Abstract
Background and Purpose
Depression is associated with stroke, but the effects of changes in depressive symptoms on stroke risk are not well understood. This study examined whether depressive symptom changes across two successive annual assessments were associated with incident stroke the following year.
Methods
We used visit data from 4,319 participants of the Cardiovascular Health Study who were stroke-free at baseline to examine whether changes in depressive symptoms classified across two consecutive annual assessments predicted incident first stroke during the subsequent year. Depressive symptoms were assessed using the 10-item Center for Epidemiologic Studies Depression scale (CES-D; high vs. low at ≥10). Survival models were inverse probability weighted to adjust for demographics, health behaviors, medical conditions, past depressive symptoms, censoring, and survival.
Results
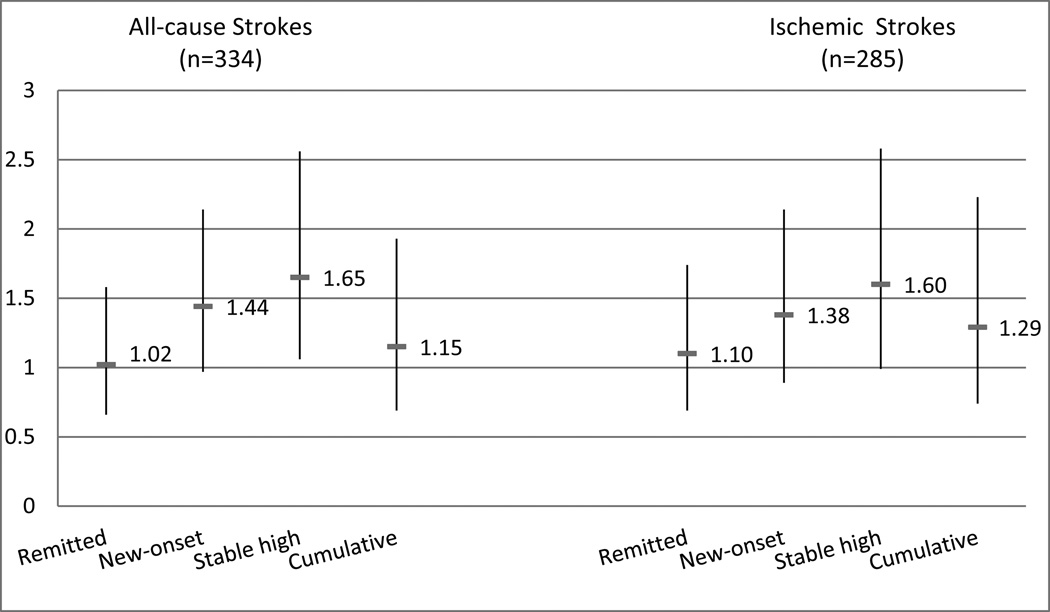
During follow-up, 334 strokes occurred. Relative to stable low scores of depressive symptoms, improved depression symptoms were associated with almost no excess risk of stroke (aHR=1.02; 95% CI: 0.66–1.58). New-onset symptoms were non-significantly associated with elevated stroke risk (aHR=1.44; 95% CI: 0.97–2.14) while persistently high depressive symptoms were associated with elevated adjusted hazard of all-cause stroke (aHR=1.65; 95% CI: 1.06–2.56). No evidence for effect modification by race, age, or sex was found.
Conclusions
Persistently high symptoms of depression predicted elevated hazard of stroke. Participants with improved depressive symptoms had no elevation in stroke risk. Such findings suggest that strategies to reduce depressive symptoms may ameliorate stroke risk.
