.1016/j.clp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *8.Mallard C, Davidson JO, Tan S, Green CR, Bennet L, Robertson NJ, Gunn AJ. Astrocytes and microglia in acute cerebral injury underlying cerebral palsy associated with preterm birth. Pediatr Res. 2013;75:234–240. doi: 10.1038/pr.2013.188. This manuscript describes the consequences of preterm birth specifically details the mechanisms associated with development of cerebral palsy. [DOI] [PubMed] [Google Scholar]
- 9.Bersani I, Thomas W, Speer CP. Chorioamnionitis--the good or the evil for neonatal outcome? J Matern Fetal Neonatal Med. 2012;25(Suppl 1):12–16. doi: 10.3109/14767058.2012.663161. [DOI] [PubMed] [Google Scholar]
- 10.Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med. 2000;343:378–384. doi: 10.1056/NEJM200008103430601. [DOI] [PubMed] [Google Scholar]
- 11.Kallen B, Finnstrom O, Nygren KG, Otterblad OP. Association between preterm birth and intrauterine growth retardation and child asthma. Eur Respir J. 2013;41:671–676. doi: 10.1183/09031936.00041912. [DOI] [PubMed] [Google Scholar]
- 12.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera GC, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- *13.Blencowe H, Lee AC, Cousens S, Bahalim A, Narwal R, Zhong N, Chou D, Say L, Modi N, Katz J, Vos T, Marlow N, Lawn JE. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res. 2013;74(Suppl 1):17–34. doi: 10.1038/pr.2013.204. Estimates the burden of preterm birth and provide recommendations for improved recording of all pregnancy outcomes and standard application of preterm definitions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *14.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. A review regarding the role inflammation and inflammatory markers in preterm birth inititation pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, Hassan SS. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- **16.Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122:369–382. doi: 10.1007/s00418-004-0677-x. This review describes the generation of oxidative and nitritive stress, mechanistic aspects oxidative stress generation and its role in pathologic complications of pregnancies. [DOI] [PubMed] [Google Scholar]
- 17.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. BJO1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 19.Kacerovsky M, Lenco J, Musilova I, Tambor V, Lamont R, Torloni MR, Menon R. Proteomic Biomarkers for Spontaneous Preterm Birth: A Systematic Review of the Literature. Reprod Sci. 2013;21:283–295. doi: 10.1177/1933719113503415. [DOI] [PubMed] [Google Scholar]
- *20.Menon R, Swan KF, Lyden TW, Rote NSFSJ. Expression of inflammatory cytokines (IL-1B, IL-6) in amniochorion. Am J Obstet Gynecol. 1995;172:493–500. doi: 10.1016/0002-9378(95)90562-6. This is one of the very first studies to show the production of inflammatory mediators by (host immune response) huamn fetal membranes, a fetal tissue in origin, during an infectious process in vitro and in clinical samples. [DOI] [PubMed] [Google Scholar]
- *21.Menon R, Fortunato SJ. The role of matrix degrading enzymes and apoptosis in rupture of membranes. J Soc Gynecol Investig. 2004;11:427–437. doi: 10.1016/j.jsgi.2004.04.001. This study using fetal membrane as a model demonstrated the association between of MMPs and apoptosis in pPROM. [DOI] [PubMed] [Google Scholar]
- 22.Menon R, Fortunato SJ. Fetal membrane inflammatory cytokines: a switching mechanism between the preterm premature rupture of the membranes and preterm labor pathways. J Perinat Med. 2004;32:391–399. doi: 10.1515/JPM.2004.134. [DOI] [PubMed] [Google Scholar]
- 23.Menon R, Fortunato SJ. Infection and the role of inflammation in preterm premature rupture of the membranes. Best Pract Res Clin Obstet Gynaecol. 2007;21:467–478. doi: 10.1016/j.bpobgyn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Menon R, Peltier MR, Eckardt J, Fortunato SJ. Diversity in cytokine response to bacteria associated with preterm birth by fetal membranes. Am J Obstet Gynecol. 2009;201:306. doi: 10.1016/j.ajog.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 25.Peltier MR, Richey LJ, Brown MB. Placental lesions caused by experimental infection of Sprague-Dawley rats with Mycoplasma pulmonis. Am J Reprod Immunol. 2003;50:254–262. doi: 10.1034/j.1600-0897.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 26.Peltier MR, Barney BM, Brown MB. Effect of experimental genital mycoplasmosis on production of matrix metalloproteinases in membranes and amniotic fluid of Sprague-Dawley rats. Am J Reprod Immunol. 2007;57:116–121. doi: 10.1111/j.1600-0897.2006.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *27.Peltier MR, Drobek CO, Bhat G, Saade G, Fortunato SJ, Menon R. Amniotic fluid and maternal race influence responsiveness of fetal membranes to bacteria. J Reprod Immunol. 2012;96:68–78. doi: 10.1016/j.jri.2012.07.006. Study showed racia disparity in immune response by fetal membranes in an in vitro model of infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velez DR, Fortunato SJ, Morgan N, Edwards TL, Lombardi SJ, Williams SM, Menon R. Patterns of cytokine profiles differ with pregnancy outcome and ethnicity. Hum Reprod. 2008;23:1902–1909. doi: 10.1093/humrep/den170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **29.Goldenberg RL, Gravett MG, Iams J, Papageorghiou AT, Waller SA, Kramer M, Culhane J, Barros F, Conde-Agudelo A, Bhutta ZA, Knight HE, Villar J. The preterm birth syndrome: issues to consider in creating a classification system. Am J Obstet Gynecol. 2012;206:113–118. doi: 10.1016/j.ajog.2011.10.865. This report highlighted the significance of prpoer definition of preterm birth phenotypes. [DOI] [PubMed] [Google Scholar]
