Abstract
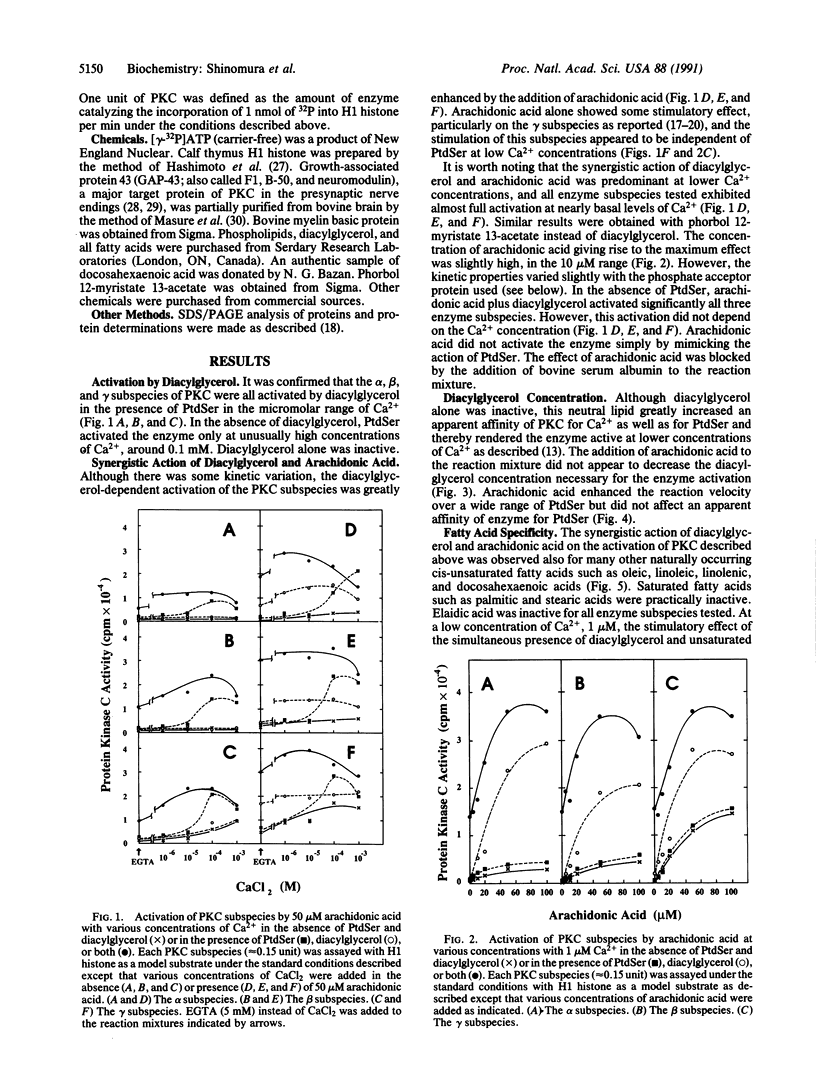
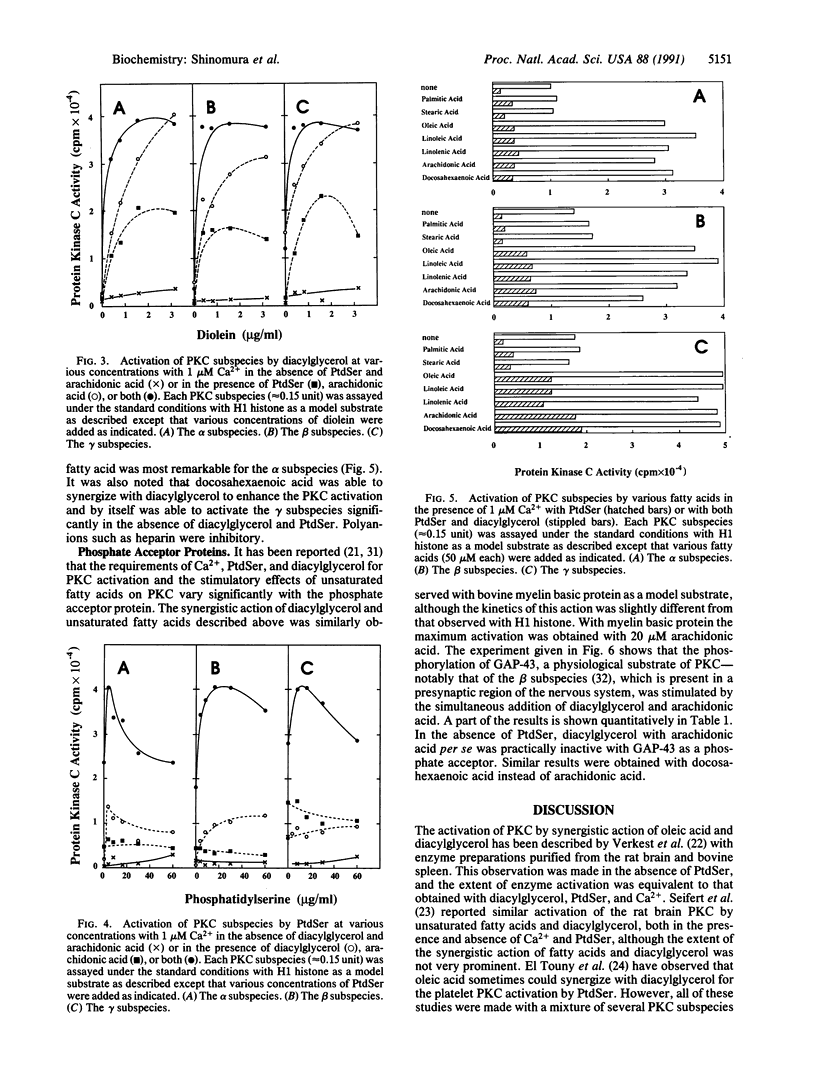
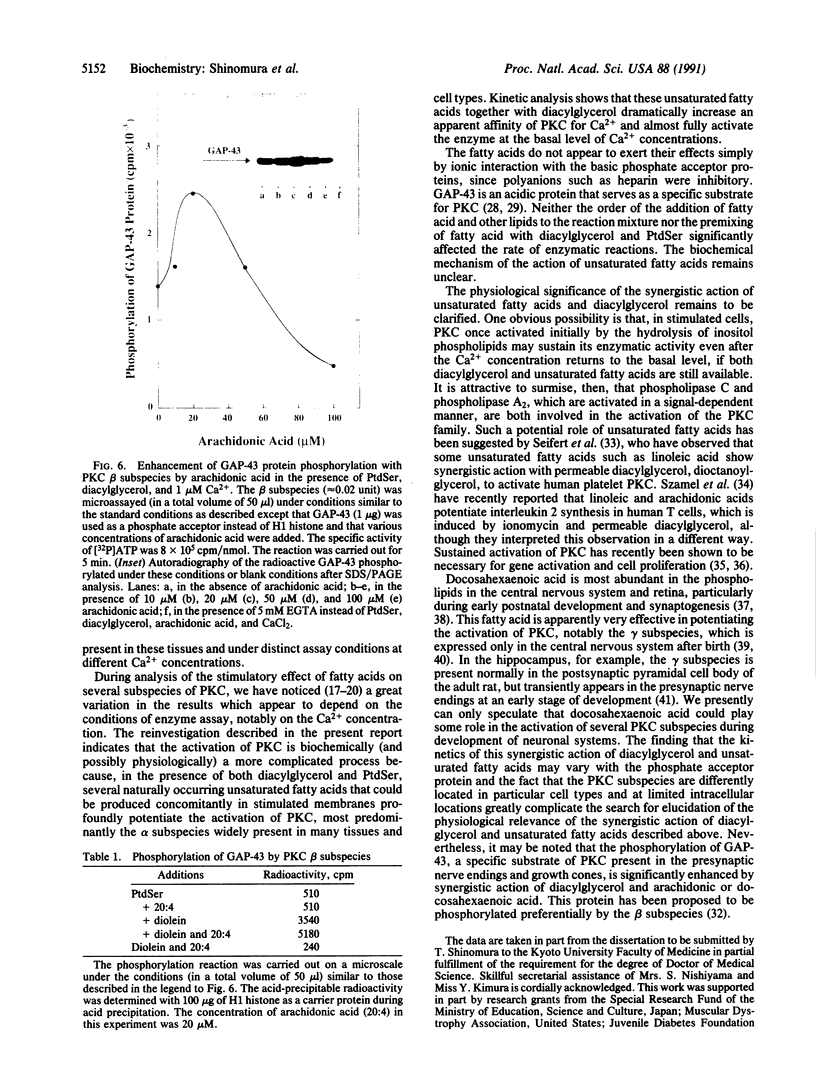
Kinetic properties of the purified alpha, beta, and gamma subspecies of protein kinase C (PKC) to respond to diacylglycerol, phosphatidylserine (PtdSer), and Ca2+ were reinvestigated in the presence of several fatty acids. Although responses of these enzyme subspecies to the lipids slightly differed from one another, the reaction velocity of these subspecies was significantly enhanced by synergistic action of diacylglycerol and a cis-unsaturated fatty acid. Arachidonic, oleic, linoleic, linolenic, and docosahexaenoic acids were active in this role, whereas saturated fatty acids such as palmitic and stearic acids were inactive. Elaidic acid was also inactive. In the presence of both PtdSer and diacylglycerol, the cis-unsaturated fatty acids increased further an apparent affinity of PKC to Ca2+ and allowed the enzyme to exhibit almost full activation at nearly basal levels of Ca2+ concentration. The concentration of fatty acid giving rise to the maximum activation of enzyme was approximately 20-50 microM. The result presented herein implies that the receptor-mediated release of unsaturated fatty acids from phospholipids may take part, in synergy with diacylglycerol, in the activation of PKC even when the Ca2+ concentration is low. A possibility arises, then, that the activation of PKC is an integral part of the signal-induced degradation cascade of various membrane phospholipids, which is initiated by the actions of phospholipase C and phospholipase A2.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agwu D. E., McPhail L. C., Chabot M. C., Daniel L. W., Wykle R. L., McCall C. E. Choline-linked phosphoglycerides. A source of phosphatidic acid and diglycerides in stimulated neutrophils. J Biol Chem. 1989 Jan 25;264(3):1405–1413. [PubMed] [Google Scholar]
- Aloyo V. J., Zwiers H., Gispen W. H. Phosphorylation of B-50 protein by calcium-activated, phospholipid-dependent protein kinase and B-50 protein kinase. J Neurochem. 1983 Sep;41(3):649–653. doi: 10.1111/j.1471-4159.1983.tb04790.x. [DOI] [PubMed] [Google Scholar]