Keywords: depression, cerebrovascular accidents, ischemic stroke, epidemiology, marginal structural models
INTRODUCTION
Major Depressive Disorder and depressive symptoms have been associated with an elevated risk of incident all-cause stroke1, 2. Debate continues regarding whether this association reflects confounding by undiagnosed vascular disease or causal effects of depression mediated by behavioral or physiologic pathways3, 4. Many studies documenting the depression-stroke association have evaluated only baseline measures of depression or depressive symptoms and confounders1. Such studies cannot assess whether effects are due to cumulative damage from years of exposure or shorter-term alterations imposed by transient depressive symptoms. Further, prior studies have rarely attempted to estimate the effects of changes in depressive symptom burden on stroke risk, partially because of methodological challenges related to assessment of time-varying confounders that may partially mediate the depression-stroke relationship5, 6. Estimating associations of stroke risk with a nuanced assessment of depressive symptoms can help elucidate timing of the effect of depression on stroke risk. This may help suggest potentially modifiable mechanisms and provide evidence on whether successful treatment of depressive symptoms is likely to reduce stroke risk. For example, if depression has acute effects on stroke risk, we might expect stroke risk to be heightened directly after onset of symptoms. Conversely, if depression alters biological processes and behaviors that influence stroke, we might expect impacts on stroke risk to emerge well after the occurrence of depressive symptoms and to remain elevated after remission.
We use data from the Cardiovascular Health Study (CHS), a large population-based cohort with annual assessment of depressive symptoms over ten years and stroke ascertainment during follow-up, to examine whether changes in depressive symptoms over two successive points in time are associated with elevated stroke risk. We adjusted for time-varying confounders, survival, and study attrition by implementing stabilized inverse probability weights (SIPWs).
For this study we defined the exposure window as two successive assessments of depressive symptoms that were one year apart. We tested the hypothesis that the risk of stroke would be greatest for those with elevated depressive symptoms at both time points. We hypothesized that stroke risk would be moderately increased for individuals with elevated depressive symptoms at only one time point.
METHODS
Study Population
Data were from the CHS, a large population-based cohort of men and women 65 and older from a sample of Medicare-eligible individuals in four Field Centers: Sacramento County, California; Washington County, Maryland; Forsyth County, North Carolina; and Pittsburgh, Pennsylvania7, 8. In 1989–1990, the Field Centers recruited 5,201 participants. In 1992–1993, an additional 687 African Americans were recruited using similar methods. Participants had annual clinical evaluations until 1998/99 with semi-annual follow-up phone interviews continuing since enrollment. The overall refusal rate for annual contact was 5.1%9. Institutional review committees at each study site approved the study, and all participants provided informed consent9. Current analyses were deemed exempt by the Harvard School of Public Health Human Subjects Committee.
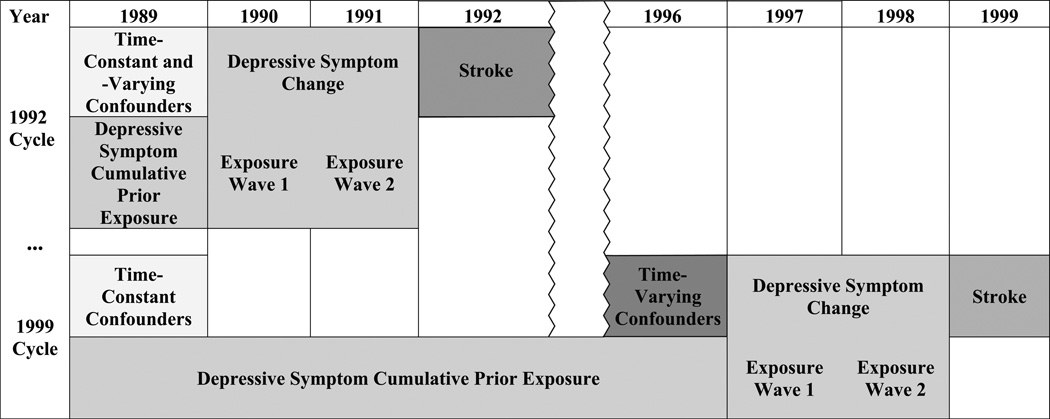
Stroke assessments examined at each study wave during 1991–1999 were linked to a depressive symptom exposure period consisting of the two consecutive annual assessments immediately preceding it. To ensure that measured confounders were temporally prior to the depression measures, we used measurements from the interview conducted three years prior to stroke assessment (Figure 1). From the 5,888 individuals enrolled, individuals were excluded for the following reasons: having a history of stroke or missing an interview prior to the end of the first exposure period (277 individuals; 4.71%), missing depression scores during either of the first two exposure waves (779 individuals; 13.23%) and missing baseline covariates (513 individuals; 8.71%). See Supplement Table I for missing data patterns at enrolment.