- *30.Behnia F, Peltier M, Getahun D, Watson C, Saade G, Menon R. High bisphenol A (BPA) concentration in the maternal, but not fetal, compartment increases the risk of spontaneous preterm delivery. J Matern Fetal Neonatal Med. 2016:1–7. doi: 10.3109/14767058.2016.1139570. This report determined the association between environmental pollutant BPA and preterm birth and pPROM. Its high concentrations in maternal vs fetal compartments and how they differentially associate with distinct adverse outcome. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson KK, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. Phthalate metabolites and bisphenol-A in association with circulating angiogenic biomarkers across pregnancy. Placenta. 2015;36:699–703. doi: 10.1016/j.placenta.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Neill MS, Osornio-Vargas A, Buxton MA, Sanchez BN, Rojas-Bracho L, Castillo-Castrejon M, Mordhukovich IB, Brown DG, Vadillo-Ortega F. Air pollution, inflammation and preterm birth in Mexico City: study design and methods. Sci Total Environ. 2013;448:79–83. doi: 10.1016/j.scitotenv.2012.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peltier MR, Koo H, Getahun D, Menon R. Does exposure to flame retardants increase the risk for preterm birth? J Reprod Immunol. 2014;107:20–25. doi: 10.1016/j.jri.2014.11.002. S0165–0378(14)00157–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen T, Kuczynski E, O’Neill LM, Funai EF, Lockwood CJ. Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J Matern Fetal Med. 2001;10:297–300. doi: 10.1080/714904361. [DOI] [PubMed] [Google Scholar]
- **35.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, Kim YM. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2014:1–16. doi: 10.3109/14767058.2014.958463. This is one of the first report on sterile inflammation with similar bichemicalsignature as infectious inflammation reported to be associated with various advrese pregnancy outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Yeo L. Prevalence and Clinical Significance of Sterile Intra-amniotic Inflammation in Patients with Preterm Labor and Intact Membranes. Am J Reprod Immunol. 2014;72:458–474. doi: 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behnia F, Sheller S, Menon R. Mechanistic Differences Leading to Infectious and Sterile Inflammation. Am J Reprod Immunol. 2016 doi: 10.1111/aji.12496. [DOI] [PubMed] [Google Scholar]
- 38.Lockwood CJ. Recent advances in elucidating the pathogenesis of preterm delivery, the detection of patients at risk, and preventative therapies. Curr Opin Obstet Gynecol. 1994;6:7–18. [PubMed] [Google Scholar]
- **39.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. Details epidemiology, pathways and strategies of understanding pregnancy complications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villar J, Gulmezoglu AM, de OM. Nutritional and antimicrobial interventions to prevent preterm birth: an overview of randomized controlled trials. Obstet Gynecol Surv. 1998;53:575–585. doi: 10.1097/00006254-199809000-00025. [DOI] [PubMed] [Google Scholar]
- 41.Harvey NC, Holroyd C, Ntani G, Javaid K, Cooper P, Moon R, Cole Z, Tinati T, Godfrey K, Dennison E, Bishop NJ, Baird J, Cooper C. Vitamin D supplementation in pregnancy: a systematic review. Health Technol Assess. 2014;18:1–190. doi: 10.3310/hta18450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berghella V, Baxter JK, Hendrix NW. Cervical assessment by ultrasound for preventing preterm delivery. Cochrane Database Syst Rev. 2013;1 doi: 10.1002/14651858.CD007235.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King J, Flenady V, Cole S, Thornton S. Cyclo-oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database Syst Rev. 2005:CD001992. doi: 10.1002/14651858.CD001992.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Flenady V, Wojcieszek AM, Papatsonis DN, Stock OM, Murray L, Jardine LA, Carbonne B. Calcium channel blockers for inhibiting preterm labour and birth. Cochrane Database Syst Rev. 2014;6:CD002255. doi: 10.1002/14651858.CD002255.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hackney DN, Olson-Chen C, Thornburg LL. What do we know about the natural outcomes of preterm labour? A systematic review and meta-analysis of women without tocolysis in preterm labour. Paediatr Perinat Epidemiol. 2013;27:452–460. doi: 10.1111/ppe.12070. [DOI] [PubMed] [Google Scholar]