- Axelrod J., Burch R. M., Jelsema C. L. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988 Mar;11(3):117–123. doi: 10.1016/0166-2236(88)90157-9. [DOI] [PubMed] [Google Scholar]
- Bazzi M. D., Nelsestuen G. L. Role of substrate in imparting calcium and phospholipid requirements to protein kinase C activation. Biochemistry. 1987 Apr 7;26(7):1974–1982. doi: 10.1021/bi00381a029. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Berry N., Ase K., Kishimoto A., Nishizuka Y. Activation of resting human T cells requires prolonged stimulation of protein kinase C. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2294–2298. doi: 10.1073/pnas.87.6.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Pai J. K., Mullmann T. J., Egan R. W., Siegel M. I. Regulation of phospholipase D in HL-60 granulocytes. Activation by phorbol esters, diglyceride, and calcium ionophore via protein kinase- independent mechanisms. J Biol Chem. 1989 May 25;264(15):9069–9076. [PubMed] [Google Scholar]
- Bocckino S. B., Blackmore P. F., Exton J. H. Stimulation of 1,2-diacylglycerol accumulation in hepatocytes by vasopressin, epinephrine, and angiotensin II. J Biol Chem. 1985 Nov 15;260(26):14201–14207. [PubMed] [Google Scholar]
- Buday L., Faragó A. Dual effect of arachidonic acid on protein kinase C isoenzymes isolated from rabbit thymus cells. FEBS Lett. 1990 Dec 10;276(1-2):223–226. doi: 10.1016/0014-5793(90)80547-v. [DOI] [PubMed] [Google Scholar]
- Chalifa V., Möhn H., Liscovitch M. A neutral phospholipase D activity from rat brain synaptic plasma membranes. Identification and partial characterization. J Biol Chem. 1990 Oct 15;265(29):17512–17519. [PubMed] [Google Scholar]
- Chan S. Y., Murakami K., Routtenberg A. Phosphoprotein F1: purification and characterization of a brain kinase C substrate related to plasticity. J Neurosci. 1986 Dec;6(12):3618–3627. doi: 10.1523/JNEUROSCI.06-12-03618.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Hashimoto E., Takeda M., Nishizuka Y., Hamana K., Iwai K. Studies on the sites in histones phosphorylated by adenosine 3':5'-monophosphate-dependent and guanosine 3':5'-monophosphate-dependent protein kinases. J Biol Chem. 1976 Oct 25;251(20):6287–6293. [PubMed] [Google Scholar]