Figure 1. Timing of depressive symptom, confounder, and stroke assessments for participants enrolled in 1989.
The first observation for participants enrolled in 1989 contains stroke assessment from 1992, changes in depressive symptoms from 1990–1991, and time-constant and –varying confounders from 1989. Their cumulative prior exposure to depressive symptoms is defined only by 1989. Assuming they remain uncensored, their final observation contains 1999 strokes assessment, 1997–1998 depressive symptom change, and cumulative prior exposure to depressive symptoms from 1989 until 1996. Their time-constant are from 1989, and time-varying confounders are from 1996.
The remaining 4,319 eligible participants contributed 24,940 observations with depressive symptom assessments. The overall median follow-up time from baseline was 8.96 years (range: 1.99–10.89); with a follow-up time of 9.69 years (range: 1.99–10.89) for participants enrolled in 1989/1990 and 6.92 years (range: 2.14–7.26) for participants enrolled in 1992/1993.
Stroke Outcomes
Strokes were identified by participant or proxy notifying CHS staff, by CHS staff asking about previous medical events during annual clinical visits and semi-annual phone interviews, or during review of medical records8. A committee comprising neurologists, a neuro-radiologist, and a physician representative from the Coordinating Center adjudicated all potential stroke events and categorized them as ischemic, hemorrhagic, or uncertain based on medical records, brain Magnetic Resonance Imaging (MRIs), patient or proxy interviews, death certificates, and the Medicare hospitalization database as described previously8, 10. Participants classified with uncertain stroke type were excluded from analyses examining effects of depressive symptoms on specific stroke types. Transient ischemic attacks were not considered as outcome events in this analysis.
Depressive Symptom Exposure
Depressive symptoms were measured using a previously validated shortened version of the CES-D scale with ten items focusing on mood, energy level, sleep, concentration, and irritability11, 12. Response options were on a 4-point scale with higher scores indicating greater frequency of symptoms during the past week (see Supplement Table II for item wording). A total depressive symptom score was calculated by summing scores across all items (reverse coding as appropriate) for a maximum score of 30. For each exposure year, a total score of at least 10 was classified as elevated depressive symptoms. This threshold has been previously validated in an older population against the 20-item CES-D (Cohen’s Kappa statistic=0.97)13.
Two-year depressive symptom exposure was classified into four categories based on a the two consecutive annual assessments immediately preceding the stroke assessment wave, which was shifted one year at a time to create a moving window for exposure categorization. These categories were: 1) stable low: no elevated symptomatology at either the first or second exposure wave; 2) remitted: elevated depressive symptoms at the first exposure wave but not the second exposure wave; 3) new-onset: elevated depressive symptoms at the second but not at the first exposure wave; and 4) stable high: persistently elevated depressive symptoms at both first and second exposure waves. The reference group for all analyses were participants with stable low depressive symptoms.
Cumulative prior exposure to depressive symptoms reflects an individual’s depressive symptom history prior to the two-year exposure window. Specifically, it was calculated as the proportion of assessment waves an individual reported elevated depressive symptoms, including all waves from baseline up to 3 waves prior to stroke assessment (i.e., through the wave prior to, but not including, the moving window described above). This cumulative exposure measure has a theoretical range from 0 (elevated depressive symptoms at none of the previous waves) to 1 (elevated depressive symptoms at all previous waves). See Figure 1 for an illustration of the data structure for an individual enrolled in 1989.