- **46.Vogel JP, Nardin JM, Dowswell T, West HM, Oladapo OT. Combination of tocolytic agents for inhibiting preterm labour. Cochrane Database Syst Rev. 2014;7:CD006169. doi: 10.1002/14651858.CD006169.pub2. Provide inconclusive evidence on combination of tocolytic drugs for preterm labour and disputes current evidence whether combination tocolytics are advantageous for women and/or newborns due to a lack of large, well-designed trials including the outcomes of interest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **47.Conde-Agudelo A, Romero R. Transdermal nitroglycerin for the treatment of preterm labor: a systematic review and metaanalysis. Am J Obstet Gynecol. 2013;209:551. doi: 10.1016/j.ajog.2013.07.022. This systematic review reports that based on state-of-the-art methods for indirect comparisons, either vaginal progesterone or cerclage are equally efficacious in the prevention of preterm birth in women with a sonographic short cervix in the mid trimester, singleton gestation, and previous preterm birth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conde-Agudelo A, Romero R, Nicolaides K, Chaiworapongsa T, O’Brien JM, Cetingoz E, da FE, Creasy G, Soma-Pillay P, Fusey S, Cam C, Alfirevic Z, Hassan SS. Vaginal progesterone vs. cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison metaanalysis. Am J Obstet Gynecol. 2013;208:42. doi: 10.1016/j.ajog.2012.10.877. S0002- [DOI] [PMC free article] [PubMed] [Google Scholar]
- **49.Saccone G, Saccone I, Berghella V. Omega-3 long-chain polyunsaturated fatty acids and fish oil supplementation during pregnancy: which evidence? J Matern Fetal Neonatal Med. 2015:1–9. doi: 10.3109/14767058.2015.1086742. This study reported not enough evidence to support the routine use of omega-3 supplementation during pregnancy to reduce the risk of preterm birth (PTB); pre-eclampsia and intrauterine growth restriction; gestational diabetes; perinatal mortality; small for gestational age and birth weight; infant eye and brain development; and postpartum depression. [DOI] [PubMed] [Google Scholar]
- 50.Shennan AH, Chandiramani M. Antibiotics for spontaneous preterm birth. BMJ. 2008;337:a3015. doi: 10.1136/bmj.a3015. [DOI] [PubMed] [Google Scholar]
- 51.Webb DA, Mathew L, Culhane JF. Lessons learned from the Philadelphia Collaborative Preterm Prevention Project: the prevalence of risk factors and program participation rates among women in the intervention group. BMC Pregnancy Childbirth. 2014;14:368. doi: 10.1186/s12884-014-0368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Duda W, Borowiack E, Roseboom T, Tomlinson J, Walczak J, Kunz R, Mol BW, Coomarasamy A, Khan KS. Interventions to reduce or prevent obesity in pregnant women: a systematic review. Health Technol Assess 2012. 2012;16:1–191. doi: 10.3310/hta16310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 54.Newnham JP, Jobe AH. Should we be prescribing repeated courses of antenatal corticosteroids? Semin Fetal Neonatal Med. 2009;14:157–163. doi: 10.1016/j.siny.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 55.Lindstrom TM, Mohan AR, Johnson MR, Bennett PR. Histone deacetylase inhibitors exert time-dependent effects on nuclear factor-kappaB but consistently suppress the expression of proinflammatory genes in human myometrial cells. Mol Pharmacol. 2008;74:109–121. doi: 10.1124/mol.107.042838. [DOI] [PubMed] [Google Scholar]
- *56.Lappas M, Yee K, Permezel M, Rice GE. Sulfasalazine and BAY 11–7082 interfere with the nuclear factor-kappa B and I kappa B kinase pathway to regulate the release of proinflammatory cytokines from human adipose tissue and skeletal muscle in vitro. Endocrinology. 2005;146:1491–1497. doi: 10.1210/en.2004-0809. Reported the usefulness of Sulfasalazine’s effect on reducing inflammation by inhibiting transcriptional activator NF-kB. [DOI] [PubMed] [Google Scholar]
- *57.De SD, Mitchell MD, Keelan JA. Inhibition of choriodecidual cytokine production and inflammatory gene expression by selective I-kappaB kinase (IKK) inhibitors. Br J Pharmacol. 2010;160:1808–1822. doi: 10.1111/j.1476-5381.2010.00839.x. This reporthighlight the need inhibition of inflammation to reduce preterm birth risk and provided evidence for transcriptional activator gene regulation to control inflammatory mediators of preterm and term labor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Filipovich Y, Lu SJ, Akira S, Hirsch E. The adaptor protein MyD88 is essential for E coli-induced preterm delivery in mice. Am J Obstet Gynecol. 2009;200:93–98. doi: 10.1016/j.ajog.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 59.McCarthy FP, Delany AC, Kenny LC, Walsh SK. PPAR-gamma -- a possible drug target for complicated pregnancies. Br J Pharmacol. 2013;168:1074–1085. doi: 10.1111/bph.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lappas M, Permezel M, Rice GE. N-Acetyl-cysteine inhibits phospholipid metabolism, proinflammatory cytokine release, protease activity, and nuclear factor-kappaB deoxyribonucleic acid-binding activity in human fetal membranes in vitro. J Clin Endocrinol Metab. 2003;88:1723–1729. doi: 10.1210/jc.2002-021677. [DOI] [PubMed] [Google Scholar]
- *61.Buhimschi IA, Buhimschi CS, Weiner CP. Protective effect of N-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. Am J Obstet Gynecol. 2003;188:203–208. doi: 10.1067/mob.2003.112. Potential effect of N-acetyl cystine in reducing oxidative stress associated adverse outcomes were reported using an in vitro model. [DOI] [PubMed] [Google Scholar]
