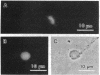
Abstract
Transit from M phase into interphase in many eukaryotic cells is preceded by an increase in intracellular free calcium ([Ca2+]i), which may act via calcium-dependent enzymes to trigger the M-phase/interphase transition. To test the role of the calcium- and phospholipid-dependent enzyme protein kinase C (PKC) in the M-phase/interphase transition, PKC was activated in M-phase-arrested Xenopus eggs by treatment with the phorbol ester phorbol 12-myristate 13-acetate under conditions that prevent a rise in [Ca2+]i and activation of other calcium-dependent enzymes. Under these conditions, several cellular events characteristic of transit into interphase occur: sperm chromatin decondenses, the Golgi and the nuclear envelope reassemble, and endocytosis resumes. These events are also triggered by treatment of eggs with the diacylglycerol 1,2-dioctanoyl-sn-glycerol. Surprisingly, the activity of M-phase-promoting factor (MPF), a universal regulator of M phase, remains high under these conditions. If [Ca2+]i is subsequently raised, MPF activity is rapidly destroyed. Similarly, lysates made from eggs treated with phorbol 12-myristate 13-acetate support sperm chromatin decondensation in vitro and yet retain high MPF activity, measured either as the ability to induce meiotic resumption in oocytes or as histone H1 kinase activity. These effects are not triggered by the 4 alpha-phorbol ester isomer, which does not activate PKC, and are sensitive to the PKC "pseudosubstrate" peptide. The results suggest that two, parallel signals are generated by the rise in [Ca2+]i both of which contribute to cell cycle regulation. One pathway inactivates MPF; the other pathway activates PKC.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arion D., Meijer L., Brizuela L., Beach D. cdc2 is a component of the M phase-specific histone H1 kinase: evidence for identity with MPF. Cell. 1988 Oct 21;55(2):371–378. doi: 10.1016/0092-8674(88)90060-8. [DOI] [PubMed] [Google Scholar]
- Bement W. M., Capco D. G. Activators of protein kinase C trigger cortical granule exocytosis, cortical contraction, and cleavage furrow formation in Xenopus laevis oocytes and eggs. J Cell Biol. 1989 Mar;108(3):885–892. doi: 10.1083/jcb.108.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bement W. M., Capco D. G. Intracellular signals trigger ultrastructural events characteristic of meiotic maturation in oocytes of Xenopus laevis. Cell Tissue Res. 1989 Jan;255(1):183–191. doi: 10.1007/BF00229080. [DOI] [PubMed] [Google Scholar]
- Bement W. M., Capco D. G. Protein kinase C acts downstream of calcium at entry into the first mitotic interphase of Xenopus laevis. Cell Regul. 1990 Feb;1(3):315–326. doi: 10.1091/mbc.1.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M., Walter R. J. Surface functions during Mitosis I: phagocytosis, pinocytosis and mobility of surface-bound Con A. Cell. 1978 Oct;15(2):327–341. doi: 10.1016/0092-8674(78)90002-8. [DOI] [PubMed] [Google Scholar]
- Busa W. B., Nuccitelli R. An elevated free cytosolic Ca2+ wave follows fertilization in eggs of the frog, Xenopus laevis. J Cell Biol. 1985 Apr;100(4):1325–1329. doi: 10.1083/jcb.100.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capco D. G., Mecca M. D. Analysis of proteins in the peripheral and central regions of amphibian oocytes and eggs. Cell Differ. 1988 Apr;23(3):155–164. doi: 10.1016/0045-6039(88)90068-1. [DOI] [PubMed] [Google Scholar]
- Chackalaparampil I., Shalloway D. Altered phosphorylation and activation of pp60c-src during fibroblast mitosis. Cell. 1988 Mar 25;52(6):801–810. doi: 10.1016/0092-8674(88)90422-9. [DOI] [PubMed] [Google Scholar]
- Chou Y. H., Bischoff J. R., Beach D., Goldman R. D. Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell. 1990 Sep 21;62(6):1063–1071. doi: 10.1016/0092-8674(90)90384-q. [DOI] [PubMed] [Google Scholar]
- Dent J. A., Polson A. G., Klymkowsky M. W. A whole-mount immunocytochemical analysis of the expression of the intermediate filament protein vimentin in Xenopus. Development. 1989 Jan;105(1):61–74. doi: 10.1242/dev.105.1.61. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G. Recovery of surface membrane in anterior pituitary cells. Variations in traffic detected with anionic and cationic ferritin. J Cell Biol. 1978 Jun;77(3):R35–R42. doi: 10.1083/jcb.77.3.r35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix M. A., Pines J., Hunt T., Karsenti E. A post-ribosomal supernatant from activated Xenopus eggs that displays post-translationally regulated oscillation of its cdc2+ mitotic kinase activity. EMBO J. 1989 Oct;8(10):3059–3069. doi: 10.1002/j.1460-2075.1989.tb08457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J., Wu M., Kirschner M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol. 1984 Apr;98(4):1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Woodgett J. R., Cooper J. A., Buss J. E., Shalloway D., Hunter T. Protein kinase C phosphorylates pp60src at a novel site. Cell. 1985 Oct;42(3):849–857. doi: 10.1016/0092-8674(85)90281-8. [DOI] [PubMed] [Google Scholar]