Covariates
We considered several potential confounders that remain stable over time and some confounders that change over time. Time-constant confounders included sex, age at enrollment (modeled as linear plus quadratic terms), self-reported race/ethnicity (non-Hispanic black, non-Hispanic white, Hispanic, or other), education (less than high school, high school, some college/vocational school, or college or more), enrollment phase (1989/1990 or 1992/1993), and clinic site. Time-updated confounders included income quartile and the following health behaviors and conditions: at least one alcoholic beverage a week (yes/no), current smoking (yes/no), blocks walked per week (continuous), total kcal expended during physical activity (continuous), obesity (body mass index≥30), heart diseases (yes/no), hypertension (yes/no), diabetes (yes/no), antidepressant use (yes/no), and cognitive function (continuous). Hypertension status was based on history of hypertension, medication use, and blood pressure measurements obtained during clinic visits. Diabetes status was based on the American Diabetes Association criteria14. Total kilocalorie expenditure was estimated during the 1st, 4th, and 8th assessments waves using self-reported frequency and duration of 16 activities including chores. Study personnel recorded antidepressant use during the two weeks prior to clinical visits by directly transcribing information from prescription bottles brought in by participants7. Cognitive function was measured using 30-point Mini-Mental State Examinations the 1st assessment wave and the 100-point Modified Mini-Mental State Examination during the remainder of follow-up15, 16. Missing values on time-updated covariates were imputed by carrying forward the most recent prior report.
Methods of analysis
We examined the distribution of depressive symptoms and time-updated confounders at each wave. In our main analysis, we used marginal structural models to concurrently estimate the hazard ratio for incident stroke associated with the depressive symptom categories in the most recent 2-wave exposure window (with stable low depressive symptoms as the reference group) and cumulative prior exposure to depressive symptoms. The model was estimated with discrete-time pooled logistic regressions to accommodate the fact that strokes were examined in one year intervals. Time-constant demographic variables were directly included in marginal structural models predicting hazard of stroke.
We controlled for time-updated confounders without conditioning on possible mediating pathways by applying stabilized inverse probability weights, which are calculated in such a way that after weighting depressive symptom patterns are unrelated to confounders. We estimated SIPWs to also adjust differential study drop-out and survival. The weights were trimmed to the 99th percentile to reduce the influence of outliers. Details regarding construction of time- and person-specific SIPWs are reported elsewhere5, 6.
We evaluated whether effects of depressive symptom categories were similar for ischemic and hemorrhagic strokes. We assessed possible multiplicative effect modification by sex, baseline age (continuous), and race on all-cause stroke. Due to the small number of participants with self-reported Hispanic ethnicity or self-reported “other” race, these groups were excluded in analyses examining possible effect modification by race/ethnicity. We also conducted sensitivity analyses requiring a change of at least six points (20% of possible score) in depressive symptom levels for participants to be classified as having new-onset or remitted depressive symptoms. We examined the association of depressive symptom change with all-cause strokes, further adjusting for the Digit Symbol Substitution Test, a measure of cognitive function. We also compared models in which we dropped the adjustment for cumulative prior depressive symptom exposure. Lastly, we examined models controlled for only demographics and weighted for survival and participation (i.e., not accounting for time-updated confounders). Participants were censored at first stroke, death, or the first time they did not respond to depressive symptom questions. Two-sided p-values less than 0.05 and less than 0.10 were considered statistically significant and marginally significant, respectively. All analyses were conducted using SAS 9.3.
RESULTS
The 4,319 participants comprising the analytic sample (Table 1) experienced 334 incident strokes (285 ischemic, 33 hemorrhagic, and 16 undetermined stroke type) during 1992–1999. Across each assessment wave, the most common exposure category was stable low depressive symptoms (Supplement Table III).
Table 1.