- 62.Ng PY, Ireland DJ, Keelan JA. Drugs to block cytokine signaling for the prevention and treatment of inflammation-induced preterm birth. Front Immunol. 2015;6:166. doi: 10.3389/fimmu.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **63.Newnham JP, Dickinson JE, Hart RJ, Pennell CE, Arrese CA, Keelan JA. Strategies to prevent preterm birth. Front Immunol. 2014;5:584. doi: 10.3389/fimmu.2014.00584. Review discusses various strategies to reduce the risk of preterm birth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim R, Lappas M. A novel role for GSK3 in the regulation of the processes of human labour. Reproduction. 2015;149:189–202. doi: 10.1530/REP-14-0493. [DOI] [PubMed] [Google Scholar]
- 65.Pirianov G, Waddington SN, Lindstrom TM, Terzidou V, Mehmet H, Bennett PR. The cyclopentenone 15-deoxy-delta 12,14-prostaglandin J(2) delays lipopolysaccharide-induced preterm delivery and reduces mortality in the newborn mouse. Endocrinology. 2009;150:699–706. doi: 10.1210/en.2008-1178. [DOI] [PubMed] [Google Scholar]
- 66.Agrawal V, Smart K, Jilling T, Hirsch E. Surfactant protein (SP)-A suppresses preterm delivery and inflammation via TLR2. PLoS One. 2013;8:e63990. doi: 10.1371/journal.pone.0063990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Devi YS, DeVine M, DeKuiper J, Ferguson S, Fazleabas AT. Inhibition of IL-6 signaling pathway by curcumin in uterine decidual cells. PLoS One. 2015;10:e0125627. doi: 10.1371/journal.pone.0125627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nadeau-Vallee M, Quiniou C, Palacios J, Hou X, Erfani A, Madaan A, Sanchez M, Leimert K, Boudreault A, Duhamel F, Rivera JC, Zhu T, Noueihed B, Robertson SA, Ni X, Olson DM, Lubell W, Girard S, Chemtob S. Novel Noncompetitive IL-1 Receptor-Biased Ligand Prevents Infection- and Inflammation-Induced Preterm Birth. J Immunol. 2015;195:3402–3415. doi: 10.4049/jimmunol.1500758. [DOI] [PubMed] [Google Scholar]
- *69.Fortunato SJ, Menon R, Swan KF, Lombardi SJ. Interleukin-10 inhibition of interleukin-6 in human amniochorionic membrane: transcriptional regulation. Am J Obstet Gynecol. 1996;175 doi: 10.1016/s0002-9378(96)80053-6. Therapeutic potential of IL-10 in preterm birth is sown in this invitro fetal membrane infection model study. [DOI] [PubMed] [Google Scholar]
- 70.Fortunato SJ, Menon R, Lombardi SJ. Interleukin-10 and transforming growth factor-beta inhibit amniochorion tumor necrosis factor-alpha production by contrasting mechanisms of action: therapeutic implications in prematurity. Am J Obstet Gynecol. 1997;177:803–809. doi: 10.1016/s0002-9378(97)70272-2. [DOI] [PubMed] [Google Scholar]
- 71.Fortunato SJ, Menon R, Lombardi SJ. The effect of transforming growth factor and interleukin-10 on interleukin-8 release by human amniochorion may regulate histologic chorioamnionitis. Am J Obstet Gynecol. 1998;179:794–799. doi: 10.1016/s0002-9378(98)70085-7. [DOI] [PubMed] [Google Scholar]
- *72.Menon R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Front Immunol. 2014;5:567. doi: 10.3389/fimmu.2014.00567. Review details the importance of oxidative stress associated damages, and not just oxidative stress, on potential adverse outcomes during pregnancy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peter ST, Scholl TO, Schluter MD, Leskiw MJ, Chen X, Spur BW, Rodriguez A. Oxidative stress early in pregnancy and pregnancy outcome. Free Radic Res. 2008;42:841–848. doi: 10.1080/10715760802510069. [DOI] [PubMed] [Google Scholar]
- 74.Menon R, Fortunato SJ, Yu J, Milne GL, Sanchez S, Drobek CO, Lappas M, Taylor RN. Cigarette smoke induces oxidative stress and apoptosis in normal term fetal membranes. Placenta. 2011;32:317–322. doi: 10.1016/j.placenta.2011.01.015. [DOI] [PubMed] [Google Scholar]
- *75.Menon R, Fortunato SJ, Milne GL, Brou L, Carnevale C, Sanchez SC, Hubbard L, Lappas M, Drobek CO, Taylor RN. Amniotic fluid eicosanoids in preterm and term births: effects of risk factors for spontaneous preterm labor. Obstet Gynecol. 2011;118:121–134. doi: 10.1097/AOG.0b013e3182204eaa. The association between oxidative stress associated damages and concentration differences of F2-Isorpostanes in the amniotic fluid between term and preterm parturitions are detialed in this manuscript. The association between of oxidative stress damage products and pregnancy outcome are detailed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *76.Menon R, Yu J, Basanta-Henry P, Brou L, Berga SL, Fortunato SJ, Taylor RN. Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS One. 2012;7:e31136. doi: 10.1371/journal.pone.0031136. This is one of the earliest studies to document telomere reduction in fetal cells and tissues associated with normal parturition as well as preterm parturition complicated by premature rupture of the membranes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dhobale M, Joshi S. Altered maternal micronutrients (folic acid, vitamin B(12)) and omega 3 fatty acids through oxidative stress may reduce neurotrophic factors in preterm pregnancy. J Matern Fetal Neonatal Med. 2012;25:317–323. doi: 10.3109/14767058.2011.579209. [DOI] [PubMed] [Google Scholar]
- 78.Chai M, Barker G, Menon R, Lappas M. Increased oxidative stress in human fetal membranes overlying the cervix from term non-labouring and post labour deliveries. Placenta. 2012;33:604–610. doi: 10.1016/j.placenta.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 79.Buhimschi IA, Buhimschi CS, Weiner CP. Protective effect of N-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. Am J Obstet Gynecol. 2003;188:203–208. doi: 10.1067/mob.2003.112. [DOI] [PubMed] [Google Scholar]