- Hashimoto T., Ase K., Sawamura S., Kikkawa U., Saito N., Tanaka C., Nishizuka Y. Postnatal development of a brain-specific subspecies of protein kinase C in rat. J Neurosci. 1988 May;8(5):1678–1683. doi: 10.1523/JNEUROSCI.08-05-01678.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Martin T. W., Michaelis K. P2-purinergic agonists stimulate phosphodiesteratic cleavage of phosphatidylcholine in endothelial cells. Evidence for activation of phospholipase D. J Biol Chem. 1989 May 25;264(15):8847–8856. [PubMed] [Google Scholar]
- Masure H. R., Alexander K. A., Wakim B. T., Storm D. R. Physicochemical and hydrodynamic characterization of P-57, a neurospecific calmodulin binding protein. Biochemistry. 1986 Nov 18;25(23):7553–7560. doi: 10.1021/bi00371a044. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Murakami K., Chan S. Y., Routtenberg A. Protein kinase C activation by cis-fatty acid in the absence of Ca2+ and phospholipids. J Biol Chem. 1986 Nov 25;261(33):15424–15429. [PubMed] [Google Scholar]
- Murakami K., Routtenberg A. Direct activation of purified protein kinase C by unsaturated fatty acids (oleate and arachidonate) in the absence of phospholipids and Ca2+. FEBS Lett. 1985 Nov 18;192(2):189–193. doi: 10.1016/0014-5793(85)80105-8. [DOI] [PubMed] [Google Scholar]
- Naor Z., Shearman M. S., Kishimoto A., Nishizuka Y. Calcium-independent activation of hypothalamic type I protein kinase C by unsaturated fatty acids. Mol Endocrinol. 1988 Nov;2(11):1043–1048. doi: 10.1210/mend-2-11-1043. [DOI] [PubMed] [Google Scholar]