Baseline characteristics of sample population, CHS (n=4,319 participants)
| Characteristics | n(%) or mean (standard deviation) |
|---|---|
| Male | 1,798 (41.63%) |
| Race/ethnicity | |
| Non-Hispanic white | 3,706 (85.81%) |
| Non-Hispanic black | 548 (12.69%) |
| Age (years) | 72.36 (5.23) |
| Married | 2,891 (66.94%) |
| Income | |
| Under $5,000 | 175 (4.05%) |
| $5,000–7,999 | 365 (8.45%) |
| $8,000–11,999 | 489 (11.32%) |
| $12,000–15,999 | 655 (15.17%) |
| $16,000–24,999 | 870 (20.14%) |
| $25,000–34,999 | 708 (16.39%) |
| $35,000–49,999 | 451 (10.44%) |
| $50,000+ | 606 (14.03%) |
| Educational attainment | |
| Less than high school | 1,131 (26.19%) |
| High school | 1,244 (28.80%) |
| Some college/vocational school | 1,008 (23.34%) |
| College+ | 936 (21.67%) |
| Depressive symptoms | |
| CES-D score (continuous)* | 4.99 (4.64) |
| CES-D score≥10 | 643 (14.89%) |
| Obese | 871 (20.17%) |
| At least one drink/week | 2,241 (51.89%) |
| Current smoker | 503 (11.65%) |
| Elevated blood pressure | 1,833 (42.44%) |
| Diabetes | 642 (14.86%) |
| Antidepressant use | 140 (3.24%) |
| Heart disease | 304 (7.04%) |
CES-D=Center for Epidemiologic Studies Depression scale
Compared to those with stable low depressive symptoms, participants with stable high depressive symptoms had significantly elevated hazard of stroke (adjusted hazards ratio (aHR)=1.65; 95% confidence interval (CI) 1.06–2.56; Figure 2). New-onset symptoms were associated with elevated stroke risk (aHR=1.44; 95% CI 0.97–2.14) although the association was not statistical significance. Remitted symptoms were not associated with excess risk (aHR=1.02; 95% CI 0.66–1.58). Cumulative depressive symptom exposure was not associated with stroke incidence when accounting for depressive symptoms change at the most recent two waves (aHR=1.15; 95% CI 0.69–1.93). Results remained similar in models further adjusting for the Digit Symbol Substitution Test (Supplement Table IV).
Figure 2. Adjusted hazard ratios for incident stroke by depressive symptom category among CHS participants (n=24,940 observations).
Models were adjusted for sex, race/ethnicity, education, age at enrollment, enrollment phase, clinic site as well as differential survival, exposure, participation, and time-varying confounders. Compared to individuals with stable low depressive symptoms, those with stable high depressive symptoms during the two previous assessments waves were at elevated risk of all-cause stroke and ischemic stroke.
When considering stroke types separately (Figure 2), individuals with stable high depressive symptoms had a 60% excess hazard of ischemic stroke, but this effect estimate did not reach statistical significance (95% CI: 0.99–2.58). Depressive symptoms were not associated with hemorrhagic strokes, but effect estimates were imprecise due to the small number of hemorrhagic events (Supplement Figure I).
In stratified analyses, stable high depressive symptoms were marginally associated with a 58% and 59% excess hazard of stroke among females and non-Hispanic whites, respectively (Table 2). Among these groups, elevated risk of stroke was also marginally associated with new-onset of depressive symptoms. While stable high depressive symptoms were associated with elevated effect estimates among the remaining subgroups, estimates were not statistically different from stable low depressive symptoms. Remitted depressive symptoms were not associated with elevated hazard of stroke among any subgroup. Cumulative prior exposure was marginally significantly associated with stroke risk among non-Hispanic blacks. The Wald Chi-square p-values for interaction terms examining possible differences in effect by sex (0.72), race (0.76), or baseline age (0.93) were not statistically significant.
Table 2.
Stratified marginal structural models: Adjusted hazard ratio of incident all-cause stroke by depressive symptom category*
| Stable Low | Remitted | New-Onset | Stable High |
Cumulative Prior Exposure |
|
|---|---|---|---|---|---|
| Sex | |||||
| Males (n=10,061 obs; 141 strokes) |
ref | 0.70 (0.30–1.65) |
1.24 (0.62–2.49) |
1.82 (0.85–3.92) |
1.23 (0.50–3.00) |
| Females (n=14,879 obs; 193 strokes) |
ref | 1.18 (0.70–1.99) |
1.54 (0.95–2.50) |
1.58 (0.93–2.68) |
1.15 (0.62–2.15) |
| Race | |||||
| NH white (n=22,070 obs; 293 strokes) |
ref | 0.89 (0.54–1.48) |
1.44 (0.94–2.20) |
1.59 (1.00–2.54) |
1.32 (0.78–2.23) |
| NH black (n=2,490 obs; 35 strokes) |
ref | 1.33 (0.45–3.91) |
1.16 (0.31–4.30) |
2.61 (0.79–8.57) |
0.14 (0.02–1.29) |
Model adjusts for selective survival, participation, and prior exposure to depressive symptoms through weights and for time-constant confounders (age at enrollment, race/ethnicity, clinic site, enrollment cohort, education) through direct inclusion in the regression.