- **80.Menon R, Torloni MR, Voltolini C, Torricelli M, Merialdi M, Betran AP, Widmer M, Allen T, Davydova I, Khodjaeva Z, Thorsen P, Kacerovsky M, Tambor V, Massinen T, Nace J, Arora C. Biomarkers of spontaneous preterm birth: an overview of the literature in the last four decades. Reprod Sci. 2011;18:1046–1070. doi: 10.1177/1933719111415548. Systematic review on biomarkers of preterm birth that showed no single biomarker can predict the risk of spontaneous preterm labor. [DOI] [PubMed] [Google Scholar]
- 81.Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, Pearson GD, Wapner RJ, Varner MW, Thorp JM, Jr, Mercer BM, Peaceman AM, Ramin SM, Carpenter MW, Samuels P, Sciscione A, Harper M, Smith WJ, Saade G, Sorokin Y, Anderson GB. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362:1282–1291. doi: 10.1056/NEJMoa0908056. Study reports lack of benefit to reduce the risk of preecplaspsia risk by vitamin supplementation during pregnancy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Conde-Agudelo A, Romero R, Kusanovic JP, Hassan SS. Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;204:503–512. doi: 10.1016/j.ajog.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Basaran A, Basaran M, Topatan B. Combined vitamin C and E supplementation for the prevention of preeclampsia: a systematic review and meta-analysis. Obstet Gynecol Surv. 2010;65:653–667. doi: 10.1097/OGX.0b013e3182095366. [DOI] [PubMed] [Google Scholar]
- **84.Behnia F, Peltier MR, Saade GR, Menon R. Environmental Pollutant Polybrominated Diphenyl Ether, a Flame Retardant, Induces Primary Amnion Cell Senescence. Am J Reprod Immunol. 2015;74:398–406. doi: 10.1111/aji.12414. This study showed flame retardants (environmental poolutant) associated with preterm birth causes p38MAPK induced senescence of human fetal cells. [DOI] [PubMed] [Google Scholar]
- **85.Bredeson S, Papaconstantinou J, Deford JH, Kechichian T, Syed TA, Saade GR, Menon R. HMGB1 Promotes a p38MAPK Associated Non-Infectious Inflammatory Response Pathway in Human Fetal Membranes. PLoS One. 2014;9:e113799. doi: 10.1371/journal.pone.0113799. Damage associated molecular pattern marker HMGB1 induced fetal cell senescence through p38MAPK mediated pathway. p38MAPK inhibitor reduced sterile inflammation induced by HMGB1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **86.Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R. Telomere Fragment Induced Amnion Cell Senescence: A Contributor to Parturition? PLoS One. 2015;10:e0137188. doi: 10.1371/journal.pone.0137188. First report to determine that telomere fragments in cell free fetal DNA can cause senescence through p38MAPK mediated pathway and sterile inflammation in invitro model of amnion epithelial cell cultures and in situ in pregnant murine models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **87.Menon R, Boldogh I, Urrabaz-Garza R, Polettini J, Syed TA, Saade GR, Papaconstantinou J, Taylor RN. Senescence of Primary Amniotic Cells via Oxidative DNA Damage. PLoS One. 2013;8:e83416. doi: 10.1371/journal.pone.0083416. This study showed that cigarette smoke extract cause fetal amnion cell senescence through p38MAPK mediated pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *88.Ruiz-Bonilla V, Perdiguero E, Gresh L, Serrano AL, Zamora M, Sousa-Victor P, Jardi M, Wagner EF, Munoz-Canoves P. Efficient adult skeletal muscle regeneration in mice deficient in p38beta, p38gamma and p38delta MAP kinases. Cell Cycle. 2008;7:2208–2214. doi: 10.4161/cc.7.14.6273. Describe the functional role of various forms of p38MAPK. [DOI] [PubMed] [Google Scholar]
- 89.Perdiguero E, Ruiz-Bonilla V, Gresh L, Hui L, Ballestar E, Sousa-Victor P, Baeza-Raja B, Jardi M, Bosch-Comas A, Esteller M, Caelles C, Serrano AL, Wagner EF, Munoz-Canoves P. Genetic analysis of p38 MAP kinases in myogenesis: fundamental role of p38alpha in abrogating myoblast proliferation. EMBO J. 2007;26:1245–1256. doi: 10.1038/sj.emboj.7601587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Risco A, del FC, Mambol A, Alsina-Beauchamp D, MacKenzie KF, Yang HT, Barber DF, Morcelle C, Arthur JS, Ley SC, Ardavin C, Cuenda A. p38gamma and p38delta kinases regulate the Toll-like receptor 4 (TLR4)-induced cytokine production by controlling ERK1/2 protein kinase pathway activation1. Proc Natl Acad Sci U S A. 2012;109:11200–11205. doi: 10.1073/pnas.1207290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang X, McGowan CH, Zhao M, He L, Downey JS, Fearns C, Wang Y, Huang S, Han J. Involvement of the MKK6-p38gamma cascade in gamma-radiation-induced cell cycle arrest. Mol Cell Biol. 2000;20:4543–4552. doi: 10.1128/mcb.20.13.4543-4552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kwong J, Chen M, Lv D, Luo N, Su W, Xiang R, Sun P. Induction of p38delta expression plays an essential role in oncogenic ras-induced senescence. Mol Cell Biol. 2013;33:3780–3794. doi: 10.1128/MCB.00784-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takeda K, Hatai T, Hamazaki TS, Nishitoh H, Saitoh M, Ichijo H. Apoptosis signal-regulating kinase 1 (ASK1) induces neuronal differentiation and survival of PC12 cells. J Biol Chem. 2000;275:9805–9813. doi: 10.1074/jbc.275.13.9805. [DOI] [PubMed] [Google Scholar]