- Naughton J. M. Supply of polyenoic fatty acids to the mammalian brain: the ease of conversion of the short-chain essential fatty acids to their longer chain polyunsaturated metabolites in liver, brain, placenta and blood. Int J Biochem. 1981;13(1):21–32. doi: 10.1016/0020-711x(81)90132-4. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Hidaka H., Shirakawa S. Possible involvement of direct stimulation of protein kinase C by unsaturated fatty acids in platelet activation. Biochem Pharmacol. 1988 Aug 15;37(16):3079–3089. doi: 10.1016/0006-2952(88)90304-8. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Scott B. L., Bazan N. G. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2903–2907. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R., Schächtele C., Rosenthal W., Schultz G. Activation of protein kinase C by cis- and trans-fatty acids and its potentiation by diacylglycerol. Biochem Biophys Res Commun. 1988 Jul 15;154(1):20–26. doi: 10.1016/0006-291x(88)90643-2. [DOI] [PubMed] [Google Scholar]
- Seifert R., Schächtele C., Schultz G. Activation of protein kinase C by cis- and trans-octadecadienoic acids in intact human platelets and its potentiation by diacylglycerol. Biochem Biophys Res Commun. 1987 Dec 16;149(2):762–768. doi: 10.1016/0006-291x(87)90433-5. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Tsukuda M., Ase K., Kikkawa U., Nishizuka Y. Mode of activation and kinetic properties of three distinct forms of protein kinase C from rat brain. J Biochem. 1988 May;103(5):759–765. doi: 10.1093/oxfordjournals.jbchem.a122343. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Tsukuda M., Ogita K., Kikkawa U., Nishizuka Y. Three distinct forms of rat brain protein kinase C: differential response to unsaturated fatty acids. Biochem Biophys Res Commun. 1987 Jun 15;145(2):797–802. doi: 10.1016/0006-291x(87)91035-7. [DOI] [PubMed] [Google Scholar]
- Shearman M. S., Naor Z., Sekiguchi K., Kishimoto A., Nishizuka Y. Selective activation of the gamma-subspecies of protein kinase C from bovine cerebellum by arachidonic acid and its lipoxygenase metabolites. FEBS Lett. 1989 Jan 30;243(2):177–182. doi: 10.1016/0014-5793(89)80125-5. [DOI] [PubMed] [Google Scholar]
- Shearman M. S., Shinomura T., Oda T., Nishizuka Y. Protein kinase C subspecies in adult rat hippocampal synaptosomes. Activation by diacylglycerol and arachidonic acid. FEBS Lett. 1991 Feb 25;279(2):261–264. doi: 10.1016/0014-5793(91)80163-w. [DOI] [PubMed] [Google Scholar]