In sensitivity analysis requiring at least a six-point change in CES-D score for depressive symptoms to be classified as changed (either new-onset or remitted), the effect estimate of stable high depressive symptoms was associated with excess elevated hazard of all-cause stroke at 77% and ischemic stroke at 69% (Table 3). New-onset depressive symptoms were associated with a 50% excess hazard of all-cause stroke. Neither remitted depressive symptoms nor cumulative prior exposure to depressive symptoms were associated with an elevated hazard of all-cause or ischemic stroke. The effect estimates of stable high depressive symptoms and new-onset depressive symptoms on hemorrhagic stroke were elevated but imprecise and not significant.
Table 3.
Adjusted hazard ratios for incident stroke, by depressive symptom category, requiring at least a six-unit change for symptom new-onset or remission (n=24,940 obs)*
| Depressive Symptom Category |
All-Cause Strokes (334 cases) aHR (95% CI) |
Ischemic Strokes (285 cases) aHR (95% CI) |
Hemorrhagic Strokes (33 cases) aHR (95% CI) |
|---|---|---|---|
| Stable low | ref | ref | ref |
| Remitted | 1.08 (0.70–1.66) | 1.15 (0.73–1.81) | 0.52 (0.07–3.88) |
| New-onset | 1.50 (1.01–2.23) | 1.44 (0.93–2.23) | 1.83 (0.61–5.47) |
| Stable high | 1.77 (1.17–2.70) | 1.69 (1.07–2.66) | 2.01 (0.68–5.97) |
| Cumulative prior exposure |
1.10 (0.67–1.81) | 1.25 (0.73–2.12) | 0.33 (0.06–1.86) |
Model adjusts for selective survival, participation, and prior exposure to depressive symptoms through weights and for time-constant confounders (age at enrollment, race/ethnicity, clinic site, enrollment cohort, education) through direct inclusion in the regression.
When we did not control for cumulative prior exposure of depressive symptoms in the models, the effect estimate for stable high depressive symptoms remained elevated (aHR=1.77; 95% CI: 1.25–2.50) while the risk associated with new-onset depressive symptoms was elevated and marginally significant (aHR=1.48; 95% CI: 1.00–2.18). Remitted depressive symptoms were not associated with stroke (aHR=1.05, 95% CI: 0.69–1.59). Models with all depressive symptoms measures but controlling for only demographics and weighted for survival and participation had similar, slightly stronger effect estimates (Supplement Table V).
DISCUSSION
In a large cohort of older Americans with adjudicated strokes, participants with persistently elevated depressive symptoms over two consecutive annual assessments had a 65% increased hazard of incident all-cause stroke during the following year compared to their counterparts with consistently low depressive symptoms after adjusting for known potential confounders, sample attrition and depressive symptom history. Relative to those with consistently low depressive symptoms, participants with new-onset of depressive symptoms during the exposure period had a 44% increase in hazard of incident all-cause stroke, though this was of marginal statistical significance. Remitted depressive symptoms and cumulative prior exposure to depressive symptoms were not significantly associated with an elevated hazard of stroke in any analysis. There was no evidence of effect modification by sex, race, or age.
Overall, these findings support the hypothesis that individuals with stable high depressive symptoms across two consecutive annual assessments have excess risk of stroke during the next year compared to individuals with stable low depressive symptoms. This is consistent with a prior study in the Health and Retirement Study (HRS) using similar methodology, which found that individuals with persistent elevated depressive symptoms across two biennial interview waves had more than double the risk of stroke in the following two-year window than individuals with stable low depressive symptoms.6 Contrary to our hypothesis and prior results in the HRS study, we did not find evidence that individuals with remitted depressive symptoms remain at elevated stroke risk during the following year. Furthermore, unlike our results in the HRS, these findings suggests that onset of depressive symptoms may also be associated with elevated risk of stroke.