- **94.Hsieh CC, Kuro-o M, Rosenblatt KP, Brobey R, Papaconstantinou J. The ASK1-Signalosome regulates p38 MAPK activity in response to levels of endogenous oxidative stress in the Klotho mouse models of aging. Aging (Albany NY) 2010;2:597–611. doi: 10.18632/aging.100194. This report showed the role of ASK-1 signalosome in the activation of p38MAPK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsieh CC, Papaconstantinou J. Dermal fibroblasts from long-lived Ames dwarf mice maintain their in vivo resistance to mitochondrial generated reactive oxygen species (ROS) Aging (Albany NY) 2009;1:784–802. doi: 10.18632/aging.100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Papaconstantinou J, Hsieh CC. Activation of senescence and aging characteristics by mitochondrially generated ROS: how are they linked? Cell Cycle. 2010;9:3831–3833. doi: 10.4161/cc.9.19.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *98.Menon R, Behnia F, Polettini J, Saade GR, Campisi J, Velarde M. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY) 2016;8:216–230. doi: 10.18632/aging.100891. The role of p38MAPK and HMGB1 in the initiation of parturition is detailed in this study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **99.Dutta EH, Behnia F, Boldogh I, Saade GR, Taylor BD, Kacerovsky M, Menon R. Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes. Mol Hum Reprod. 2016;22:143–157. doi: 10.1093/molehr/gav074. Study showed the contrbution of p38MAPK in preterm birth and preterm premature rupture of the fetal membranes. [DOI] [PubMed] [Google Scholar]
- **100.Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells. 2003;8:131–144. doi: 10.1046/j.1365-2443.2003.00620.x. The role of p38MAPK in senescence activation is described. [DOI] [PubMed] [Google Scholar]
- 101.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **102.Polettini J, Dutta EH, Behnia F, Saade GR, Torloni MR, Menon R. Aging of intrauterine tissues in spontaneous preterm birth and preterm premature rupture of the membranes: A systematic review of the literature. Placenta. 2015;36:969–973. doi: 10.1016/j.placenta.2015.05.003. Systematic review identifies knowledge gaps in the literaure on aging associated pathologies and adverse pregnancy outcomes. [DOI] [PubMed] [Google Scholar]
- 103.Yeung YT, Bryce NS, Adams S, Braidy N, Konayagi M, McDonald KL, Teo C, Guillemin GJ, Grewal T, Munoz L. p38 MAPK inhibitors attenuate pro-inflammatory cytokine production and the invasiveness of human U251 glioblastoma cells. J Neurooncol. 2012;109:35–44. doi: 10.1007/s11060-012-0875-7. [DOI] [PubMed] [Google Scholar]
- **104.Guo X, Ma N, Wang J, Song J, Bu X, Cheng Y, Sun K, Xiong H, Jiang G, Zhang B, Wu M, Wei L. Increased p38-MAPK is responsible for chemotherapy resistance in human gastric cancer cells. BMC Cancer. 2008;8:375. doi: 10.1186/1471-2407-8-375. Deleterious role of p38MAPK activation is reported in this study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Graziosi L, Mencarelli A, Santorelli C, Renga B, Cipriani S, Cavazzoni E, Palladino G, Laufer S, Burnet M, Donini A, Fiorucci S. Mechanistic role of p38 MAPK in gastric cancer dissemination in a rodent model peritoneal metastasis. Eur J Pharmacol. 2012;674:143–152. doi: 10.1016/j.ejphar.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 106.Bendotti C, Bao CM, Cheroni C, Grignaschi G, Lo CD, Peviani M, Tortarolo M, Veglianese P, Zennaro E. Inter- and intracellular signaling in amyotrophic lateral sclerosis: role of p38 mitogen-activated protein kinase. Neurodegener Dis. 2005;2:128–134. doi: 10.1159/000089617. [DOI] [PubMed] [Google Scholar]
- *107.Kompa AR, See F, Lewis DA, Adrahtas A, Cantwell DM, Wang BH, Krum H. Long-term but not short-term p38 mitogen-activated protein kinase inhibition improves cardiac function and reduces cardiac remodeling post-myocardial infarction. J Pharmacol Exp Ther. 2008;325:741–750. doi: 10.1124/jpet.107.133546. Benefits of p38MAPK inhibiton tin cardiac medicine is discussed. [DOI] [PubMed] [Google Scholar]
- **108.Liu C, Lin J, Wrobleski ST, Lin S, Hynes J, Wu H, Dyckman AJ, Li T, Wityak J, Gillooly KM, Pitt S, Shen DR, Zhang RF, McIntyre KW, Salter-Cid L, Shuster DJ, Zhang H, Marathe PH, Doweyko AM, Sack JS, Kiefer SE, Kish KF, Newitt JA, McKinnon M, Dodd JH, Barrish JC, Schieven GL, Leftheris K. Discovery of 4-(5-(cyclopropylcarbamoyl)-2-methylphenylamino)-5-methyl-N-propylpyrrolo[1,2-f][ 1,2,4]triazine-6-carboxamide (BMS-582949), a clinical p38alpha MAP kinase inhibitor for the treatment of inflammatory diseases. J Med Chem. 2010;53:6629–6639. doi: 10.1021/jm100540x. Discovery of p38α MAP kinase inhibitor, a prodrug, 2 (BMS-751324), featuring a carbamoylmethylene linked promoiety containing hydroxyphenyl acetic acid (HPA) derived ester and phosphate functionalities, was reported. [DOI] [PubMed] [Google Scholar]
- 109.Ferrari F, Facchinetti F, Saade G, Menon R. Placental telomere shortening in stillbirth: a sign of premature senescence? J Matern Fetal Neonatal Med. 2015:1–6. doi: 10.3109/14767058.2015.1046045. [DOI] [PubMed] [Google Scholar]