- Shearman M. S., Shinomura T., Oda T., Nishizuka Y. Synaptosomal protein kinase C subspecies: A. Dynamic changes in the hippocampus and cerebellar cortex concomitant with synaptogenesis. J Neurochem. 1991 Apr;56(4):1255–1262. doi: 10.1111/j.1471-4159.1991.tb11419.x. [DOI] [PubMed] [Google Scholar]
- Sheu F. S., Marais R. M., Parker P. J., Bazan N. G., Routtenberg A. Neuron-specific protein F1/GAP-43 shows substrate specificity for the beta subtype of protein kinase C. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1236–1243. doi: 10.1016/0006-291x(90)90818-8. [DOI] [PubMed] [Google Scholar]
- Szamel M., Rehermann B., Krebs B., Kurrle R., Resch K. Activation signals in human lymphocytes. Incorporation of polyunsaturated fatty acids into plasma membrane phospholipids regulates IL-2 synthesis via sustained activation of protein kinase C. J Immunol. 1989 Nov 1;143(9):2806–2813. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Verkest V., McArthur M., Hamilton S. Fatty acid activation of protein kinase C: dependence on diacylglycerol. Biochem Biophys Res Commun. 1988 Apr 29;152(2):825–829. doi: 10.1016/s0006-291x(88)80112-8. [DOI] [PubMed] [Google Scholar]
- William F., Wagner F., Karin M., Kraft A. S. Multiple doses of diacylglycerol and calcium ionophore are necessary to activate AP-1 enhancer activity and induce markers of macrophage differentiation. J Biol Chem. 1990 Oct 25;265(30):18166–18171. [PubMed] [Google Scholar]
- Yoshida Y., Huang F. L., Nakabayashi H., Huang K. P. Tissue distribution and developmental expression of protein kinase C isozymes. J Biol Chem. 1988 Jul 15;263(20):9868–9873. [PubMed] [Google Scholar]
- el Touny S., Khan W., Hannun Y. Regulation of platelet protein kinase C by oleic acid. Kinetic analysis of allosteric regulation and effects on autophosphorylation, phorbol ester binding, and susceptibility to inhibition. J Biol Chem. 1990 Sep 25;265(27):16437–16443. [PubMed] [Google Scholar]