Pathways linking depressive symptoms and stroke remain unclear but may be categorized as operating in the shorter and longer term. Depression has been associated with stroke triggers such as infection17, 18; some work has suggested depression may down regulate the immune system thereby increasing risk of infection19, 20. The few mediation analyses that have been conducted in prior literature examined possible pathways that may operate over a longer time frame. One possibility is that depression leads to higher inflammation or greater metabolic dysregulation which increases risk of stroke21–24. One study found evidence suggesting a composite of major metabolic diseases (hypertension, hyperlipidemia, and diabetes) partially mediated the association between major depressive disorder and all-cause stroke23. However, the various metabolic diseases were not examined separately, leaving the precise pathway unclear. Depressive symptoms or diagnosis have also been associated with physiological dysregulation which develops over a long period of time (e.g. atherosclerosis25, white matter hypertensities26) and may directly increase risk of stroke. However, depression and stroke could simply share risk factors with stroke. Future research should continue to examine possible mediators of the relationship between depressive symptoms and stroke functioning both in the short- and long-terms.
A strength of our study was the ability to consider dynamic aspects of depressive symptoms due to the annual assessment of depressive symptoms. Rigorous assessment of stroke incidence and type reduces the risk of measurement bias. With information on a wide range of possible confounders of the association between depressive symptoms and stroke, death, and study participation, we can also appropriately control for time-varying confounders and account for selective attrition through SIPWs.
Regarding study limitations, our sample did not allow for separate consideration of individuals who identified as Hispanic or a race other than black or white. A plausible set of unmeasured confounders relate to subclinical vascular diseases, which have been hypothesized to increase depression risk3, and other unmeasured confounders as well as residual confounding remain possible. This analysis included only one depressive symptom assessment per year and did not address how trajectories of symptoms over a longer period of time might impact stroke risk. This study also does not examine if effects on stroke risk differ by reason for depressive symptom remission (e.g. psychotherapy, pharmacotherapy, or natural resolution).
CONCLUSIONS
In conclusion, our study provides valuable information to public health and medical professionals by examining a dynamic relationship between two prevalent conditions: elevated depressive symptoms and incidence of stroke. We contribute to the research by showing evidence that sustained elevated depressive symptoms are associated with increases in stroke risk and suggesting that risk of stroke may decrease if depressive symptoms remit. Further research should consider even more comprehensively the effects of depressive symptom trajectories, depressive symptom variability, the consequences of different forms of depression treatment, the association between depression and subtypes of ischemic stroke, the mechanisms that may underlie the associations between depression and stroke, and over what time frame interventions are most effective.
Supplementary Material
Acknowledgments
Financial support: This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The authors gratefully acknowledge financial support from the following NIH institutes: National Heart, Lung, and Blood Institute [F31HL112613 to P.G.]; the National Institute of Mental Health [RC4MH092707 to L.K. and M.G.]; National Institute of Aging [R21AG03438502 to M.G.]; National Institute of Allergy and Infectious Diseases [AI113251 and AI104459 to E.T.T.]; and National Institute of Environmental Health Science [AI113251 to E.T.T.] as well as financial support from the Initiative for Maximizing Student Development [R25GM055353 to P.G.]; the Yerby Postdoctoral Fellowship [to P.G.]; and the American Heart Association [10SDG2640243 to M.G. and P.G.]. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health. The study funders had no role in the design, execution, or interpretation of these analyses.
Footnotes
Disclosures: None.