- 110.Coulthard LR, White DE, Jones DL, McDermott MF, Burchill SA. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol Med. 2009;15:369–379. doi: 10.1016/j.molmed.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *111.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. This report describe MPAK as potential target for intervention for inflammatory conditions. [DOI] [PubMed] [Google Scholar]
- 112.Montalban AG, Boman E, Chang CD, Ceide SC, Dahl R, Dalesandro D, Delaet NG, Erb E, Ernst J, Gibbs A, Kahl J, Kessler L, Lundstrom J, Miller S, Nakanishi H, Roberts E, Saiah E, Sullivan R, Wang Z, Larson CJ. KR-003048, a potent, orally active inhibitor of p38 mitogen-activated protein kinase. Eur J Pharmacol. 2010;632:93–102. doi: 10.1016/j.ejphar.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 113.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- **114.Parasrampuria DA, de BP, Desai-Krieger D, Chow AT, Jones CR. Single-dose pharmacokinetics and pharmacodynamics of RWJ 67657, a specific p38 mitogen-activated protein kinase inhibitor: a first-in-human study. J Clin Pharmacol. 2003;43:406–413. doi: 10.1177/0091270002250615. This study examined ppharmacokinetics and ex vivo pharmacodynamics of increasing doses of RWJ 67657, a specific p38 mitogen-activated protein kinase inhibitor and demonstrated that RWJ 67657 has acceptable safety and pharmacokinetics. [DOI] [PubMed] [Google Scholar]
- 115.Hsieh CC, Papaconstantinou J. The effect of aging on p38 signaling pathway activity in the mouse liver and in response to ROS generated by 3-nitropropionic acid. Mech Ageing Dev. 2002;123:1423–1435. doi: 10.1016/s0047-6374(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 116.Mannam P, Zhang X, Shan P, Zhang Y, Shinn AS, Zhang Y, Lee PJ. Endothelial MKK3 is a critical mediator of lethal murine endotoxemia and acute lung injury. J Immunol. 2013;190:1264–1275. doi: 10.4049/jimmunol.1202012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **117.Hope HR, Anderson GD, Burnette BL, Compton RP, Devraj RV, Hirsch JL, Keith RH, Li X, Mbalaviele G, Messing DM, Saabye MJ, Schindler JF, Selness SR, Stillwell LI, Webb EG, Zhang J, Monahan JB. Anti-inflammatory properties of a novel N-phenyl pyridinone inhibitor of p38 mitogen-activated protein kinase: preclinical-to-clinical translation. J Pharmacol Exp Ther. 2009;331:882–895. doi: 10.1124/jpet.109.158329. This study described the pharmacological properties of the N-phenyl pyridinone p38 MAP kinase inhibitor PH-797804, an ATP-competitive, readily reversible inhibitor of the alpha isoform of human p38 MAP kinase in reducing the risk of chronic inflammatory disease. [DOI] [PubMed] [Google Scholar]
- 118.Xing L, Shieh HS, Selness SR, Devraj RV, Walker JK, Devadas B, Hope HR, Compton RP, Schindler JF, Hirsch JL, Benson AG, Kurumbail RG, Stegeman RA, Williams JM, Broadus RM, Walden Z, Monahan JB. Structural bioinformatics-based prediction of exceptional selectivity of p38 MAP kinase inhibitor PH-797804. Biochemistry. 2009;48:6402–6411. doi: 10.1021/bi900655f. [DOI] [PubMed] [Google Scholar]
- *119.Selness SR, Devraj RV, Devadas B, Walker JK, Boehm TL, Durley RC, Shieh H, Xing L, Rucker PV, Jerome KD, Benson AG, Marrufo LD, Madsen HM, Hitchcock J, Owen TJ, Christie L, Promo MA, Hickory BS, Alvira E, Naing W, Blevis-Bal R, Messing D, Yang J, Mao MK, Yalamanchili G, Vonder ER, Hirsch J, Saabye M, Bonar S, Webb E, Anderson G, Monahan JB. Discovery of PH-797804, a highly selective and potent inhibitor of p38 MAP kinase. Bioorg Med Chem Lett. 2011;21:4066–4071. doi: 10.1016/j.bmcl.2011.04.121. This manuscript described the synthesis and SAR studies of a novel N-aryl pyridinone class of p38 kinase inhibitors. [DOI] [PubMed] [Google Scholar]
- **120.Hasumi K, Sato S, Saito T, Kato JY, Shirota K, Sato J, Suzuki H, Ohta S. Design and synthesis of 5-[(2-chloro-6-fluorophenyl)acetylamino]-3-(4-fluorophenyl)-4-(4-pyrimidinyl)isox azole (AKP-001), a novel inhibitor of p38 MAP kinase with reduced side effects based on the antedrug concept. Bioorg Med Chem. 2014;22:4162–4176. doi: 10.1016/j.bmc.2014.05.045. Study describe antedrug compund AKP-001), a novel inhibitor of p38 MAP kinase and its function. [DOI] [PubMed] [Google Scholar]
- **121.Shirota K, Kaneko M, Sasaki M, Minato K, Fujikata A, Ohta S, Hisaka A, Suzuki H. Analysis of the disposition of a novel p38 MAPK inhibitor, AKP-001, and its metabolites in rats with a simple physiologically based pharmacokinetic model. Drug Metab Dispos. 2015;43:217–226. doi: 10.1124/dmd.114.060046. Animal model studies tested the pharmacokinetics of ante-drug properties of 5-[(2-Chloro-6-fluorophenyl)acetylamino]-3-(4-fluorophenyl)-4-(4-pyrimidinyl)isoxazole (AKP-001), a potent p38 MAPK inhibitor. [DOI] [PubMed] [Google Scholar]
- 122.Biggadike K, Angell RM, Burgess CM, Farrell RM, Hancock AP, Harker AJ, Irving WR, Ioannou C, Procopiou PA, Shaw RE, Solanke YE, Singh OM, Snowden MA, Stubbs RJ, Walton S, Weston HE. Selective plasma hydrolysis of glucocorticoid gamma-lactones and cyclic carbonates by the enzyme paraoxonase: an ideal plasma inactivation mechanism. J Med Chem. 2000;43:19–21. doi: 10.1021/jm990436t. [DOI] [PubMed] [Google Scholar]
- 123.Freyne EJ, Lacrampe JF, Deroose F, Boeckx GM, Willems M, Embrechts W, Coesemans E, Willems JJ, Fortin JM, Ligney Y, Dillen LL, Cools WF, Goossens J, Corens D, De GA, Van Wauwe JP. Synthesis and biological evaluation of 1,2,4-triazinylphenylalkylthiazolecarboxylic acid esters as cytokine-inhibiting antedrugs with strong bronchodilating effects in an animal model of asthma. J Med Chem. 2005;48:2167–2175. doi: 10.1021/jm049479m. [DOI] [PubMed] [Google Scholar]