- Hepler P. K. Calcium transients during mitosis: observations in flux. J Cell Biol. 1989 Dec;109(6 Pt 1):2567–2573. doi: 10.1083/jcb.109.6.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Kline D. Calcium-dependent events at fertilization of the frog egg: injection of a calcium buffer blocks ion channel opening, exocytosis, and formation of pronuclei. Dev Biol. 1988 Apr;126(2):346–361. doi: 10.1016/0012-1606(88)90145-5. [DOI] [PubMed] [Google Scholar]
- Kubota H. Y., Yoshimoto Y., Yoneda M., Hiramoto Y. Free calcium wave upon activation in Xenopus eggs. Dev Biol. 1987 Jan;119(1):129–136. doi: 10.1016/0012-1606(87)90214-4. [DOI] [PubMed] [Google Scholar]
- Laurent A., Basset M., Dorée M., Le Peuch C. J. Involvement of a calcium-phospholipid-dependent protein kinase in the maturation of Xenopus laevis oocytes. FEBS Lett. 1988 Jan 4;226(2):324–330. doi: 10.1016/0014-5793(88)81448-0. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Maller J. L. Induction of nuclear envelope breakdown, chromosome condensation, and spindle formation in cell-free extracts. J Cell Biol. 1985 Aug;101(2):518–523. doi: 10.1083/jcb.101.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Effects of Ca2+ ions on the formation of metaphase chromosomes and sperm pronuclei in cell-free preparations from unactivated Rana pipiens eggs. Dev Biol. 1984 Jun;103(2):434–442. doi: 10.1016/0012-1606(84)90331-2. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983 May 13;220(4598):719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- Louvard D., Reggio H., Warren G. Antibodies to the Golgi complex and the rough endoplasmic reticulum. J Cell Biol. 1982 Jan;92(1):92–107. doi: 10.1083/jcb.92.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989 May 25;339(6222):275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Dominoes and clocks: the union of two views of the cell cycle. Science. 1989 Nov 3;246(4930):614–621. doi: 10.1126/science.2683077. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989 May 25;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Peter M., Nakagawa J., Dorée M., Labbé J. C., Nigg E. A. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990 May 18;61(4):591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- Sagata N., Watanabe N., Vande Woude G. F., Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989 Nov 30;342(6249):512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- Shen S. S., Buck W. R. A synthetic peptide of the pseudosubstrate domain of protein kinase C blocks cytoplasmic alkalinization during activation of the sea urchin egg. Dev Biol. 1990 Aug;140(2):272–280. doi: 10.1016/0012-1606(90)90077-v. [DOI] [PubMed] [Google Scholar]
- Shenoy S., Choi J. K., Bagrodia S., Copeland T. D., Maller J. L., Shalloway D. Purified maturation promoting factor phosphorylates pp60c-src at the sites phosphorylated during fibroblast mitosis. Cell. 1989 Jun 2;57(5):763–774. doi: 10.1016/0092-8674(89)90791-5. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Ward G. E., Kirschner M. W. Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C. Cell. 1990 May 18;61(4):561–577. doi: 10.1016/0092-8674(90)90469-u. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Vande Woude G. F., Ikawa Y., Sagata N. Specific proteolysis of the c-mos proto-oncogene product by calpain on fertilization of Xenopus eggs. Nature. 1989 Nov 30;342(6249):505–511. doi: 10.1038/342505a0. [DOI] [PubMed] [Google Scholar]
- Whitaker M., Patel R. Calcium and cell cycle control. Development. 1990 Apr;108(4):525–542. doi: 10.1242/dev.108.4.525. [DOI] [PubMed] [Google Scholar]
- Wilson K. L., Newport J. A trypsin-sensitive receptor on membrane vesicles is required for nuclear envelope formation in vitro. J Cell Biol. 1988 Jul;107(1):57–68. doi: 10.1083/jcb.107.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeligs J. D., Wollman S. H. Mitosis in rat thyroid epithelial cells in vivo. I. Ultrastructural changes in cytoplasmic organelles during the mitotic cycle. J Ultrastruct Res. 1979 Jan;66(1):53–77. doi: 10.1016/s0022-5320(79)80065-9. [DOI] [PubMed] [Google Scholar]