References
- 1.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA. 2011;306:1241–1249. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong JY, Zhang YH, Tong J, Qin LQ. Depression and risk of stroke: a meta-analysis of prospective studies. Stroke. 2012;43:32–37. doi: 10.1161/STROKEAHA.111.630871. [DOI] [PubMed] [Google Scholar]
- 3.Brodtmann A. Vascular risk, depression, and stroke: post hoc ergo propter hoc … or not. Neurology. 2014;83:1688–1689. doi: 10.1212/WNL.0000000000000968. [DOI] [PubMed] [Google Scholar]
- 4.Kubzansky LD, Winning A, Kawachi I. Affective states and health. In: Berkman LF, Kawachi I, Glymour MM, editors. Social epidemiology. 2014. pp. 320–364. [Google Scholar]
- 5.Robins JM, Hernán MÁ, Brumback B. Marginal Structural Models and Causal Inference in Epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Gilsanz P, Walter S, Tchetgen Tchetgen EJ, Patton KK, Moon JR, Capistrant BD, et al. Changes in Depressive Symptoms and Incidence of First Stroke Among Middle-Aged and Older US Adults. J Am Heart Assoc. 2015;4:e001923. doi: 10.1161/JAHA.115.001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 8.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 9.Strotmeyer ES, Arnold AM, Boudreau RM, Ives DG, Cushman M, Robbins JA, et al. Long-Term Retention of Older Adults in the Cardiovascular Health Study: Implications for Studies of the Oldest Old. Journal of the American Geriatrics Society. 2010;58:696–701. doi: 10.1111/j.1532-5415.2010.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longstreth WT, Jr, Bernick C, Fitzpatrick A, Cushman M, Knepper L, Lima J, et al. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology. 2001;56:368–375. doi: 10.1212/wnl.56.3.368. [DOI] [PubMed] [Google Scholar]
- 11.Steffens DC, Helms MJ, Krishnan KRR, Burke GL. Cerebrovascular Disease and Depression Symptoms in the Cardiovascular Health Study. Stroke. 1999;30:2159–2166. doi: 10.1161/01.str.30.10.2159. [DOI] [PubMed] [Google Scholar]
- 12.Radloff LS. The CES-D Scale. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 13.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D. Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 14.Diagnosis TECot, Mellitus CoD. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 15.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Adams TB, Wharton CM, Quilter L, Hirsch T. The association between mental health and acute infectious illness among a national sample of 18- to 24-year-old college students. J Am Coll Health. 2008;56:657–663. doi: 10.3200/JACH.56.6.657-664. [DOI] [PubMed] [Google Scholar]
- 18.Falagas ME, Karamanidou C, Kastoris AC, Karlis G, Rafailidis PI. Psychosocial factors and susceptibility to or outcome of acute respiratory tract infections [Review article] The International Journal of Tuberculosis and Lung Disease. 2010;14:141–148. [PubMed] [Google Scholar]
- 19.Herbert TB, Cohen S. Depression and immunity: A meta-analytic review. Psychological Bulletin. 1993;113:472–486. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- 20.Kiecolt-Glaser JK, Glaser R. Depression and immune function: Central pathways to morbidity and mortality. J Psychosom Res. 2002;53:873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 21.Empana JP, Sykes DH, Luc G, Juhan-Vague I, Arveiler D, Ferrieres J, et al. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2005;111:2299–2305. doi: 10.1161/01.CIR.0000164203.54111.AE. [DOI] [PubMed] [Google Scholar]
- 22.Arbelaez JJ, Ariyo AA, Crum RM, Fried LP, Ford DE. Depressive symptoms, inflammation, and ischemic stroke in older adults: a prospective analysis in the cardiovascular health study. Journal of the American Geriatrics Society. 2007;55:1825–1830. doi: 10.1111/j.1532-5415.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 23.Li C-T, Bai Y-M, Tu P-C, Lee Y-C, Huang Y-L, Chen T-J, et al. Major Depressive Disorder and Stroke Risks: A 9-Year Follow-Up Population-Based, Matched Cohort Study. PLoS One. 2012;7:e46818. doi: 10.1371/journal.pone.0046818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pizzi C, Costa GM, Santarella L, Flacco ME, Capasso L, Bert F, et al. Depression symptoms and the progression of carotid intima–media thickness: A 5-year follow-up study. Atherosclerosis. 2014;233:530–536. doi: 10.1016/j.atherosclerosis.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79:619–624. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.