- 124.Khan MO, Park KK, Lee HJ. Antedrugs: an approach to safer drugs. Curr Med Chem. 2005;12:2227–2239. doi: 10.2174/0929867054864840. [DOI] [PubMed] [Google Scholar]
- 125.Shroot B, Caron JC, Ponec M. Glucocorticoid specific binding: structure-activity relationships. Br J Dermatol. 1982;107(Suppl 23):30–34. doi: 10.1111/j.1365-2133.1982.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 126.Roy SM, Grum-Tokars VL, Schavocky JP, Saeed F, Staniszewski A, Teich AF, Arancio O, Bachstetter AD, Webster SJ, Van Eldik LJ, Minasov G, Anderson WF, Pelletier JC, Watterson DM. Targeting human central nervous system protein kinases: An isoform selective p38alphaMAPK inhibitor that attenuates disease progression in Alzheimer’s disease mouse models. ACS Chem Neurosci. 2015;6:666–680. doi: 10.1021/acschemneuro.5b00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Satoh T, Hosokawa M. The mammalian carboxylesterases: from molecules to functions. Annu Rev Pharmacol Toxicol. 1998;38:257–288. doi: 10.1146/annurev.pharmtox.38.1.257. [DOI] [PubMed] [Google Scholar]
- 128.Imai T. Human carboxylesterase isozymes: catalytic properties and rational drug design. Drug Metab Pharmacokinet. 2006;21:173–185. doi: 10.2133/dmpk.21.173. [DOI] [PubMed] [Google Scholar]
- 129.Lee HJ, Cooperwood JS, You Z, Ko DH. Prodrug and antedrug: two diametrical approaches in designing safer drugs. Arch Pharm Res. 2002;25:111–136. doi: 10.1007/BF02976552. [DOI] [PubMed] [Google Scholar]
- 130.O’Donnell S, O’Morain CA. Therapeutic benefits of budesonide in gastroenterology. Ther Adv Chronic Dis. 2010;1:177–186. doi: 10.1177/2040622310379293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *131.Schreiber S, Feagan B, D’Haens G, Colombel JF, Geboes K, Yurcov M, Isakov V, Golovenko O, Bernstein CN, Ludwig D, Winter T, Meier U, Yong C, Steffgen J. Oral p38 mitogen-activated protein kinase inhibition with BIRB 796 for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:325–334. doi: 10.1016/j.cgh.2005.11.013. This study investigated the efficacy and safety of BIRB 796, a highly potent inhibitor of p38 MAPK, in chronic active Crohn’s disease in a randomized, double-blind, placebo-controlled trial and reports no efficacy. [DOI] [PubMed] [Google Scholar]
- 132.Sears MR. Descriptive epidemiology of asthma. Lancet. 1997;350(Suppl 2):SII1–SII4. doi: 10.1016/s0140-6736(97)90028-3. [DOI] [PubMed] [Google Scholar]
- 133.Barnes PJ. Therapeutic strategies for allergic diseases. Nature. 1999;402:B31–B38. doi: 10.1038/35037026. [DOI] [PubMed] [Google Scholar]
- 134.Wong ES, Le GX, Demidov ON, Marshall NT, Wang ST, Krishnamurthy J, Sharpless NE, Dunn NR, Bulavin DV. p38MAPK controls expression of multiple cell cycle inhibitors and islet proliferation with advancing age. Dev Cell. 2009;17:142–149. doi: 10.1016/j.devcel.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 135.Collins CJ, Sedivy JM. Involvement of the INK4a/Arf gene locus in senescence. Aging Cell. 2003;2:145–150. doi: 10.1046/j.1474-9728.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- **136.Papaconstantinou J, Wang CZ, Zhang M, Yang S, Deford J, Bulavin DV, Ansari NH. Attenuation of p38alpha MAPK stress response signaling delays the in vivo aging of skeletal muscle myofibers and progenitor cells. Aging (Albany NY) 2015;7:718–733. doi: 10.18632/aging.100802. This study used the dominant negative haploinsufficient p38α mouse (DN-p38α(AF/+)) to demonstrate that in vivo attenuation of p38α activity in the gastrocnemius of the aged mutant delays age-associated processes that include: a) the decline of the juvenile protective factors, BubR1, aldehyde dehydrogenase 1A (ALDH1A1), and aldehyde dehydrogenase 2 (ALDH2); b) attenuated expression of p16(Ink4a) and p19(Arf) tumor suppressor genes of the Cdkn2a locus; c) decreased levels of hydroxynonenal protein adducts, expression of COX2 and iNOS; d) decline of the senescent progenitor cell pool level and d) the loss of gastrocnemius muscle mass. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hsieh CC, Papaconstantinou J. Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. FASEB J. 2006;20:259–268. doi: 10.1096/fj.05-4376com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **138.Menon R, Boldogh I, Hawkins HK, Woodson M, Polettini J, Syed TA, Fortunato SJ, Saade GR, Papaconstantinou J, Taylor RN. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. Am J Pathol. 2014;184:1740–1751. doi: 10.1016/j.ajpath.2014.02.011. This report histologicaly evaluated the association between p38MAPK in normal and preterm parturition and reports the role of oxidative stress induced p38MAPK activation in term and preterm parturition when complicated with fetal membrane ruptyure. [DOI] [PubMed] [Google Scholar]
- 139.Brobey RK, German D, Sonsalla PK, Gurnani P, Pastor J, Hsieh CC, Papaconstantinou J, Foster PP, Kuro-o M, Rosenblatt KP. Klotho Protects Dopaminergic Neuron Oxidant-Induced Degeneration by Modulating ASK1 and p38 MAPK Signaling Pathways. PLoS One. 2015;10:e0139914. doi: 10.1371/journal.pone.0139914. [DOI] [PMC free article] [PubMed] [Google Scholar]